Please cite this paper as:
Banerjee, A., Chattopadhyay, A. and Bandyopadhyay, D. 2021. Melatonin: an ancient note in a contemporary wrap. Melatonin Research. 4, 3 (Sept. 2021), 453-478. DOI:https://doi.org/https://doi.org/10.32794/mr112500105.
Review
Melatonin: an ancient note in a contemporary wrap
Adrita Banerjee1, 2, Aindrila Chattopadhyay2*, Debasish Bandyopadhyay1#*
1Oxidative Stress and Free Radical Biology Laboratory, Department of Physiology, University of Calcutta, 92, APC Road, Kolkata-700009, India
2Department of Physiology, Vidyasagar College, 39, Sankar Ghosh Lane, Kolkata-700006, India
#Principal Investigator, Centre with Potential for Excellence in a Particular Area, (CPEPA), University of Calcutta, 92, APC Road, Kolkata-700009, India
*Correspondence: dbphys@caluniv.ac.in; debashisbaner@gmail.com, Tel: +91-9433072066, aindrila63@gmail.com, Tel: +91-9836060830
Running Title: Functional evolution of melatonin
Received: March 9, 2021; Accepted: September 18, 2021
ABSTRACT
At the beginning of life, natural selection is and still the principal driving force for the evolution of all organisms to adapt in the particular environments of the earth. As a result, ultimately neither the strongest, nor the supreme intelligent but the most adaptable species win the race. Not only the organisms, but also the elements which are necessary for survival of them also undergo extreme evolution. These include DNA, proteins and other biochemical molecules. However, melatonin, an indoleamine, presents in the early life form remains unchanged in its structure from unicellular organisms to mammals. When it was discovered, it was considered to be a neuronal hormone produced exclusively in the pineal gland of vertebrates. The latter discovery of its presence in primitive bacteria drives the melatonin research in different directions. Its primary function is serving as an antioxidant in all organisms. Its chemical structure is perfect to scavenge free radicals and thus, this molecule is preserved from bacteria to mammals. However, this molecule acquired many additional functions during evolution. These include circadian regulation, immuno-enhancement, oncostatic, anti-inflammatory and anti-aging activities. In the review, we are trying to present hypothetical and most plausible chronological events in the functional evolvements of melatonin during the process of evolution.
Key words: Origin of life, melatonin, evolution, pineal gland, receptor, antioxidant, oxidative stress.
__________________________________________________________________________
1. INTRODUCTION
The environments, in which we inhabit, have undergone drastic changes in both of its physical and chemical context with time and these lead to adaptation of its habitats. Since nature is the primary cue to all such amendments, natural selection has been the driving force for evolution.
At the early stages of life, completely anaerobic environment facilitates the existence of obligatory anaerobes only, who can survive merely without oxygen (O2) (1). With advancement of time, primitive bacteria, which rely upon anoxygenic photosynthesis for carbon assimilation, evolved to facultative anaerobe like proteobacteria, where O2 no longer remains toxic for existing organisms. This change was triggered by the environment itself since the oxygenated atmosphere was gradually formed in the earth (2). The poorly reduced primordial anoxic atmosphere was predominated by carbon dioxide (CO2) (3, 4) and gradually O2 became one of the most widespread elements of earth (5). A photochemistry model speculated the formation of O2 rich atmosphere as a result of abiotic photolysis of water (H2O) and CO2 (6). In contrast, abiotically produced hydrogen peroxide (H2O2) from pyrite/aqueous suspension has also been considered as the source of O2 (7). In addition, the activities of the microbes, particularly cyanobacteria also contribute to the formation of oxygenated atmosphere (8). The extreme alterations of the environment took place about 2.5 billion years ago, in early Proterozoic era with happening of Great Oxygen Event that drove extinction of many obligatory anaerobic species and ensured the survival of aerobic organisms (9, 10). This landmark event was triggered by cyanobacteria, an organism from one of the most ancient evolutionary lineages. Cyanobacteria was the first to be evolved with photosystem II, advancing towards oxygenic photosynthesis which leads to the appearance of oxygen in atmosphere and subsequent changes in earth system (8, 11, 12). Along with this atmospheric modulation, due to the continuous exposure of organisms to oxygen and owing to oxygen dependent energy metabolism, deleterious free radicals also generated and inevitably cause oxidative stress to these organisms (13). To reduce the deleterious impact of the oxidative stress, some oxygen consuming species have to give rise to antioxidants to help them coping with reactive oxygen species (ROS) for their survival. Eventually, some molecules with reduced capacity have been selected to be antioxidants. Around 1.5 to 2 billion years ago, the pro-eukaryotes have been emerged. Based on the theory of endosymbiosis, a pro-eukaryote engulfs an alpha-proteobacterium. This alpha-proteobacterium avoids digestion by the host cell and symbiotically lives inside the host cell. This bacterium has the capacity of oxidative phosphorylation and generates ATP with high efficiency and provides extra energy to the host cell. The pro-eukaryote and alpha-proteobacterium are beneficial to each other. This endosymbiotic relationship between the host and intruder finally results in the mitochondrial formation from the alpha-proteobacterium (14). It is hypothesized that the secondary endosymbiosis of the eukaryotes with cyanobacteria leads to the generation of plants and the cyanobacteria are the precursors of the chloroplasts (15, 16). Currently, evidence has been shown that archaea may involve in the origin of eukaryote and mitochondria (17, 18). Interestingly, in animals and plants, both mitochondria and chloroplasts are the major sites for synthesis of melatonin (19), a molecule having both free radical scavenging and endogenous antioxidant enzyme stimulating properties (20-22) while mitochondria and chloroplasts are the main sources of ROS production (19).
Since its discovery (23), scientists have continuously extended their investigations to establish the functional relevance of its existence. It is broadly present from primitive bacteria to high ranks of eukaryotes and this indicates the pleiotropic roles of this indole among species (24). Though the presence of this molecule in archaea is still under investigation, the horizontal gene transfer from bacteria to archaea (25, 26) as well as collateral emergence of both archaea and bacteria bring speculation on its presence in the domain of archaea (26). Moreover, the emergence of eukaryotes from archaea carries a confirmatory note of melatonin synthesis in archaea (27, 28) since eukaryotes possess well developed machineries for melatonin synthesis (29). While its unchanged chemical structure throughout evolution leads to the concept of its primary function to be an antioxidant, its circadian rhythm observed in most of the species also suggests it as an entrainer of ‘biological clock’ (30). For example, dinoflagellates as well as several microalgae, chlorophyceans, the photoautotrophs all possess melatonin biorhythm with prominence (26, 31-33). This rhythm has been identified also in many plants, irrespective of their classifications (34, 35). The protection of plants from various biotic and abiotic stressors during daylight has been assumed to be the factor behind such a diurnal rhythm (26). In contrast, evolution has brought a dedicated pineal gland in vertebrates to secrete melatonin with circadian rhythm (23). This molecule seems to regulate the sleep/wake cycle (36, 37), peripheral organ oscillation (38) and reproductive physiology of vertebrates (39, 40) via master clock, the suprachiasmatic nucleus (SCN) in hypothalamus (41). The actions are mediated by melatonin receptor. For example, anti-inflammatory and anti-cancerous effects of melatonin are receptor-dependent (42-44). Hence, functional evolution of melatonin in organisms for adaptive responses make them surviving under different (or changeable) environments.
2. MELATONIN: THE OMNIPRESENT MOLECULE IN CHRONICLE OF LIFE
Despite the co-existence of aerobic and anaerobic species, oxygen-centric surroundings drive the environment in favour of oxygen dependent aerobic organisms. During early emergence of lives, the discharged oxygen was utilized in making up the organic materials and earth crust (1). Progressively, the increased oxygen level in atmosphere trigged a mass extinction of anaerobes (2, 45, 46) while selected aerobic organisms. However, when the one electron reduction of oxygen (known as the ROS) reaches some levels it can induce deleterious effects even on the aerobic organisms. This can happen because these ROS, particularly the hydroxyl radical can injure macromolecular structures rapidly (13, 47, 48). Thus, the organisms have to develop mechanisms to tolerate ROS and reduce oxidative damage. Cyanobacteria were reported as the most ancient oxygen tolerant species (1) to counter superoxide anion radical, hydroxyl radical and many other reactive species. Thus, the endogenous antioxidants become necessary for those organisms who survive in the oxygenated ambience. Here, melatonin becomes most relevant to be discussed due to its diverse antioxidative properties and its ubiquitous presence throughout lineages of organisms (21, 49) (Figures 1, 2).
Melatonin has been first isolated from the pineal gland of cow in Twentieth century (23) to concrete the idea of its existence in high-ranking organisms. However, with advancement of research, a unicellular flagellate has also been reported as a residence of the indole (31, 50, 51). When a dinoflagellate Lingulodinium polyedrum exposed to a temperature, below its optimal one, its antioxidant enzyme level was reduced along with a sudden elevation in its melatonin production (52, 53). Moreover, physiological dose of melatonin in culture medium protects these algae against hydrogen peroxide induced oxidative damage (52). Subsequently, presence of melatonin has been identified in photosynthetic bacteria and fungi including euglenoids, trypanosomids, chlorophyceans (54-56) as well as in plants (57- 60). Although there is scarcity of evidence regarding the synthesis of melatonin in the domain of archaea, the tolerance of archaea on harsh environments including extremely high temperature (61) similar to some extremophil bacteria might be relevant for the presence of melatonin in this domain (62). In vascular plants, melatonin was first detected mainly in mono- and dicotyledon edible plant families in their roots, shoots, leaves and seeds (35, 58, 63-66). All of these discoveries have raised a question whether melatonin is conserved in evolution either due to necessity of all organisms or it is only beneficial for those species who acquire it from food chain.
Endosymbiotic theory of mitochondria and chloroplast origin explains the crucial presence of melatonin in organisms. The earliest alpha-proteobacterium, a precursor of mitochondrion and cyanobacterium, a precursor of chloroplast have already had the capacity to synthesize melatonin. As a result, organelles evolved from these bacteria also retain this capacity and became specific sites for melatonin synthesis in all organisms. Mitochondria and chloroplasts both are the heart of energy metabolism in animal and plant, respectively which have made these structures highly vulnerable to oxidative stress (19, 67). Hence, de novo melatonin synthesis in these organelles add advantages for them to detoxify on-site generated ROS. This mystery was unveiled by the breakthrough discovery of antioxidant property of the indoleamine (20) and led to the extensive investigations of its diverse antioxidant mechanisms in microorganisms, plants and animals (68-70). The natural selection has preserved this bio-weapon as an obvious requisite to combat with noxious free radicals in all organisms.

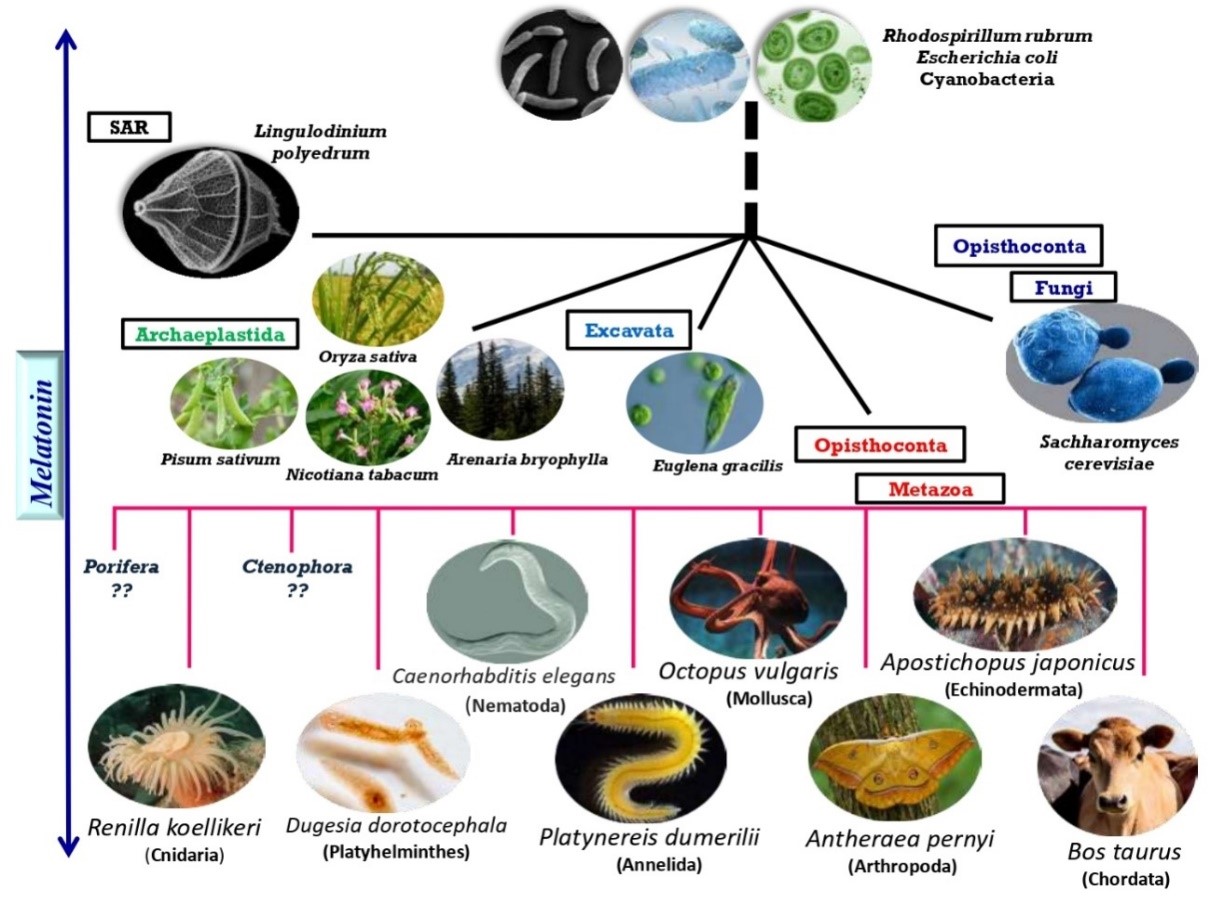
Fig.1. Ubiquitous presence of melatonin from Prokaryotes to Eukaryotes.
From Prokaryotes to Eukaryotes including Plants, Fungi and Animalia, melatonin has emerged in all life forms. At the top of the figure, presence of melatonin has been shown in unicellular prokaryotes such as in R. rubrum (24), E. coli (56), Cyanobacteria (102,26). With evolution, multicellular Eukaryotes have preserved their melatonin synthetic ability. In the superclade SAR (Stramenopila, Alveolata, Rhizaria), the dinoflagellate Lingulodinium polyedrum have developed melatonin circadian rhythm. In modern taxonomy, another superclade of eukaryotes mentioned as Archaeaplastida, comprises of green plants. When plants emerged in evolution, melatonin acquires more diverse actions including controlling photoperiodic reproduction, flowering along with its primary antioxidative actions evidenced in P. sativum (147), O. sativa (91), N. tabacum (57), A. bryophylla (35,64). The melatonin production in another eukaryote Euglena gracilis of superclade excavate exhibits circadian rhythm (54,56,62,148). Fungi, which belongs to superclade Opisthoconta, possess melatonin in members including S. cerevisiae (149) and N. crassa (56) as well as diurnal rhythm was evidenced with melatonin synthetic function in the latter. Presence of melatonin became more pertinent in Metazoans, from the same supergroup Opisthoconta. With appearance of invertebrates, melatonin showed its excellence in preserving their behavioural pattern and locomotor activities. The need of melatonin become more relevant when vertebrates appear with evolutionary alterations. Prominent sleep/wake cycle maintenance, rhythmic behavioural pattern preservation, anti-inflammatory as well as anti-cancerous activities have been affixed for this molecule in many dorotocephala species e.g., in R. koellikeri (150, 151), D. Dorotocephala (152), C. elegans (153), P. dumerilii (154), O. vulgaris (155), A. pernyi (156), A. japonicas (157), B. taurus (23). The horizontal lines are not indicative of distance.
3. DIVERSE PATHWAYS CONVERGE AT MELATONIN SYNTHESIS
Bacteria, fungi and plants are capable of synthesizing melatonin at their own through shikimic acid pathway utilizing either carbon dioxide or erythrose-4-phosphate or phosphoenolpyruvate as the source (42, 71, 72). However, the amino acid tryptophan has been identified as the initial precursor of melatonin synthesis in animals. For some animals, due to lack of tryptophan synthesizing enzymes they can only obtain it from the dietary source (42).
Even though melatonin remains structurally unchanged throughout evolutionary adaptations, its synthetic pathways have been diversified depending on the species. For example, in the initial step of melatonin synthesis, tryptophan undergoes decarboxylation by tryptophan decarboxylase to produce tryptamine in plants, while animals utilize tryptophan hydroxylase to hydrolyse tryptophan to form 5-hydroxytryptophan. Involvement of different chemical reactions toward formation of a single amine molecule indicates the diversity of enzyme-substrate specificity as well as of enzyme coding genes among different taxa. The tryptamine and 5-hydroxytryptophan, two different molecules converge to serotonin through catalytic actions of tryptamine-5-hydroxylase and aromatic amino acid decarboxylase in plants and animals respectively (29, 73-75). Serotonin undergoes two steps catalytic process to produce melatonin where, two enzymes serotonin N-acetyltransferase (SNAT) and N-acetylserotonin O-methyltransferase (ASMT) work consecutively but order of their catalysis depends on the substrate specificity of enzyme in particular species. In plants and bacteria, serotonin seemsto show more specificity towards ASMT while the terminal step has been found to be catalyzed by SNAT. Nevertheless, in a contrasting manner, in animals, acetylation has been energetically favoured over methylation of serotonin and thus the final step of melatonin synthesis has been catalysed by ASMT (76-79). Hence, it seems that evolution has selected the enzymes to act upon their substrates.
Here, the question arises that even if all organisms possess the similar capacity to synthesize melatonin, why the selectivity of enzymes to substrates has evolved with time? A variety of homologs of SNAT and ASMT discovered in plants and animals have answered this important evolutionary question. These diversities may come from the horizontal gene transferring from different bacteria and archaea. However, in vertebrates, vertical inheritance of SNAT has been identified and the homology with catalytic regions of the gene is from the phylogenic ancestors. The differences in structure and DNA sequences of N-terminal as well as C-terminal regulatory regions of SNAT in vertebrates were considered as the results of natural selection during evolution (26). Only one isoform of SNAT has been identified in mammals while non-mammalian vertebrates possess various isoforms as evidenced in fish. Exclusive expression of SNAT2 has been found in pineal gland of fish (80) whereas SNAT1a and SNAT1b were reported to be expressed in retina and other sites, respectively (81). In a similar manner, two isoforms of SNAT have been identified in rice. These OsSNAT1 and OsSNAT2, have dissimilar DNA sequences and kinetic patterns, experimented to respond in different environmental cues (82). Additionally, diversification in stimulants for various SNAT homologs has also been observed. For example, some are upregulated by photoperiodic changes and others by oxidative stress (83). Apart from melatonin synthesis, SNAT in vertebrates also involves in sclerotization of insects, indicating the functional diversity of this enzyme (84, 85). The homologs of SNAT found in unicellular green algae, fungi and bacteria possess distinct sequence pattern from its vertebrate homolog. Though catalytic domain of vertebrate SNAT shares the similarity with bacterial and/or fungal SNAT, the regulatory region, being dissimilar, confirms the natural selective power during evolution (86). Striking dissimilarities have been demonstrated between SNAT sequences of different domains of life. Vertebrate SNAT has been reported to have two histidine residues (at position 120 and 122) whereas fungi have only one and bacteria don’t possess any (86, 87). Presences of various isoforms such as SNAT1a, SANAT1b and SNAT2 have been detailed in Drosophila melanogaster (85) when another two forms SNAT5b and SNAT7 have been evidenced in Aedes aegypti (88, 89). Both SNAT and ASMT in plants showed a wide temperature tolerance range of 4 °C to 95 °C while their animal isoform exhibited a narrow range of tolerance between 25 °C to 45 °C (86, 90- 93). This variation also authenticated the evolutionary selection of enzymes with different optimum temperature since immobile plants have to face tremendous atmospheric alterations (1, 94). Other than SNAT, contribution of another form of N-acetyltransferase on melatonin synthesis has been explained in mammals (95, 96). For example, NAT-1 was reported to be involved in melatonin synthesis in mammalian skin (97), especially in the situation of SNAT deficiency (98). In addition, NAT-2 mediated N-acetylation of serotonin has been found in hamster skin (99).
The NATs of cyanobacteria and archaea share few amino acid residue homologies while sequence homologies of NATs have been confirmed between archaea and eukaryotes (62, 100, 101). Cloning and characterization of a thermophilic archaeal hypothetical acetyltransferase (NAT) that belongs to GCN5-related N-acetyltransferase (GNAT) superfamily has the function similar to SNAT of cyanobacteria (100, 102). Therefore, despite of having sequence dissimilarities, the functional resemblance as well as appearance of GNAT in both bacteria and archaea surmises the potential melatonin synthetic function in archaea.
The diversities of NATs among plants, animals and microorganisms suggest horizontal gene transfer among those taxa (85, 103, 104). However, scientists assume presence of divergent evolution also due to the limited residual similarities (105). It suggests independent emergence of NAT in different taxa with post endosymbiotic acquired modifications. Experimental evidence of considerable similarities in NAT DNA sequence and protein residues of cyanobacteria Synechocystis and plant species Oryza sativa (rice) supported symbiotic association between them (62, 91, 102). The rate limiting enzyme of melatonin synthesis has been found to be resided in plant's chloroplast and in DNA of its precursor, cyanobacteria which further supports the theory of endosymbiosis (73, 91, 106, 107). On the other hand, sharing striking homology in DNA sequences of NAT gene between Rhodospirillum rubrum and different eukaryotes again bring confirmation to endosymbiotic theory since Rhodospirillum is assumed to be the precursor of mitochondria (85, 105).
Consequently, the trail of origin of melatonin has been embedded some billion years ago which is running forward owing to its high functional relevance and regard in present day.
4. THE SITES OF MELATONIN SYNTHESIS
Endosymbiotic theory explains the presence and synthesis of melatonin in chloroplast and mitochondria. Both organelles are the major sources of ROS formation. This substantiates the presence of melatonin in both of them. The natural selection thus, preserves melatonin synthetic capacity in these most metabolically active organelles in plants and animals.
The existence of melatonin in chloroplasts is suggested from its presence in cyanobacteria, but also confirmed by the presence of rate limiting enzyme SNAT in them (73, 91, 106). Chloroplast is the site of plant photosynthesis, the major energy harvesting house of plant. During photosynthesis, as an obvious consequence, the oxygen is excited to form singlet oxygen which triggers peroxidation of poly unsaturated fatty acids in thylakoid membrane resulting into declination in photosynthetic efficiency of plants (1, 71, 108, 109). In addition to the singlet oxygen, hydrogen peroxide is another ROS to cause oxidative stress in plants. Hydrogen peroxide is formed in thylakoid membrane and chloroplast stroma by superoxide anion dismutation either enzymatically or automatically (108). In some conditions, the deficiency of catalase, glutathione peroxidase and ascorbate peroxidase (110) result in an accumulation of hydrogen peroxide within chloroplast causing injury to photosynthetic system (111). The efficacy of melatonin in scavenging hydrogen peroxide and subsequent hydroxyl radical from chloroplast has made this molecule critical for plant survival (70, 112-114) and this is also the advantage of natural selection. Apart from chloroplast, plant mitochondria also participate in the melatonin synthesis. SNAT has been identified in plant mitochondria and isolated apple mitochondria have been verified to generate melatonin (92). Since plants have to combat with countless biotic and abiotic stressors, perhaps natural selection has chosen both organelles to synthesize melatonin to ensure much high magnitude of melatonin level in plants comparing to animals. Melatonin protects plants against a variety of environmental insults including heat, cold, draught, water stress, soil pollutants etc. which goes parallel with protection by exogenously administered melatonin against chlorophyll degradation (26, 62, 115-122). Very high melatonin level of 230 μg/gm has been evidenced in pistachio nut (123). Various vegetables, herbs and fruits have been identified as source of melatonin (109, 124, 125). Moreover, evolution has loaded plants with all the enzymes required to synthesize tryptophan, the precursor of melatonin so that scarcity of tryptophan can’t limit the synthesis of the indoleamine in plants (72) such as in animals. Hence, exposure of plants to severe oxidative stress is the driving force in selecting melatonin as an on-site antioxidant to protect them from environmental insults (126, 127).
In animals, mitochondria are energy generating house and also melatonin synthetic site. Melatonin can effectively protect mitochondria from ROS damage (67, 128, 129). For example, respiratory electron transport chain (ETC) inhibition can be alleviated by melatonin (130-133). Melatonin can also reduce ruthenium red induced mitochondrial calcium channel blockage and subsequent mitochondrial damage (134). Most importantly, melatonin can maintain normal mitochondrial milieu to retard aging and several disorders (135, 136). In addition to mitochondria and chloroplasts, melatonin synthetic enzymes have been found in cytoplasm including ASMT (91), tryptophan hydroxylase (73, 75). Since melatonin synthesis requires the substrate acetyl-coenzyme A which is mainly generated in the mitochondria and chloroplasts, from the point of view of substrate availability, the cytoplasm melatonin synthesis is less efficient than that occurs in mitochondria and chloroplasts (137). Cytoplasm melatonin synthesis may play a complementary role to the two organelles in organisms.
The varied subcellular localizations of melatonin synthesis make the cell be much stronger to combat stress. Assay of level of melatonin in rodent cerebrocortical cells indicates highest concentration of melatonin within mitochondria followed by membrane, nuclei and cytosol (129). Its presence has been recognized beyond mitochondria and chloroplasts. Most of the cells and tissues have the capacity to synthesize melatonin due to the fact that they contain mitochondria. For example, gastrointestinal tract is a huge source of melatonin where enterochromaffin cells of gastric mucosa secrete melatonin. The amount of melatonin generated by gastric tissue is estimated to be several hundred-fold than pineal melatonin (138). An exocrine gland, Harderian gland in some mammals secretes melatonin with circadian rhythm (139, 140). Skin produces melatonin at a very high concentration (141). This extra pineal melatonin synthesis indicates the evolutionary choice to alleviate oxidative attack on site where free radicals produced rather than to expense time to obtain melatonin from other places.
5. FUNCTIONAL EVOLUTION OF MELATONIN
Melatonin was first isolated from pineal gland of cows with circadian rhythm (142). With further progress in research, retina was identified as another source of this indoleamine (143- 145). Both pineal gland and retina share many common features comprising their neural origin (146). Subsequently, many tissues and organs have been identified also to produce melatonin. A turning point come from the discovery of presence of melatonin in unicellular organisms of algae and bacteria (31, 52, 54, 55). It appears that melatonin may function with different purposes in early life forms rather than maintaining light-dark biorhythm in the vertebrates.
Even though the primitive photosynthetic bacteria Rhodospirillum rubrum exhibit light/dark melatonin circadian rhythm similar to the vertebrates (24), this rhythm has not been observed in other microorganisms including Escherichia coli, fungi (Saccharomyces cerevisiae) (56, 158). In Saccharomyces cerevisiae, melatonin’s production is governed by availability of its precursors, but not by the photoperiod (123). In contrast, night time surge in melatonin level has been evidenced in Neurospora crassa, whereas no indoleamine has been traced in photophase (56). It is now understandable that the absence of melatonin in photophase and high level in dark phase in some microorganisms has a different reason from vertebrates. It is hypothesized that the bacteria including Rhodospirillum rubrum, synthesize melatonin in a constant rate during the photo phase or scotophase. The reduced level of melatonin in photo phase is due to melatonin consumption as an antioxidant to scavenge free radicals induced by UV irradiation and photosynthesis (159). Thus, the primary function of melatonin in early life form is not circadian regulator but serves as the first line defence as potent antioxidant. Other functions are acquired during evolution (Figure 2).
As discussed earlier, plants have already equipped with dual melatonin synthesizing organelles to ensure high level of melatonin production. This high level of melatonin in plants significantly strengthens antioxidative capacity of plants for their survival under biotic and abiotic stressful environments (104). Melatonin protects Pisum sativum from excess copper toxicity (66). Under the UV irradiation, Nicotiana tabacum and Glycyrrhiza uralensis shield themselves by elevating their endogenous melatonin concentrations to combat the UV induced oxidative injury (57, 160). It appears as a common feature that plants under harsh environments always do enhance their melatonin production to protect themselves (35, 64). This phenomenon has also been observed in plants exposed to the chemical and metal pollutants including NaCl, ZnSo4 and H2O2 (122). Melatonin can preserve photosystem by hindering chlorophyll degradation (161) and collaborate with other phytohormones including auxin, salicylic acid, jasmonic acid, ethylene, etc. (162-64).
Melatonin is distributed in all parts of plants including seeds, leaves, fruits and roots (35, 165, 166). It not only plays a defensive role but also promotes plant growth. It acts parallelly to the major plant growth hormone auxin stimulating growth of plants (167- 170). These stimulatory actions include promoting plant growth rate, number and length of lateral and adventitious roots (170- 172), flower development (173). In some species, such as Chenopodium rubrum and Arabidopsis thaliana melatonin may delay their flowering depending on the length of the photoperiods (33, 174). This may relate to the responses of different isoforms of melatonin synthetic enzymes on different light exposure conditions (175). The different functions of melatonin in plants are evolved to make the plants to adapt to the environmental changes (159).
For animals, the functional varieties are even more colourful than those in the plants. Melatonin and its diurnal oscillation modulate ciliary swimming, locomotor activity and behaviour pattern in diverse species of invertebrates (176-181). An anti-inflammatory activity of melatonin is another example which helps the organisms to adapt the perceived stimuli. Inflammation is a normal physiological response of organisms against viral or bacterial invasions. Overaction will damage tissues and sometimes has the lethal outcome. Melatonin can suppress the excessive inflammatory reaction by up-regulating anti-inflammatory cytokines along with promoting down-regulation of TNF-α, pro-inflammatory cytokines, pro-inflammatory enzymes like COX2 (26,182- 184). This anti-inflammatory property of melatonin contributes in alleviating many diseased conditions like cardiomyopathy, diabetes, ischaemia, gastrointestinal injury and even neurodegenerative disorders (184-187). Inhibition of eosinophil peroxidase by melatonin has been reported in human, which suggests its role in blocking phagocytosis (188).
As mentioned above, mitochondria are the major site of melatonin synthesis. The question is why melatonin is also present in the red blood cells (RBC) which are mitochondria free (189). In addition, RBCs are always exposed to high concentration of oxygen and enormous ROS and need melatonin’s protection. The fact is that RBC not only can extract melatonin from blood stream but they also have the capacity to synthesize melatonin per se (190). Melatonin can protect RBC from haemolysis and prolong their storage time (190).
During evolution, the pineal gland of vertebrates evolves into a specific organ to exclusively produce melatonin (23). Unlikely other organs, pineal gland releases its melatonin into circulation as an environmental photoperiodic clue to other tissues and organs which cannot directly detect the light. The other pathway is that pineal gland directly releases melatonin into the third ventricle and acts on the melatonin receptors located in the SCN to entrain dark/light information (191). Hence, functional diversification of melatonin comes with evolution based on its spatial differentiation (Figure. 2).

Fig. 2. The functional acquirements of melatonin during evolution.
Melatonin undergoes tremendous functional acquirements during evolution. Melatonin originally acts as an antioxidant, then it acquired other functions that have been shown here starting from Pisces to Mammalia.
6. RELEVANCE OF PINEAL GLAND IN EVOLUTION
Since, animals have evolved their unique metabolic systems, they do not depend on photon energy assimilation for energy generation any more (26). At that time, retina became the sensor of light to synchronize the physiological activities of the internal tissues and organs of organisms to adapt the light and dark cycle with the help of melanopsin pigment (26, 192). In this respect, melatonin is selected as the chemical expression of day/night cycle to regulate physiological function of organisms. It is obvious that vertebrates inherit this signalling pathway from their ancestors. In this pathway, the ganglion cells of retina act as the sole light sensors or photoreceptors to convert it as a neural signal to reach SCN of hypothalamus (26, 158, 193). Although retina can synthesize melatonin, this melatonin will not release into circulation (194, 195), except some exceptions (196) such as in amphibian, pinealectomy doesn’t cause complete elimination of melatonin from circulation due to retinal contribution (194, 197, 198). Fish circadian clock is also regulated by melatonin from both pineal gland and retina (199, 200). For amphibians and fish, their retina and pineal gland seem to have the similar functions, that is to serve as the photoreceptor as well as to release melatonin into circulation as the circadian regulator. In the mammals, retina has dedicated to photo-perception and pineal gland evolves to an organ for exclusive melatonin synthesis (201, 202). Both organs are differentiated from diencephalon, and exhibit the intricacy rhythm, even in absence of external cues such as in humans residing in underground bunker (203). Evidence shows that pineal gland may evolve from retina since remnants of rods and cones have been identified within pineal gland (204). In contrast, in some lower vertebrates, and even in higher vertebrates like birds, pineal gland still has the ability to sense light (205, 206). In invertebrates, those are devoid of pineal gland, the locally synthesized melatonin may serve as autocrine and paracrine signal which perhaps did not respond to diurnal rhythm (42). In some unicellular organisms, they also have melatonin circadian rhythm. Whether the circadian pattern of melatonin production in vertebrates inherits from ancient microorganisms is debatable. Melatonin secreted from pineal gland exclusively performs as an endocrine signal to provide light/dark information to all organs in order to synchronize their physiological functions (42, 50, 207, 208). Melatonin circadian rhythm as the photoperiodic signal can help the organisms protect against various disease conditions including diabetes (209). It also has the important physiological relevance. For example, in seasonal breeders, their reproductive activity depended on environmental photoperiodic information and melatonin usually provides this information (210). The seasonal breeders identify the season with high nocturnal melatonin level and duration as winter and short dark period with low nocturnal melatonin as summer. The deer, goat, hamster respond to this melatonin circadian rhythm to adjust their breeding time (211-213). The significance of pineal gland became more evident. For example, when the signal of reproductive season is interrupted in seasonal breeding animals by pinealectomy, this makes them breeding at the wrong season which will increase the mortality of their offspring (214). In some seasonal breeders including Syrian hamsters and palm squirrels, high melatonin level and duration inhibit their reproductive activity by acting on the hypothalamo hypophyseal gonadal axis (215-217). This has great impact on long photoperiodic reproductive animals. The winter associated high melatonin information inhibits them to give birth in the cold winter and increases the survival rate of their offspring (159, 218). Thus, the emergence of pineal melatonin as the biological rhythm regulator brings pulse to the life of organisms, necessary for their survival.
Rhythmical melatonin secretion from pineal gland also influences sleep-wake cycle by binding to master clock SCN as a ligand. The night-time decrease in temperature is associated with nocturnal melatonin surge and subsequent sleep (219). In diurnal organisms, the daily clock during dark period, promote sleep while in nocturnal animals, the high melatonin level reminds them about foraging activities (220) and thus this molecule brings temporal synchronization in activities of different organisms.
7. MELATONIN RECEPTOR: PERSPECTIVE OF ITS EMERGANCE
In unicellular organism, the secretion of melatonin and its action were limited within a small boundary where the indole acted as the on-site protector against free radicals. This action is receptor-independent since melatonin receptors have not been reported in unicellular organisms (26, 159). But, melatonin binding site may be present in these organisms (221).
In multicellular organisms, for the long-distance signal relay, melatonin receptors have been emerged. Non-mammalian vertebrates have initiated MT3 receptor with relatively low affinity for melatonin ligand, thereafter, all mammals have developed high affinity MT1 and MT2 which are predominant in brain and retina (222-224). Other peripheral tissues including Harderian gland, spleen, gut, testis, ovary and smooth muscle, cells of immune system all have melatonin receptors (225, 226). The presence of melatonin receptors in SCN is selected for regulation of bio-clock and homoeostasis by nature (227, 228).
The MT1 and MT2 subtypes have distinct molecular structures, chromosomal sites and pharmacological specifications (229, 230-232). They can function in monomeric, in homodimeric or even in heterodimeric forms (233). These complexes of receptor configurations make the functional variable of these receptors. For example, considering the involvement of melatonin in learning process, MT2 knock-down in mice results in cognitive dysfunction (234); however, MT1/MT2 double knockout displays better performance in learning and memory in mice (235). Presence of both MT1 and MT2 receptors in pancreas reduces excessive insulin secretion and insulin resistance (209). The cardio-protective effects of melatonin require both MT1 and MT2 participation. MT1 activation suppresses adenylate cyclase and reduction of intracellular cAMP level (236) while effects of MT2 are mitochondria dependent. Hence, distinction in involvement of receptor sub-types in diverse pathological situations has been evidenced based on their locations.
8. CONCLUSION REMARKS
In summary, the pleiotropic nature of melatonin makes this molecule more acceptable and appropriate for organisms. Due to its multiple functions acquired during evolution, melatonin gradually gains the advantages over other naturally occurring molecules. For example, the synthetic ability of vitamin C and vitamin E has been lost in many mammals during evolution while the melatonin synthetic capacity has been preserved and even strengthened in all animals. This can be reflected by the diversities of melatonin synthetic pathways and enzymes which converge to generate the final product, melatonin and secure the sufficient melatonin level for life surviving and thriving. This review projects the chronological events of genesis of melatonin as an essential and indispensable molecule of life.
ACKNOWLEDGEMENTS
A Junior Research Fellowship (JRF) under WBDST [304(Sanc.)/STP/S&T/1G-67/2017DATED 29.03.2018] is greatly acknowledged by AB. Dr. AC is supported by funds available to her from Department of Science and Technology, Govt. of West Bengal. Dr. DB gratefully acknowledges the support he received from Departmental BI Grant and DST-PURSE Program awarded to the University of Calcutta. Dr. DB also extends grateful thanks to UGC, Govt. of India, for being awarded as a principal investigator under Centre with Potential for Excellence in a Particular Area (CPEPA) at University of Calcutta. Dr. DB also gratefully acknowledges the contribution of Dr. DunXian Tan, Editor-In-Chief, Melatonin Research, for critically reading the manuscript, making thought provoking editorial corrections which has surely increased the scientific and readership quality of the article.
AUTHOR’S CONTRIBUTION
DB initiated the concept, revised the manuscript and approved it. AC contributed in critical review and approval. AB prepared, drafted and edited the manuscript and figure.
CONFLICT OF INTEREST
Authors declare no conflict of interests.
REFERENCES
Manchester LC, Coto‐Montes A, Boga JA, Andersen LP, Zhou Z, Galano A, Vriend J, Tan DX, Reiter RJ (2015) Melatonin: an ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 59 (4): 403-419. DOI: 10.1111/jpi.12267.
Kump LR, Barley ME (2007) Increased subaerial volcanism and the rise of atmospheric oxygen 2.5 billion years ago. Nature 448 (7157): 1033-1036. DOI: 10.1038/nature06058.
Kasting JF (1993) Earth's early atmosphere. Science 259 (5097): 920-926. DOI: 10.1126/science.11536547
Shaw GH (2008) Earth's atmosphere–Hadean to early Proterozoic. Geochemistry 68 (3): 235-264. DOI: 10.1016/j.chemer.2008.05.001.
Ślesak I, Ślesak H, Kruk J (2012) Oxygen and hydrogen peroxide in the early evolution of life on earth: in silico comparative analysis of biochemical pathways. Astrobiology 12 (8): 775-784. DOI: 10.1089/ast.2011.0704.
Selsis F, Despois D, Parisot JP (2002) Signature of life on exoplanets: Can Darwin produce false positive detections?. Astron. Astrophysi. 388 (3): 985-1003. DOI: 10.1051/0004-6361:20020527.
Borda MJ, Elsetinow AR, Schoonen MA, Strongin DR (2001) Pyrite-induced hydrogen peroxide formation as a driving force in the evolution of photosynthetic organisms on an early Earth. Astrobiology 1 (3): 283-288. DOI: 10.1089/15311070152757474.
Schirrmeister BE, Gugger M, Donoghue PC (2015) Cyanobacteria and the Great Oxidation Event: evidence from genes and fossils. Palaeontology 58 (5): 769-785. DOI: 10.1111/pala.12178.
Embley TM, Williams TA (2015) Steps on the road to eukaryotes. Nature 521 (7551): 169-170. DOI: 10.1038/nature14522.
Embley TM, Martin W (2006) Eukaryotic evolution, changes and challenges. Nature 440 (7084): 623-630. DOI: 10.1038/nature04546.
Holland HD (2006) The oxygenation of the atmosphere and oceans. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 361 (1470): 903-915. DOI: 10.1098/rstb.2006.1838.
Van Kranendonk MJ, Altermann W, Beard BL, Hoffman PF, Johnson CM, Kasting JF, Melezhik VA, Nutman AP, Papineau D, Pirajno, F (2012) Chapter 16. A chronostratigraphic division of the Precambrian: possibilities and challenges. In: F. M. Gradstein, J. G. Ogg, M. D. Schmitz and G. M. Ogg (eds). The Geologic Time Scale 2012. pp. 299– 392. Elsevier, Boston, MA. DOI: 10.1016/B978-0-444-59425-9.00016-0.
Fridovich I (2013) Oxygen: how do we stand it?. Med. Princ. Pract. 22 (2): 131-137. DOI: 10.1159/000339212.
Margulis L (1975) Symbiotic theory of the origin of eukaryotic organelles; criteria for proof. In: Symposia of the Society for Experimental Biology 29: 21-38. PMID: 822529.
Rockwell NC, Lagarias JC, Bhattacharya D (2014) Primary endosymbiosis and the evolution of light and oxygen sensing in photosynthetic eukaryotes. Front. Ecol. Evol. 2: 66. DOI: 10.3389/fevo.2014.00066.
Wernegreen JJ (2012) Endosymbiosis. Curr. Biol. 22 (14): R555-R561. DOI: 10.1016/j.cub.2012.06.010.
Gray MW, Burger G, Lang BF (1999) Mitochondrial evolution. Science 283 (5407): 1476-1481. DOI: 10.1126/science.283.5407.1476.
Koonin EV (2010) The origin and early evolution of eukaryotes in the light of phylogenomics. Genome Biol. 11 (5): 1-2. DOI: 10.1186/gb-2010-11-5-209.
Tan DX, Manchester LC, Liu X, Rosales‐Corral SA, Acuna‐Castroviejo D, Reiter RJ (2013) Mitochondria and chloroplasts as the original sites of melatonin synthesis: a hypothesis related to melatonin's primary function and evolution in eukaryotes. J. Pineal Res. 54 (2): 127-138. DOI: 10.1111/jpi.12026.
Tan DX et al. (1993) Melatonin: a potent, endogenous hydroxyl radical scavenger. Endocr. J. 1:57-60.
Galano A, Tan DX, Reiter RJ (2011) Melatonin as a natural ally against oxidative stress: a physicochemical examination. J. Pineal Res. 51 (1): 1-6.DOI: 10.1111/j.1600-079X.2011.00916.x.
Fischer TW,Kleszczyński K, Hardkop LH, Kruse N, Zillikens D (2013) Melatonin enhances antioxidative enzyme gene expression (CAT, GPx, SOD), prevents their UVR-induced depletion, and protects against the formation of DNA damage (8-hydroxy-2' deoxyguanosine) in ex vivo human skin. J. Pineal Res. 54: 303–312. DOI: 10.1111/jpi.12018.
Lerner AB, Case JD, Takahashi Y, Lee TH, Mori W (1958) Isolation of melatonin, the pineal gland factor that lightens melanocyte. J. Am. Chem. Soc. 80 (10): 2587-2587. DOI: 10.1021/ja01543a060.
Manchester LC, Poeggeler B, Alvares FL, Ogden GB, Reiter RJ (1995) Melatonin immunoreactivity in the photosynthetic prokaryote Rhodospirillum rubrum: implications for an ancient antioxidant system. Cell Mol. Biol. Res. 41 (5): 391-395. PMID: 8867786.
Lurie-Weinberger MN, Peeri M, Tuller T, Gophna U (2012) Extensive inter-domain lateral gene transfer in the evolution of the human commensal Methanosphaerastadtmanae. Front. Genet. 3: 182. DOI: 10.3389/fgene.2012.00182.
Tan DX, Zheng X, Kong J, Manchester LC, Hardeland R, Kim SJ, Xu X, Reiter RJ (2014) Fundamental issues related to the origin of melatonin and melatonin isomers during evolution: relation to their biological functions. Int. J. Mol. Sci. 15 (9):15858-15890. DOI: 10.3390/ijms150915858. DOI: 10.3390/ijms150915858.
Spang A, Martijn J, Saw JH, Lind AE, Guy L, Ettema TJ (2013) Close encounters of the third domain: the emerging genomic view of archaeal diversity and evolution. Archaea 2013: 202358. DOI: 10.1155/2013/202358.
Williams TA, Foster PG, Cox CJ, Embley TM (2013) An archaeal origin of eukaryotes supports only two primary domains of life. Nature 504 (7479): 231-236. DOI: 10.1038/nature12779.
Tan DX, Hardeland R, Back K, Manchester LC, Alatorre‐Jimenez MA, Reiter RJ (2016) On the significance of an alternate pathway of melatonin synthesis via 5‐methoxytryptamine: comparisons across species. J. Pineal Res. 61 (1): 27-40. DOI: 10.1111/jpi.12336.
Klein DC (2007) Arylalkylamine N-acetyltransferase: “the Timezyme” J. Biol. Chem. 282 (7): 4233-4237. DOI: 10.1074/jbc.R600036200.
Poeggeler B, Balzer I, Hardeland R, Lerchl A (1991) Pineal hormone melatonin oscillates also in the dinoflagellate Gonyaulaxpolyedra. Naturwissenschaften 78 (6): 268-269. DOI: 10.1007/BF01134354.
Balzer, I., Poeggeler,Hardeland, R. (1993) Circadian rhythms of indoleamines in a dinoflagellate, Gonyaulaxpolyedra: Persistence of melatonin rhythm in constant darkness and relationship to 5- methoxytryptamine. In: Y. Touitou, j. Arendt and P. Pevet, ed., Melatonin and the Pineal Gland. From Basic Science to Clinical Application. 183-186. Elsevier, Amsterdam.
Kolář J, Macháčková I (2005) Melatonin in higher plants: occurrence and possible functions. J. Pineal Res. 39 (4): 333-341. DOI: 10.1111/j.1600-079X.2005.00276.x.
Kolár J, Johnson CH, Machácková I (1999) Presence and possible role of melatonin in a short-day flowering plant, Chenopodium rubrum. Adv. Exp. Med. Biol. 460: 391-393. DOI: 10.1007/0-306-46814-x_46.
Hardeland R, Pandi-Perumal SR, Poeggeler B (2007) Melatonin in plants–Focus on a vertebrate night hormone with cytoprotective properties. Funct. Plant Sci. Biotechnol. 1 (1): 32-45.
Pandi-Perumal SR, Zisapel N, Srinivasan V, Cardinali DP (2005) Melatonin and sleep in aging population. Exp. Gerontol. 40 (12): 911-925. DOI: 10.1016/j.exger.2005.08.009.
Luboshizsky R, Lavie P (1998) Sleep-inducing effects of exogenous melatonin administration. Sleep Med. Rev. 2 (3): 191-202. DOI: 10.1016/s1087-0792(98)90021-1.
Hardeland R, Cardinali DP, Srinivasan V, Spence DW, Brown GM, Pandi-Perumal SR (2011) Melatonin—A pleiotropic, orchestrating regulator molecule. Prog. Neurobiol. 93 (3): 350-384. DOI: 10.1016/j.pneurobio.2010.12.004.
Reiter RJ, Tan DX, Sharma R (2018) Historical perspective and evaluation of the mechanisms by which melatonin mediates seasonal reproduction in mammals. Melatonin Res.1 (1): 59-77. DOI: 10.32794/mr11250004.
Kriegsfeld LJ, Ubuka T, Bentley GE, Tsutsui K (2015) Seasonal control of gonadotropin-inhibitory hormone (GnIH) in birds and mammals. Front. Neuroendocrinol. 37: 65-75. DOI: 10.1016/j.yfrne.2014.12.001.
Gillette MU, McArthur AJ (1995) Circadian actions of melatonin at the suprachiasmatic nucleus. Behav. Brain Res.73 (1-2): 135-139. DOI: 10.1016/0166-4328(96)00085-x.
Zhao D, Yu Y, Shen Y, Liu Q, Zhao Z, Sharma R, Reiter RJ (2019) Melatonin synthesis and function: evolutionary history in animals and plants. Front. Endocrinol. 10: 249. DOI: 10.3389/fendo.2019.00249.
Najafi M, Shirazi A, Motevaseli E, Rezaeyan AH, Salajegheh A, Rezapoor S (2017) Melatonin as an anti-inflammatory agent in radiotherapy. Inflammopharmacology 25 (4): 403-413. DOI: 10.1007/s10787-017-0332-5.
Bondy SC, Campbell A (2018) Mechanisms underlying tumor suppressive properties of melatonin. Int. J. Mol. Sci. 19 (8): 2205. DOI: 10.3390/ijms19082205.
Luo G, Ono S, Beukes NJ, Wang DT, Xie S, Summons RE (2016) Rapid oxygenation of Earth’s atmosphere 2.33 billion years ago. Sci. Adv. 2 (5): e1600134. DOI: 10.1126/sciadv.1600134.
Lyons TW, Reinhard CT, Planavsky NJ (2014) The rise of oxygen in Earth’s early ocean and atmosphere. Nature 506 (7488): 307-315. DOI: 10.1038/nature13068.
Papa S, Skulachev VP (1997) Reactive oxygen species, mitochondria, apoptosis and aging. In: Detection of mitochondrial diseases. 305-319. Springer, Boston, MA. PMID: 9309704.
Halliwell B (1996) Free radicals, proteins and DNA: oxidative damage versus redox regulation. Biochem. Soc. Trans 24 (4):1023-1027. DOI: 10.1042/bst0241023.
Reiter RJ (1996) Functional aspects of the pineal hormone melatonin in combating cell and tissue damage induced by free radicals. Eur. J. Endocrinol. 134 (4): 412-420. DOI: 10.1530/eje.0.1340412.
Hardeland R, Fuhrberg B, Uria H, Behrmann G, Meyer TJ, Burkhardt S, Poeggeler B (1996) Chronobiology of indoleamines in the dinoflagellate Gonyaulaxpolyedra: metabolism and effects related to circadian rhythmicity and photoperiodism. Braz. J. Med. Biol. Res. 29 (1): 119-123. PMID: 8731341.
Poeggeler B, Balzer I, Fischer J, Behrmann G, Hardeland R (1989) A role of melatonin in dinoflagellates?. Eur. J Endocrinol. 120 (3 Suppl): S97. DOI: 10.1530/acta.0.120S097.
Antolín I, Obst B, Burkhardt S, Hardeland R (1997) Antioxidative protection in a high‐melatonin organism: the dinoflagellate Gonyaulaxpolyedra is rescued from lethal oxidative stress by strongly elevated, but physiologically possible concentrations of melatonin. J. Pineal Res. 23 (4): 182-190. DOI: 10.1111/j.1600-079x.1997.tb00353.x.
Fuhrberg B, Hardeland R, Poeggeler B, Behrmann C (1997) Dramatic rises of melatonin and 5-methoxytryptamine in Gonyaulax exposed to decreased temperature. Biol. Rhythm Res. 28 (1): 144-150. DOI: 10.1076/brhm.28.1.144.12978.
Hardeland R (1996) Ubiquitous melatonin-presence and effects in unicells, plants and animals. Trends Comp. Biochem. Physiol. 2: 25-45.
Macias M, Rodrigueez‐Cabezas MN, Reiter RJ, Osuna A, Acuña‐Castrovejo D (1999) Presence and effects of melatonin in Trypanosomacruzi. J. Pineal Res. 27 (2): 86-94. DOI: 10.1111/j.1600-079x.1999.tb00601.x.
Balzer I, Höcker B, Kapp H, Bartolomaeus B (2000) Occurrence and comparative physiology of melatonin in evolutionary diverse organisms. In: Driessche TV, Guisset JL, Petiau-de Vries GM ed., The Redox State and Circadian Rhythms. 95-119. Springer, Dordrecht. DOI: 10.1007/978-94-015-9556-8_6.
Dubbels R, Reiter RJ, Klenke E, Goebel A, Schnakenberg E, Ehlers C, Schiwara HW, Schloot W (1995) Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography‐mass spectrometry. J. Pineal Res. 18 (1): 28-31. DOI: 10.1111/j.1600-079x.1995.tb00136.x.
Hattori A, Migitaka H, Iigo M, Itoh M, Yamamoto K, Ohtani-Kaneko R, Hara M, Suzuki T, Reiter RJ (1995) Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem. Mol. Biol. Int. 35 (3): 627. PMID: 7773197.
Reiter RJ, Tan DX, Manchester LC, Simopoulos AP, Maldonado MD, Flores LJ, Terron MP (2007) Melatonin in edible plants (phytomelatonin): identification, concentrations, bioavailability and proposed functions. World Rev. Nutr. Diet. 97: 211-230. DOI: 10.1159/000097917.
Paredes SD, Korkmaz A, Manchester LC, Tan DX, Reiter RJ (2009) Phytomelatonin: a review. J. Exp. Bot. 60 (1): 57-69. DOI: 10.1093/jxb/ern284.
Reed CJ, Lewis H, Trejo E, Winston V, Evilia C (2013) Protein adaptations in archaeal extremophiles. Archaea 2013: 373275. DOI: 10.1155/2013/373275.
Hardeland R (2019) Melatonin in the evolution of plants and other phototrophs. Meletonin Res. 2 (3): 10-36. DOI: 10.32794/mr11250029.
Manchester LC, Tan DX, Reiter RJ, Park W, Monis K, Qi W (2000) High levels of melatonin in the seeds of edible plants: possible function in germ tissue protection. Life Sci. 67 (25): 3023-3029. DOI: 10.1016/s0024-3205(00)00896-1.
Conti A, Tettamanti C, Singaravel M, Haldar C, Pandi-Perumal RS, Maestroni GJ (2002) Melatonin: An ubiquitous and evolutionary hormone. In: Haldar C, Singaravel M, Maitra SK ed., Treatise on pineal gland and melatonin. 105-143. Enfield, NH: Science Publishers.
Van Tassel DL, Roberts N, Lewy A, O'Neill SD (2001) Melatonin in plant organs. J. Pineal Res. 31 (1): 8-15. DOI: 10.1034/j.1600-079x.2001.310102.x.
Tan DX, Manchester LC, Helton P, Reiter RJ (2007) Phytoremediative capacity of plants enriched with melatonin. Plant Signal Behav. 2 (6): 514-516. DOI: 10.4161/psb.2.6.4639.
Reiter RJ, Rosales-Corral S, Tan DX, Jou MJ, Galano A, Xu B (2017) Melatonin as a mitochondria-targeted antioxidant: one of evolution’s best ideas. Cell Mol. Life Sci. 74 (21): 3863-3881. DOI: 10.1007/s00018-017-2609-7.
Rodriguez C, Mayo JC, Sainz RM, Antolín I, Herrera F, Martín V, Reiter RJ (2004) Regulation of antioxidant enzymes: a significant role for melatonin. J. Pineal Res. 36 (1): 1-9. DOI: 10.1046/j.1600-079x.2003.00092.x.
Ghosh AK, Naaz S, Bhattacharjee B, Ghosal N, Chattopadhyay A, Roy S, Reiter RJ, Bandyopadhyay D (2017) Mechanism of melatonin protection against copper-ascorbate-induced oxidative damage in vitro through isothermal titration calorimetry. Life Sci. 180: 123-136. DOI: 10.1016/j.lfs.2017.05.022.
Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre‐Jimenez M, Qin L (2016) Melatonin as an antioxidant: under promises but over delivers. J. Pineal Res. 61 (3): 253-278. DOI: 10.1111/jpi.12360.
Hardeland R (2015) Melatonin in plants and other phototrophs: advances and gaps concerning the diversity of functions. J. Exp. Bot. 66 (3): 627-646. DOI: 10.1093/jxb/eru386.
Bochkov DV, Sysolyatin SV, Kalashnikov AI, Surmacheva IA (2012) Shikimic acid: review of its analytical, isolation, and purification techniques from plant and microbial sources. J. Chem. Biol. 5 (1): 5-17. DOI: 10.1007/s12154-011-0064-8.
Back K, Tan DX, Reiter RJ (2016) Melatonin biosynthesis in plants: multiple pathways catalyze tryptophan to melatonin in the cytoplasm or chloroplasts. J. Pineal Res. 61 (4): 426-437. DOI: 10.1111/jpi.12364.
De Luca V, Marineau C, Brisson N (1989) Molecular cloning and analysis of cDNA encoding a plant tryptophan decarboxylase: comparison with animal dopa decarboxylases. Proc. Nat. Acad. Sci. 86 (8): 2582-2586. DOI: 10.1073/pnas.86.8.2582.
Park M, Kang K, Park S, Back K (2008) Conversion of 5-hydroxytryptophan into serotonin by tryptophan decarboxylase in plants, Escherichia coli, and yeast. Biosci. Biotechnol. Biochem. 72 (9): 2456-2458. DOI: 10.1271/bbb.80220.
Axelrod J, Weissbach H (1960) Enzymatic O-methylation of N-acetylserotonin to melatonin. Science 131 (3409): 1312. DOI: 10.1126/science.131.3409.1312.
Kang K, Lee K, Park S, Byeon Y, Back K (2013) Molecular cloning of rice serotonin N‐acetyltransferase, the penultimate gene in plant melatonin biosynthesis. J. Pineal Res. 55 (1): 7-13. DOI: 10.1111/jpi.12011.
Weissbach H (1960) Biosynthesis of melatonin: enzymic conversion of serotonin to N-acetylserotonin. Biochim. Biophys. Acta 43: 352-353. DOI: 10.1016/0006-3002(60)90453-4.
Byeon Y, Lee HJ, Lee HY, Back K (2016) Cloning and functional characterization of the Arabidopsis N‐acetylserotonin O‐methyltransferase responsible for melatonin synthesis. J. Pineal Res. 60 (1): 65-73. DOI: 10.1111/jpi.12289.
Isorna E, El M’Rabet A, Confente F, Falcón J, Muñoz-Cueto JA (2009) Cloning and expression of arylalkylamine N-acetyltranferase-2 during early development and metamorphosis in the soleSoleasenegalensis. Gen. Comp. Endocrinol. 161 (1): 97-102. DOI: 10.1016/j.ygcen.2008.10.007.
Isorna E, Aliaga‐Guerrero M, M’Rabet AE, Servili A, Falcón J, Muñoz‐Cueto JA (2011) Identification of two arylalkylamine N‐acetyltranferase 1 genes with different developmental expression profiles in the flatfish Soleasenegalensis. J. Pineal Res. 51 (4): 434-444. DOI: 10.1111/j.1600-079X.2011.00907.x.
Byeon Y, Lee HY, Back K (2016) Cloning and characterization of the serotonin N‐acetyltransferase‐2 gene (SNAT2) in rice (Oryza sativa). J. Pineal Res. 61 (2): 198-207. DOI: 10.1111/jpi.12339.
Tan DX, Manchester LC, Esteban-Zubero E, Zhou Z, Reiter RJ (2015) Melatonin as a potent and inducible endogenous antioxidant: synthesis and metabolism. Molecules 20 (10): 18886-18906. DOI: 10.3390/molecules201018886.
Amherd R, HintermannE, Walz D, Affolter M, Meyer UA (2000) Purification, cloning, and characterization of a second arylalkylamine N-acetyltransferase from Drosophila melanogaster. DNA Cell Biol. 19 (11): 697-705. DOI: 10.1089/10445490050199081.
Coon SL, Klein DC (2006) Evolution of arylalkylamine N-acetyltransferase: emergence and divergence. Mol. Cell Endocrinol. 252 (1-2): 2-10. DOI: 10.1016/j.mce.2006.03.039.
Ganguly S, Mummaneni P, Steinbach PJ, Klein DC, Coon SL (2001) Characterization of the Saccharomyces cerevisiae homolog of the melatonin rhythm enzyme arylalkylamine N-acetyltransferase (EC 2.3. 1.87). J. Biol. Chem. 276 (50): 47239-47247. DOI: 10.1074/jbc.M107222200.
Iyer LM, AravindL, Coon SL, Klein DC, Koonin EV (2004) Evolution of cell–cell signaling in animals: did late horizontal gene transfer from bacteria have a role?. Trends Genet. 20 (7): 292-299. DOI: 10.1016/j.tig.2004.05.007.
Pavlicek J, Sauzet S, Besseau L, Coon SL, Weller JL, Boeuf G, Gaildrat P, Omelchenko MV, Koonin EV, Falcón J, Klein DC (2010) Evolution of AANAT: expansion of the gene family in the cephalochordate amphioxus. BMC Evol. Biol. 10 (1): 154. DOI: 10.1186/1471-2148-10-154.
Han Q, Robinson H, Ding H, Christensen BM, Li J (2012) Evolution of insect arylalkylamine N-acetyltransferases: structural evidence from the yellow fever mosquito, Aedes aegypti. Proc. Natl. Acad. Sci. 109 (29): 11669-11674. DOI: 10.1073/pnas.1206828109.
Tan DX, Manchester LC, Reiter RJ, Qi WB, Karbownik M, Calvo JR (2000) Significance of melatonin in antioxidative defense system: reactions and products. Biol. Signals Recept. 9 (3-4): 137-159. DOI: 10.1159/000014635.
Byeon Y, Lee HY, Lee K, Park S, Back K (2014) Cellular localization and kinetics of the rice melatonin biosynthetic enzymes SNAT and ASMT. J. Pineal Res. 56 (1): 107-114. DOI: 10.1111/jpi.12103.
Wang L, Feng C, Zheng X, Guo Y, Zhou F, Shan D, Liu X, Kong J (2017) Plant mitochondria synthesize melatonin and enhance the tolerance of plants to drought stress. J. Pineal Res. 63 (3): e12429. DOI: 10.1111/jpi.12429.
Yu Y, Bian L, Jiao Z, Yu K, Wan Y, Zhang G, Guo D (2019) Molecular cloning and characterization of a grapevine (Vitis vinifera L.) serotonin N-acetyltransferase (VvSNAT2) gene involved in plant defense. BMC genomics 20 (1): 1-3. DOI: 10.1186/s12864-019-6085-3.
Reiter RJ, Tan DX, Zhou Z, Cruz MH, Fuentes-Broto L, Galano A (2015) Phytomelatonin: assisting plants to survive and thrive. Molecules 20 (4): 7396-437. DOI: 10.3390/molecules20047396.
Gaudet SJ, Hayden BJ, Chader GJ, Namboodiri MA (1993) Differential regulation of arylamine and arylalkylamine N-acetyltransferases in human retinoblastoma (Y-79) cells. Neurochem. Int. 22 (3): 271-275. DOI: 10.1016/0197-0186(93)90055-a.
Gaudet SJ, Tsilou E, Chader GJ (1993) Identification and characterization of arylamine N-acetyltransferase activity from the bovine retinal pigment epithelium. Curr. Eye Res. 12 (3): 271-278. DOI: 10.3109/02713689308999473.
Slominski A, Fischer TW, Zmijewski MA, Wortsman J, Semak I, Zbytek B, Slominski RM, Tobin DJ (2005) On the role of melatonin in skin physiology and pathology. Endocrine 27 (2): 137-147. DOI: 10.1385/ENDO:27:2:137.
Slominski A, Pisarchik A, Semak I, Sweatman T, Wortsman J (2003) Characterization of the serotoninergic system in the C57BL/6 mouse skin. Eur. J. Biochem. 270 (16): 3335-3344. DOI: 10.1046/j.1432-1033.2003.03708.x.
Gaudet SJ, Slominski A, Etminan M, Pruski D, Paus R, Namboodiri MA (1993) Identification and characterization of two isozymic forms of arylamine N-acetyltransferase in Syrian hamster skin. J. Invest. Dermatol. 101 (5): 660-665. DOI: 10.1111/1523-1747.ep12371672.
Biarrotte-Sorin S, Mayer C (2005) Cloning, purification, crystallization and preliminary crystallographic analysis of a hypothetical acetyltransferase from Pyrococcusfuriosus ActaCrystallogr. Sect. F. Struct. Biol. Cryst. Commun. 61 (3): 269-270. DOI: 10.1107/S174430910500223X.
Ma C, Pathak C, Jang S, Lee SJ, Nam M, Kim SJ, Im H, Lee BJ (2014) Structure of Thermoplasma volcanium Ard1 belongs to N-acetyltransferase family member suggesting multiple ligand binding modes with acetyl coenzyme A and coenzyme A. Biochim. Biophys. Acta. 1844 (10): 1790-1797. DOI: 10.1016/j.bbapap.2014.07.011.
Byeon Y, Lee K, Park YI, Park S, Back K (2013) Molecular cloning and functional analysis of serotonin N‐acetyltransferase from the cyanobacterium Synechocystis sp. PCC 6803. J. Pineal Res. 55 (4): 371-376. DOI: 10.1111/jpi.12080.
Zheng X, Xie G, Zhao A, Zhao L, Yao C, Chiu NH, Zhou Z, Bao Y, Jia W (2011) Nicholson JK, Jia W. The footprints of gut microbial–mammalian co-metabolism. J. Proteome Res. 10 (12): 5512-22.DOI: 10.1021/pr2007945.
Tan DX, Reiter RJ (2020) An evolutionary view of melatonin synthesis and metabolism related to its biological functions in plants. J. Exp. Bot. 71 (16): 4677-4689. DOI: 10.1093/jxb/eraa235.
Choi GH, Lee HY, Back K (2017) Chloroplast overexpression of rice caffeic acid O‐methyltransferase increases melatonin production in chloroplasts via the 5‐methoxytryptamine pathway in transgenic rice plants. J. Pineal Res. 63 (1): e12412. DOI: 10.1111/jpi.12412.
Zheng X, Tan DX, Allan AC, Zuo B, Zhao Y, Reiter RJ, Wang L, Wang Z, Guo Y, Zhou J, Shan D (2017) Chloroplastic biosynthesis of melatonin and its involvement in protection of plants from salt stress. Sci. Rep. 7 (1): 1-2. DOI: 10.1038/srep41236.
Lee HY,Byeon Y, Lee K, Lee HJ, Back K (2014) Cloning of A rabidopsis serotonin N‐acetyltransferase and its role with caffeic acid O‐methyltransferase in the biosynthesis of melatonin in vitro despite their different subcellular localizations. J. Pineal Res. 57 (4): 418-426. DOI: 10.1111/jpi.12181.
Del Río LA (2015) ROS and RNS in plant physiology: an overview. J. Exp. Bot. 66 (10): 2827-2837. DOI: 10.1093/jxb/erv099.
Hardeland R (2016) Melatonin in plants–diversity of levels and multiplicity of functions. Front. Plant Sci. 7: 198. DOI: 10.3389/fpls.2016.00198.
RahantaniainaMS, Tuzet A, Mhamdi A, Noctor G (2013) Missing links in understanding redox signaling via thiol/disulfide modulation: how is glutathione oxidized in plants?. Front. Plant Sci. 4: 477. DOI: 10.3389/fpls.2013.00477.
Takahashi S, Murata N (2008) How do environmental stresses accelerate photoinhibition?. Trends Plant Sci. 13 (4): 178-182. DOI: 10.1016/j.tplants.2008.01.005.
Tan DX, Reiter RJ, Manchester LC, Yan MT, El-Sawi M, Sainz RM, Mayo JC, Kohen R, Allegra MC, Hardeland R (2002) Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr. Top. Med. Chem. 2 (2): 181-197. DOI: 10.2174/1568026023394443.
Tan DX, Manchester LC, Reiter RJ, Plummer BF, Limson J, Weintraub ST, Qi W (2000) Melatonin directly scavenges hydrogen peroxide: a potentially new metabolic pathway of melatonin biotransformation. Free Radic. Biol. Med. 29 (11): 1177-1185. DOI: 10.1016/s0891-5849(00)00435-4.
Stasica P, Paneth P, Rosiak JM (2000) Hydroxyl radical reaction with melatonin molecule: a computational study. J. Pineal Res. 29 (2): 125-127. DOI: 10.1034/j.1600-079x.2000.290209.x.
Wang P, Sun X, Li C, Wei Z, Liang D, Ma F (2013) Long‐term exogenous application of melatonin delays drought‐induced leaf senescence in apple. J. Pineal Res. 54 (3): 292-302. DOI: 10.1111/jpi.12017.
Xiang-dong X, Yan S, Bo S, Jian Z, Xiao-qin G (2010) Effects of exogenous melatonin on active oxygen metabolism of cucumber seedlings under high temperature stress. Ying Yong Sheng Tai XueBao. 21 (5): 1295-1300. PMID: 20707116.
Uchendu EE, Shukla MR, Reed BM, Saxena PK (2013) Melatonin enhances the recovery of cryopreserved shoot tips of A merican elm (U lmus americana L.). J. Pineal Res. 55 (4): 435-442. DOI: 10.1111/jpi.12094.
Bajwa VS, Shukla MR, Sherif SM, Murch SJ, Saxena PK (2014) Role of melatonin in alleviating cold stress in Arabidopsis thaliana. J. Pineal Res. 56 (3): 238-245. DOI: 10.1111/jpi.12115.
Zhang N, Zhao B, Zhang HJ, Weeda S, Yang C, Yang ZC, Ren S, Guo YD (2013) Melatonin promotes water‐stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.). J. Pineal Res. 54 (1): 15-23. DOI: 10.1111/j.1600-079X.2012.01015.x.
Wang P, Yin L, Liang D, Li C, Ma F, Yue Z (2012) Delayed senescence of apple leaves by exogenous melatonin treatment: toward regulating the ascorbate–glutathione cycle. J. Pineal Res. 53 (1): 11-20. DOI: 10.1111/j.1600-079X.2011.00966.x.
Park S, Lee DE, Jang H, Byeon Y, Kim YS, Back K (2013) Melatonin‐rich transgenic rice plants exhibit resistance to herbicide‐induced oxidative stress. J. Pineal Res. 54 (3): 258-263. DOI: 10.1111/j.1600-079X.2012.01029.x.
Arnao MB, Hernández‐Ruiz J (2009) Chemical stress by different agents affects the melatonin content of barley roots. J. Pineal Res. 46 (3): 295-299. DOI: 10.1111/j.1600-079X.2008.00660.x.
Oladi E, Mohamadi M, Shamspur T, Mostafavi A (2014) Spectrofluorimetric determination of melatonin in kernels of four different Pistacia varieties after ultrasound-assisted solid–liquid extraction. Spectrochim. Acta A Mol. Biomol. Spectrosc. 132: 326-329. DOI: 10.1016/j.saa.2019.04.006.
Murch SJ, Simmons CB (1997) Melatonin in feverfew and other medicinal plants. Lancet 350 (9091): 1598-1599. DOI: 10.1016/S0140-6736(05)64014-7.
Brown PN, Turi CE, Shipley PR, Murch SJ (2012) Comparisons of large (Vaccinium macrocarpon Ait.) and small (Vacciniumoxycoccos L., Vaccinium vitis-idaea L.) cranberry in British Columbia by phytochemical determination, antioxidant potential, and metabolomic profiling with chemometric analysis. Planta med. 78 (06): 630-640. DOI: 10.1055/s-0031-1298239.
Arnao MB, Hernández-Ruiz J (2013) Growth conditions influence the melatonin content of tomato plants. Food chem. 138 (2-3): 1212-1214. DOI: 10.1016/j.foodchem.2012.10.077.
Arnao MB, Hernández‐Ruiz J (2013) Growth conditions determine different melatonin levels in L upinusalbus L. J. Pineal Res. 55 (2): 149-155. DOI: 10.1111/jpi.12055.
Reiter RJ, Tan DX, Galano A (2014) Melatonin: exceeding expectations. Physiology 29 (5): 325-33. DOI: 10.1152/physiol.00011.2014. DOI: 10.1152/physiol.00011.2014.
Venegas C, García JA, Escames G, Ortiz F, López A, Doerrier C, García‐Corzo L, López LC, Reiter RJ, Acuña‐Castroviejo D (2012) Extrapineal melatonin: analysis of its subcellular distribution and daily fluctuations. J. Pineal Res. 52 (2): 217-227. DOI: 10.1111/j.1600-079X.2011.00931.x.
Yamamoto HA, Tang HW (1996) Preventive effect of melatonin against cyanide-induced seizures and lipid peroxidation in mice. Neuroscilett. 207 (2): 89-92. DOI: 10.1016/0304-3940(96)12493-9.
Dabbeni-Sala FE, DI Santo ST, Franceschini D, D. Skaper ST, Pietro Giusti AN (2001) Melatonin protects against 6‐OHDA‐induced neurotoxicity in rats: a role for mitochondrial complex I activity. FASEB J. 15 (1): 164-170. DOI: 10.1096/fj.00-0129com.
Dabbeni-Sala F, Floreani M, Franceschini D, Skaper SD, Giusti P (2001) Kainic acid induces selective mitochondrial oxidative phosphorylation enzyme dysfunction in cerebellar granule neurons: protective effects of melatonin and GSH ethyl ester. FASEB J. 15 (10): 1786-1788. DOI: 10.1096/fj.00-0427fje.
Absi E, Ayala A, Machado A, Parrado J (2000) Protective effect of melatonin against the 1‐methyl‐4‐phenylpyridinium‐induced inhibition of complex I of the mitochondrial respiratory chain. J. Pineal Res. 29 (1): 40-47. DOI: 10.1034/j.1600-079x.2000.290106.x.
Martin M, Macias M, Escames G, Reiter RJ, Agapito MT, Ortiz GG, Acuña‐Castroviejo D (2000) Melatonin‐induced increased activity of the respiratory chain complexes I and IV can prevent mitochondrial damage induced by ruthenium red in vivo. J. Pineal Res. 28 (4): 242-248. DOI: 10.1034/j.1600-079x.2000.280407.x.
Reiter R, Paredes S, Korkmaz A, JouMJ, Tan DX (2008) Melatonin combats molecular terrorism at the mitochondrial level. Interdiscip. Toxicol. 1 (2): 137-149. DOI: 10.2478/v10102-010-0030-2.
Hardeland R (2013) Melatonin and the theories of aging: a critical appraisal of melatonin's role in antiaging mechanisms. J. Pineal Res. 55 (4): 325-356. DOI: 10.1111/jpi.12090.
Tan DX, Hardeland R (2020) Targeting host defensesystem and rescuing compromised mitochondria to increase tolerance against pathogens by melatonin may impact outcome of deadly virus infection pertinent to COVID-19. Molecules 25 (19): 4410. DOI: 10.3390/molecules25194410.
Huether GE (1993) The contribution of extrapineal sites of melatonin synthesis to circulating melatonin levels in higher vertebrates. Experientia 49 (8): 665-670. DOI: 10.1007/BF01923948.
Pang SF, Brown GM, Grota LJ, Chambers JW, Rodman RL (1977) Determination of N-acetylserotonin and melatonin activities in the pineal gland, retina, Harderian gland, brain and serum of rats and chickens. Neuroendocrinology 23 (1): 1-3. DOI: 10.1159/000122649. DOI: 10.1159/000122649.
Reiter RJ, Richardson BA, Matthews SA, Lane SJ, Ferguson BN (1983) Rhythms in immunoreactive melatonin in the retina and Harderian gland of rats: persistence after pinealectomy. Life sci. 32 (11): 1229-1236. DOI: 10.1016/0024-3205(83)90192-3.
Slominski A, Tobin DJ, Zmijewski MA, Wortsman J, Paus R (2008) Melatonin in the skin: synthesis, metabolism and functions. Trends Endocrinol. Metab. 19 (1): 17-24. DOI: 10.1016/j.tem.2007.10.007.
Pévet P (2014) The internal time-giver role of melatonin. A key for our health. Rev. Neurol. 170 (11): 646-652. DOI: 10.1016/j.neurol.2014.05.008.
Nagle CA, Cardinali DP, Rosner JM (1973) Retinal and pineal hydroxyindole-o-methyl transferases in the rat: changes following cervical sympathectomy, pinealectomy or blinding. Endocrinology 92 (5): 1560-1564. DOI: 10.1210/endo-92-5-1560. DOI: 10.1210/endo-92-5-1560.
Quay WB (1965) Indole derivatives of pineal and related neural and retinal tissues. Pharmacol. Rev. 17 (3): 321-345. PMID: 5318083.
Cardinali DP, Rosner JM (1971) Retinal localization of the hydroxyindole-O-methyl transferase (HIOMT) in the rat. Endocrinology 89 (1): 301-303. DOI: 10.1210/endo-89-1-301.
Eakin RM, Westfall JA (1959) Fine structure of the retina in the reptilian third eye. J. Biophys. Biochem. Cytol. 6 (1): 133. DOI: 10.1083/jcb.6.1.133.
Szafrańska K, Reiter RJ, Posmyk MM (2017) Melatonin improves the photosynthetic apparatus in pea leaves stressed by paraquat via chlorophyll breakdown regulation and its accelerated de novo synthesis. Front. Plant Sci. 8: 878. DOI: 10.3389/fpls.2017.00878.
Balzer I, Fuhrberg B, Hardeland R, Elsner N, Schnitzler HU (1996) The neurohormone melatonin oscillates in a circadian fashion already in unicells. In: Brain and Evolution. Elsner N, Schnitzler HU, eds., pp. 228. Thieme, Stuttgart, New York.
Sprenger J, Hardeland R, Fuhrberg B, Han SZ (1999) Melatonin and other 5-methoxylated indoles in yeast: presence in high concentrations and dependence on tryptophan availability. Cytologia 64 (2): 209-213. DOI: 10.1508/cytologia.64.209.
Mechawar N, Anctil M (1997) Melatonin in a primitive metazoan: seasonal changes of levels and immunohistochemical visualization in neurons. J. Comp. Neurol. 387 (2): 243-54. PMID: 9336226.
Roopin M, Levy O (2012) Melatonin distribution reveals clues to its biological significance in basal metazoans. PLoS one 7 (12): e52266. DOI: 10.1371/journal.pone.0052266.
Morita M, Best JB (1984) Effects of photoperiods and melatonin on planarian asexual reproduction. J. Exp. Zool. 231 (2): 273-282. DOI: 10.1002/jez.1402310212.
Migliori ML, Romanowski A, Simonetta SH, Valdez D, Guido M, Golombek DA (2012) Daily variation in melatonin synthesis and arylalkylamine N‐acetyltransferase activity in the nematode Caenorhabditis elegans. J. Pineal Res. 53 (1): 38-46. DOI: 10.1111/j.1600-079X.2011.00969.x.
Tosches MA, Bucher D, Vopalensky P, Arendt D (2014) Melatonin signaling controls circadian swimming behavior in marine zooplankton. Cell 159 (1): 46-57. DOI: 10.1016/j.cell.2014.07.042.
Munoz JL, Patino MA, Hermosilla C, Conde-Sieira M,Soengas JL, Rocha F, Míguez JM (2011) Melatonin in octopus (Octopus vulgaris): tissue distribution, daily changes and relation with serotonin and its acid metabolite. J. Comp. Physiol. A. Neuroethol. Sens. Neural. Behav. Physiol. 197 (8): 789-797. DOI: 10.1007/s00359-011-0641-x.
Wang Q, Egi Y, Takeda M, Oishi K, Sakamoto K (2015) Melatonin pathway transmits information to terminate pupal diapause in the Chinese oak silkmoth A ntheraeapernyi and through reciprocated inhibition of dopamine pathway functions as a photoperiodic counter. Entomologic. Sci. 18 (1): 74-84. DOI: 10.1111/ens.12083.
Ding K, Zhang L, Zhang T, Yang H, Brinkman R (2019) The Effect of melatonin on locomotor behavior and muscle physiology in the sea cucumber apostichopusjaponicus. Front. Physiol. 10: 221. DOI: 10.3389/fphys.2019.00221.
Hardeland R, Poeggeler B (2003) Non‐vertebrate melatonin. J. Pineal Res. 34 (4): 233-241. DOI: 10.1034/j.1600-079x.2003.00040.x.
Tan DX, Hardeland R, Manchester LC, Paredes SD, Korkmaz A, Sainz RM, Mayo JC, Fuentes‐Broto L, Reiter RJ (2010) The changing biological roles of melatonin during evolution: from an antioxidant to signals of darkness, sexual selection and fitness. Biol. Rev.Camb. Philos. Soc. 85 (3): 607-623. DOI: 10.1111/j.1469-185X.2009.00118.x.
AfreenF, Zobayed SM, Kozai T (2006) Melatonin in Glycyrrhiza uralensis: response of plant roots to spectral quality of light and UV‐B radiation. J. Pineal Res. 41 (2): 108-115. DOI: 10.1111/j.1600-079X.2006.00337.x.
Arnao MB, Hernández‐Ruiz J (2009) Protective effect of melatonin against chlorophyll degradation during the senescence of barley leaves. J. Pineal Res. 46 (1): 58-63. DOI: 10.1111/j.1600-079X.2008.00625.x.
Ullah A, Manghwar H, Shaban M, Khan AH, Akbar A, Ali U, Ali E, Fahad S (2018) Phytohormones enhanced drought tolerance in plants: a coping strategy. Environ. Sci. Pollut. Res. Int. 25 (33): 33103-33118. DOI: 10.1007/s11356-018-3364-5.
Ku YS, Sintaha M, Cheung MY, Lam HM (2018) Plant hormone signaling crosstalks between biotic and abiotic stress responses. Int. J. Mol. Sci. 19 (10): 3206. DOI: 10.3390/ijms19103206.
Erland LA, Shukla MR, Singh AS, Murch SJ, Saxena PK (2018) Melatonin and serotonin: mediators in the symphony of plant morphogenesis. J. Pineal Res. 64 (2): e12452. DOI: 10.1111/jpi.12452.
Hardeland R, Pandi-Perumal SR (2005) Melatonin, a potent agent in antioxidative defense: actions as a natural food constituent, gastrointestinal factor, drug and prodrug. Nutr. Metab. 2 (1): 2 2. DOI: 10.1186/1743-7075-2-22.
Burkhardt S, Tan DX, Manchester LC, Hardeland R, Reiter RJ (2001) Detection and quantification of the antioxidant melatonin in Montmorency and Balaton tart cherries (Prunus cerasus). J. Agric. Food Chem. 49 (10): 4898-4902. DOI: 10.1021/jf010321+.
Arnao MB, Hernández-Ruiz J (2018) Melatonin and its relationship to plant hormones. Ann. Bot. 121 (2): 195-207. DOI: 10.1093/aob/mcx114.
Arnao MB, Hernández-Ruiz J (2019) Melatonin: a new plant hormone and/or a plant master regulator?. Trends Plant Sci. 24 (1): 38-48. DOI: 10.1016/j.tplants.2018.10.010.
Hernandez-Ruiz J, Cano A, Arnao MB (2004) Melatonin: a growth-stimulating compound present in lupin tissues. Planta 220 (1): 140-144. DOI: 10.1007/s00425-004-1317-3.
Liang C, Li A, Yu H, Li W, Liang C, Guo S, Zhang R, Chu C (2017) Melatonin regulates root architecture by modulating auxin response in rice. Front. Plant Sci. 8: 134. DOI: 10.3389/fpls.2017.00134.
Arnao MB, Hernández‐Ruiz J (2007) Melatonin promotes adventitious‐and lateral root regeneration in etiolated hypocotyls of Lupinus albus L. J. Pineal Res. 42 (2): 147-152. DOI: 10.1111/j.1600-079X.2006.00396.x.
Mao J, Niu C, Li K, Chen S, Tahir MM, Han M, Zhang D (2020) Melatonin promotes adventitious root formation in apple by promoting the function of MdWOX11. BMC Plant Biol. 20 (1): 1-1. DOI: 10.1186/s12870-020-02747-z.
Murch SJ, Saxena PK (2002) Mammalian neurohormones: potential significance in reproductive physiology of St. John's wort (Hypericum perforatum L.)?. Naturwissenschaften 89 (12): 555-560. DOI: 10.1007/s00114-002-0376-1.
Kolář J, Johnson CH, Macháčková I (2003) Exogenously applied melatonin (N‐acetyl‐5‐methoxytryptamine) affects flowering of the short‐day plant Chenopodium rubrum. Physiologia Plantarum 118 (4): 605-612. DOI: 10.1034/j.1399-3054.2003.00114.x.
Murch SJ, KrishnaRaj S, Saxena PK (2000) Tryptophan is a precursor for melatonin and serotonin biosynthesis in in vitro regenerated St. John's wort (Hypericum perforatum L. cv. Anthos) plants. Plant Cell Rep. 19 (7): 698-704. DOI: 10.1007/s002990000206.
Maitra SK, Hasan KN (2016) The role of melatonin as a hormone and an antioxidant in the control of fish reproduction. Front. Endocrinol. 7: 38. DOI: 10.3389/fendo.2016.00038.
Axelrod J, QUAY WB, BAKER PC (1965) Enzymatic synthesis of the skin-lightening agent, melatonin, in amphibians. Nature 208 (5008): 386. DOI: 10.1038/208386a0.
Foa A, Janik D, Minutini L (1992) Circadian rhythms of plasma melatonin in the ruin lizard Podarcissicula: effects of pinealectomy. J. Pineal Res. 12 (3): 109-113. DOI: 10.1111/j.1600-079x.1992.tb00036.x.
Cassone VM, Westneat DF (2012) The bird of time: cognition and the avian biological clock. Front. Mol. Neurosci. 5: 32. DOI: 10.3389/fnmol.2012.00032.
Ubuka T, Bentley GE, Ukena K, Wingfield JC, Tsutsui K (2005) Melatonin induces the expression of gonadotropin-inhibitory hormone in the avian brain. Proc. Nat. Acad. Sci. 102 (8): 3052-3057. DOI: 10.1073/pnas.0403840102.
Grivas TB, Savvidou OD (2007) Melatonin the" light of night" in human biology and adolescent idiopathic scoliosis. Scoliosis 2 (1): 6. DOI: 10.1186/1748-7161-2-6.
Mayo JC, Sainz RM, Tan DX, Hardeland R, Leon J, Rodriguez C, Reiter RJ (2005) Anti-inflammatory actions of melatonin and its metabolites, N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) and N1-acetyl-5-methoxykynuramine (AMK), in macrophages. J. Neuroimmunol. 165 (1-2): 139-149. DOI: 10.1016/j.jneuroim.2005.05.002.
Nabavi SM, Nabavi SF, Sureda A, Xiao J, Dehpour AR, Shirooie S, Silva AS, Baldi A, Khan H, Daglia M (2019) Anti-inflammatory effects of Melatonin: A mechanistic review. Crit. Rev. Food Sci. Nutr. 59 (sup1): S4-S16. DOI: 10.1080/10408398.2018.1487927.
Pal PK, Sarkar S, Mishra S, Chattopadhyay S, Chattopadhyay A, Bandyopadhyay D (2020) Amelioration of adrenaline induced oxidative gastrointestinal damages in rat by melatonin through SIRT1-NFκB and PGC1α-AMPKα cascades. Melatonin Res. 3 (4): 482-502. DOI: 10.32794/mr11250074
Crespo E, Macías M, Pozo D, Escames G, Martín M, Vives F, Guerrero JM, Acuña‐Castroviejo D (1999) Melatonin inhibits expression of the inducible NO synthase II in liver and lung and prevents endotoxemia in lipopolysaccharide‐induced multiple organ dysfunction syndrome in rats. FASEB J. 13 (12): 1537-1546. PMID: 10463945.
Hardeland R (2019) Aging, melatonin, and the pro-and anti-inflammatory networks. Int. J. Mol. Sci. 20 (5): 1223. DOI: 10.3390/ijms20051223.
Prado NJ, Ferder L, Manucha W, Diez ER (2018) Anti-inflammatory effects of melatonin in obesity and hypertension. Curr. Hypertens Rep. 20 (5): 45. DOI: 10.1007/s11906-018-0842-6.
Lu T, Galijasevic S, Abdulhamid I, Abu‐Soud HM (2008) Analysis of the mechanism by which melatonin inhibits human eosinophil peroxidase. Br. J. Pharmacol. 154 (6): 1308-1317. DOI: 10.1038/bjp.2008.173.
Zhang ZW, Cheng J, Xu F, Chen YE, Du JB, Yuan M, Zhu F, Xu XC, Yuan S (2011) Red blood cell extrudes nucleus and mitochondria against oxidative stress. IUBMB life 63 (7): 560-565. DOI: 10.1002/iub.490.
Banerjee A, Chattopadhyay A, Pal PK, Bandyopadhyay D (2020) Melatonin is a potential therapeutic molecule for oxidative stress induced red blood cell (RBC) injury: A review. Melatonin Res. 3 (1): 1-31. DOI: 10.32794/mr11250045.
Cassone VM, Warren WS, Brooks DS, Lu J (1993) Melatonin, the pineal gland, and circadian rhythms. J. Biol. Rhythms 8: S73-S81.PMID: 8274765.
Pozdeyev N, Taylor C, Haque R, Chaurasia SS, Visser A, Thazyeen A, Du Y, Fu H, Weller J, Klein DC, Iuvone PM (2006) Photic regulation of arylalkylamine N-acetyltransferase binding to 14-3-3 proteins in retinal photoreceptor cells. J. Neurosci. 26 (36): 9153-9161. DOI: 10.1523/JNEUROSCI.1384-06.2006.
Hardeland R (2013) Chronobiology of melatonin beyond the feedback to the suprachiasmatic nucleus—consequences to melatonin dysfunction. Int. J. Mol. Sci. 14 (3): 5817-5841. DOI: 10.3390/ijms14035817.
Green CB (2003) Molecular control of Xenopus retinal circadian rhythms. J. Neuroendocrinol. 15 (4): 350-354. DOI: 0.1046/j.1365-2826.2003.00999.x.
Tosini G, Baba K, Hwang CK, Iuvone PM (2012) Melatonin: an underappreciated player in retinal physiology and pathophysiology. Exp. Eye Res. 103: 82-89. DOI: 10.1016/j.exer.2012.08.009.
Underwood H, Binkley S, Siopes T, Mosher K (1984) Melatonin rhythms in the eyes, pineal bodies, and blood of Japanese quail (Coturnixcoturnix japonica). Gen. Comp. Endocrinol. 56 (1): 70-81. DOI: 10.1016/0016-6480(84)90063-7.
Menaker M (1985) Eyes-the second (and third) pineal glands. Ciba. Found. Symp.117: 78-92. DOI: 10.1002/9780470720981.ch6.
Isorna E, Besseau L, Boeuf G, Desdevises Y, Vuilleumier R, Alonso-Gómez AL, Delgado MJ, Falcón J (2006) Retinal, pineal and diencephalic expression of frog arylalkylamine N-acetyltransferase-1. Mol. Cell Endocrinol. 252 (1-2): 11-18. DOI: 10.1016/j.mce.2006.03.032.
Falcon J, Migaud H, Munoz-Cueto JA, Carrillo M (2010) Current knowledge on the melatonin system in teleost fish. Gen. Comp. Endocrinol. 165 (3): 469-482. DOI: 10.1016/j.ygcen.2009.04.026.
Acuña-Castroviejo D, Escames G, Venegas C, Díaz-Casado ME, Lima-Cabello E, López LC, Rosales-Corral S, Tan DX, Reiter RJ (2014) Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol. Life Sci. 71 (16): 2997-3025. DOI: 10.1007/s00018-014-1579-2.
Gern WA (1981) Evolution of melatonin function: a hypothesis. In: N. Birau W. Schloot ed., Melatonin: Current status and perspectives. 85-87. Pergamon.
Aulinas A (2019) Physiology of the pineal gland and melatonin. Endotext [Internet]. PMID: 31841296.
AschoffJ (1960) Exogenous and endogenous components in circadian rhythms. In: Cold Spring Harbor symposia on quantitative biology. 25: 11-28. Cold Spring Harbor Laboratory Press.
Kramm CM, De Grip WJ, Korf HW (1993) Rod-opsin immunoreaction in the pineal organ of the pigmented mouse does not indicate the presence of a functional photopigment. Cell Tissue Res. 274 (1): 71-78. DOI: 10.1007/BF00327987.
Oksche A (1991) The development of the concept of photoneuroendocrine systems: historical perspective. In: Klein DC, Moor RY, Reppert SM. ed., Suprachiasmatic Nucleus. New York, NY: Oxford University Press pp. 5–11.
Menaker M, Roberts R, Elliott J, Underwood H (1970) Extraretinal light perception in the sparrow, III: the eyes do not participate in photoperiodic photoreception. Proc. Natl. Acad. Sci. 67 (1): 320-325. DOI: 10.1073/pnas.67.1.320.
Binkley SA (1980) In: RJ Reiter ed., The Pineal Gland: Its Anatomy and Biochemistry. CRC Press, West Palm Beach, Florida.
Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, Berson DM (2006) Central projections of melanopsin‐expressing retinal ganglion cells in the mouse. J. Comp. Neurol. 497 (3): 326-349. DOI: 10.1002/cne.20970.
Banerjee A, Chattopadhyay A, Bandyopadhyay D (2020) Biorhythmic and receptor mediated interplay between melatonin and insulin: its consequences on diabetic erythrocytes. Melatonin Res. 3 (2): 243-263. DOI: 10.32794/mr12250060.
Reiter RJ (1993) The melatonin rhythm: both a clock and a calendar. Experientia 49 (8): 654-664. DOI: 10.1007/BF01923947.
Lincoln GA (1998) Photoperiod-melatonin relay in deer. Acta Vet. Hung. 46 (3): 341-356. PMID: 9704533.
Chemineau P, Pelletier J, Guérin Y, Colas G, Ravault JP, Toure G, Almeida G, Thimonier J, Ortavant R, Daveau A, Maurice F (1988) Photoperiodic and melatonin treatments for the control of seasonal reproduction in sheep and goats. Reprod. Nutr. Dev. 28 (2B): 409-422. DOI: 10.1051/rnd:19880307.
Powers JB, Jetton AE, Mangels RA, Bittman EL (1997) Effects of photoperiod duration and melatonin signal characteristics on the reproductive system of male syrian hamsters. J. Neuroendocrinol. 9 (6): 451-466. DOI: 10.1046/j.1365-2826.1997.t01-1-00602.x.
Reiter RJ (1974) Influence of pinealectomy on the breeding capability of hamsters maintained under natural photoperiodic and temperature conditions. Neuroendocrinology 13 (6): 366-370. DOI: 10.1159/000122222.
Reiter RJ (1973) Pineal control of a seasonal reproductive rhythm in male golden hamsters exposed to natural daylight and temperature. Endocrinology 92 (2): 423-430. DOI: 10.1210/endo-92-2-423.
Haldar C, Vidhu S (1997) Effect of pinealectomy and testosterone on gonadal regression and accessory sex organs in Indian palm squirrel, Funambuluspennanti. Indian J. Exp. Biol. 35 (6): 594-596. PMID: 9357162
Chen HJ (1981) Spontaneous and melatonin-induced testicular regression in male golden hamsters: augmented sensitivity of the old male to melatonin inhibition. Neuroendocrinology 33 (1): 43-46. DOI: 10.1159/000123198.
Reiter RJ (1980) The pineal and its hormones in the control of reproduction in mammals. Endocr. Rev. 1 (2): 109-131. DOI: 10.1210/edrv-1-2-109.
Samejima M, Shavali S, Tamotsu S, Uchida K, Morita Y, Fukuda A (2000) Light-and temperature-dependence of the melatonin secretion rhythm in the pineal organ of the lamprey, Lampetra japonica. Jpn. J. Physiol. 50 (4): 437-442. DOI: 10.2170/jjphysiol.50.437.
Fisher SP, Sugden D (2010) Endogenous melatonin is not obligatory for the regulation of the rat sleep-wake cycle. Sleep 33 (6): 833-840. DOI: 10.1093/sleep/33.6.833.
Mueller U, Hardeland R (1999) Transient accumulations of exogenous melatonin indicate binding sites in the dinoflagellate Gonyaulaxpolyedra. In: Studies on Antioxidants and Their Metabolites. Hardeland, R., Ed. pp. 140-147Cuvillier: Göttingen, Germany.
Nosjean O, Ferro M, Cogé F, Beauverger P, Henlin JM, Lefoulon F, Fauchère JL, Delagrange P, Canet E, Boutin JA (2000) Identification of the melatonin-binding site MT 3 as the quinone reductase 2. J. Biol. Chem. 275 (40): 31311-31317. DOI: 10.1074/jbc.M005141200.
Reppert SM, Godson C, Mahle CD, Weaver DR, Slaugenhaupt SA, Gusella JF (1995) Molecular characterization of a second melatonin receptor expressed in human retina and brain: the Mel1b melatonin receptor. Proc. Nat. Acad. Sci. 92 (19): 8734-8738. DOI: 10.1073/pnas.92.19.8734.
Dubocovich ML, Yun K, Al‐Ghoul WM, Benloucif S, Masana MI (1998) Selective MT2 melatonin receptor antagonists block melatonin‐mediated phase advances of circadian rhythms. FASEB J. 12 (12): 1211-1220. DOI: 10.1096/fasebj.12.12.1211.
Liu J, Clough SJ, Hutchinson AJ, Adamah-Biassi EB, Popovska-Gorevski M, Dubocovich ML (2016) MT1 and MT2 melatonin receptors: a therapeutic perspective. Annu. Rev. Pharmacol. Toxicol. 56: 361-383. DOI: 10.1146/annurev-pharmtox-010814-124742.
Mayo JC, Cernuda R, Quiros I, Rodriguez P, Garcia JI, Hevia D, Sainz RM (2019) Understanding the role of melatonin in cancer metabolism. Melatonin Res. 2 (3): 76-104. DOI: 10.32794/11250032.
Mazzucchelli C, Pannacci M, Nonno R, Lucini V, Fraschini F, Stankov BM (1996) The melatonin receptor in the human brain: cloning experiments and distribution studies. Mol. Brain Res. 39 (1-2): 117-126. DOI: 10.1016/0169-328x(96)00017-4.
Dubocovich ML (2007) Melatonin receptors: role on sleep and circadian rhythm regulation. Sleep med. 8: 34-42. DOI: 10.1016/j.sleep.2007.10.007.
Reppart SM, Weaver DR, Godson C (1996) Melatonin receptors step into the light: cloning and classification of subtypes. Trends Pharmacol. Sci. 17 (3): 100-102. DOI: 10.1016/0165-6147(96)10005-5.
Slaugenhaupt SA, Roca AL, Liebert CB, Altherr MR, Gusella JF, Reppert SM (1995) Mapping of the gene for the Mel1a-melatonin receptor to human chromosome 4 (MTNR1A) and mouse chromosome 8 (Mtnr1a). Genomics 27 (2): 355-357. DOI: 10.1006/geno.1995.1056.
Audinot V, Mailliet F, Lahaye-Brasseur C, Bonnaud A, Le Gall A, Amossé C, Dromaint S, Rodriguez M, Nagel N, Galizzi JP, Malpaux B (2003) New selective ligands of human cloned melatonin MT 1 and MT 2 receptors. NaunynSchmiedebergs Arch. Pharmacol. 367 (6): 553-561. DOI: 10.1007/s00210-003-0751-2.
Dubocovich ML, Masana MI, Iacob S, Sauri DM (1997) Melatonin receptor antagonists that differentiate between the human Mel1a and Mel1b recombinant subtypes are used to assess the pharmacological profile of the rabbit retina ML1 presynaptic heteroreceptor. NaunynSchmiedebergs Arch. Pharmacol. 355 (3): 365-375. DOI: 10.1007/pl00004956.
Ayoub MA, Levoye A, Delagrange P, Jockers R (2004) Preferential formation of MT1/MT2 melatonin receptor heterodimers with distinct ligand interaction properties compared with MT2 homodimers. Mol. Pharmacol. 66 (2): 312-321. DOI: 10.1124/mol.104.000398.
Wang LM, Suthana NA, Chaudhury D, Weaver DR, Colwell CS (2005) Melatonin inhibits hippocampal long‐term potentiation. Eur. J. Neurosci. 22 (9): 2231-2237. DOI: 10.1111/j.1460-9568.2005.04408.x.
O’Neal-Moffitt G, Pilli J, Kumar SS, Olcese J (2014) Genetic deletion of MT1/MT2 melatonin receptors enhances murine cognitive and motor performance. Neuroscience 277: 506-521. DOI: 10.1016/j.neuroscience.2014.07.018.
Chern CM, Liao JF, Wang YH, Shen YC (2012) Melatonin ameliorates neural function by promoting endogenous neurogenesis through the MT2 melatonin receptor in ischemic-stroke mice. Free Radic. Biol. Med. 52 (9): 1634-1647. DOI: 10.1016/j.freeradbiomed.2012.01.030.

This work is licensed under a Creative Commons Attribution 4.0 International License