Pal, P., Bhattacharjee, B., Chattopadhyay, A. and Bandyopadhyay, D. 2019. Melatonin as an armament against non-steroidal anti-inflammatory drug induced gastric injury: An overview. Melatonin Research. 2, 1 (Feb. 2019), 115-137. DOI:https://doi.org/https://doi.org/10.32794/mr11250015.
Review
Melatonin as an armament against non-steroidal anti-inflammatory drug (NSAID) induced gastric injury: An overview
Palash Kumar Pala, Bharati Bhattacharjeea, Aindrila Chattopadhyayb, Debasish Bandyopadhyaya*
aOxidative Stress and Free Radical Biology Laboratory, Department of Physiology, University of Calcutta, 92, APC Road, Kolkata-700009
bDepartment of Physiology, Vidyasagar College,39, Sankar Ghosh Lane, Kolkata-700006
*Correspondence:debasish63@gmail.com; Tel:+91-9433072066
Running title: Melatonin protects against NSAIDs
Received: December 19, 2018; Accepted: February 20, 2019
ABSTRACT
Non-steroidal anti-inflammatory drugs (NSAIDs) are the most widely prescribed medicines to treat numerous pathophysiological conditions clinically. However, growing evidence indicates the adverse effects of NSAIDs on the different vital organs, among which gastrointestinal (GI) tract seems to be the utmost target in most of the cases. NSAIDs promote over production of harmful reactive oxygen species (ROS) and reactive nitrogen species (RNS) in the gastric mucosa. These toxic species cause microvascular damage, increasing intestinal permeability, leading to the development of gastric lesions including ulcerations. Several strategies have been proposed to reduce the side effects of NSAIDs on the GI tissue, but most of them have failed to achieve this goal. Identification of an appropriate therapeutic strategy is urgently required. It is our opinion that this novel strategy to target GI damage induced by NSAID should include both anti-inflammatory and antioxidant properties. Under such a circumstance melatonin probably is the best choice for this purpose. Melatonin is a broad spectrum antioxidant and anti-inflammatory molecule. Numerous studies have reported the protective role of melatonin against gastric tissue damages caused by NSAIDs in animals or clinically. However, the underlying molecular mechanisms are not fully clarified. Thus, the present review attempts to gather the available information on this topic to provide a clear understanding on the exact scenario of this aspect.
Keywords: NSAIDs, cyclooxygenase, gastrointestinal tract, oxidative stress, tissue damage, melatonin, antioxidant
__________________________________________________________________________________________
1. INTRODUCTION
Nonsteroidal anti-inflammatory drugs (NSAIDs) are regularly prescribed globally for their anti-inflammatory, antipyretic and analgesic properties (1-3) to relieve pain and fever in rheumatic degenerative joint diseases and accidental injuries (4, 5). Despite of the beneficial effects, the uncontrolled application of NSAIDs has become a concerning due to their diverse harmful side effects on different organs, especially on the gastrointestinal (GI) tract (3). NSAIDs are reported to cause nephrotoxicity, hepatotoxicity and interrupted platelet functions. However, their serious toxicity frequently occurs in GI tract which could lead to death with high dose (6). The mechanisms of GI toxicity are complicated. For example, mercaptomethyl-imidazole (MMI) stimulates acid secretion in GI tract (7). In contrast, other NSAIDs block acid secretion (8), therefore, to cause hypergastrinemia which triggers hyperplasia in gastric enterochromaffin like cells (9) and ultimately results in gastric lesions (10) and gastric carcinoma (11). To make the mechanisms even more complex is that these drugs are also known to interact with the cytochrome P-450 system and alter the metabolic pattern of several other drugs (12). Although numerous studies have been conducted to enlighten the underlying mechanisms as to how NSAIDs cause such detrimental pathophyiological conditions in the GI tract and what is the best protective remedy, a limited success has been achieved. The reason behind such failure might be due to the complex interplay of several organ specific factors and the functional characteristics of different NSAIDs that vary between individuals.
One of such major factors consistently found to be involved in various gastric pathology induced by NSAIDs is reactive oxygen species (ROS) or reactive nitrogen species (RNS) (13-15). NSAIDs, for example indomethacin, increase ROS production (16) and irreversibly inactivate peroxidases in the gastric mucosa (17). RNS also involve in gastric ulcerogenesis induced by NSAIDs in human as well as in various animal models (18). Gene expression analyses on gastric tissues in different animal models support such contention (18, 19). The overproduction of ROS/ RNS induced by NSAIDs may overwhelm the antioxidant defence system in diverse types of GI cells (20) and ultimately lead to cell death. Thus, the prime focus is to identify a potent antioxidant molecule with minimal or no toxicity to neutralize the ROS/RNS and protects the cells/tissues/organs from the deleterious effects of NSAIDs. These potential molecules should exhibit three properties of anti-secretory, antiulcer and antioxidant.
In this endeavor, discovery of melatonin (N-acetyl-5-methoxytryptamine) from bovine pineal gland (21) was a major breakthrough in a pharmacological aspect. In addition to its diverse physiological roles, melatonin is found to be a potent antioxidant and direct free radical scavenger (22-23) with minimal or no toxicity even at high pharmacological doses (24), It has drawn a great attention from researchers in context of protection against NSAIDs induced side effects. Considering its direct (as a free radical scavenger) and indirect (receptor dependent) actions of melatonin (22, 23) researchers have continuously tested this small molecule and tried to establish its therapeutic use in GI tract damage. Consequently, its beneficial effects on oxidative stress and drug-induced damage in gastric tissues have been observed (15, 25). The protective mechanism of melatonin on gastric injury associated with NSAIDs is not yet clearly understood and remains a topic of debating. It is important to gather the existing information to further understand its protective mechanisms on NSAIDs GI toxicity. Hence, this review summarizes the development of melatonin research from animal studies to human clinical trials. The information and discussion will definitely enhance our knowledge regarding the molecular and cellular mechanisms involving the development of gastric ulcers caused by NSAIDs and highlighting melatonin as its potentially primary therapeutic remedy.
2. NSAIDS PROMOTE DIVERSE PATHOPHYSIOLOGICAL CONDITIONS IN DIFFERENT ORGANS AND TISSUES.
NSAIDs including sulindac, sulindac sulfide, sulindac sulfone, aspirin, indomethacin, acemetacin, tolmetin, etodolac, ketorolac, and oxaprozin possess therapeutic benefits for pain relief, inhibition of inflammation, H2O2 detoxification (26,27,28), antipyretic and antithrombotic activities (28) and neuroprotection (29). However, they also caused detrimental side effects in different tissues/organs. For example, NSAIDs exhibit neurotoxic effects on the central nervous system (29), alter the night-time levels of melatonin and body temperature (30), cause cardiovascular and musculoskeletal complications (31), platelet dysfunction, convulsions (32), stroke, high blood pressure, incontinence in urinary functions, increase in fall risk, and even cancer in diverse organs, such as in the prostate (33), endometrium (34), oesophageal, head and neck (35). Long-term use of NSAIDs may also develop dementia, depression, impaired cognitive function and other psychiatricdisorders (31). Among them, the most adverse effect of NSAIDs occurs on the GI tract from initial tissue damage and gastrointestinal bleeding to serious gastric inflammation and ulcers (15, 36). The differential side effects of NSAIDs on GI tract is due to the pharmacokinetics of these molecules and the local environments of GI. Biochemically NSAIDs contain monocarboxylic acid group which exhibits weak organic acidosis (pKa 3–5). At the acidic condition of GI tract, the NSAIDs are extracted (37). The carboxylic group, a basic structure of most of the NSAIDs, makes them to have high water solubility and detergent properties, thus they can interact with the surface membrane phospholipids and enter efficiently into the gastric cells causing unusual intracellular ion trapping (38).
3. MULTIPLE PATHWAYS ASSOCIATED WITH GASTRIC INJURIES INDUCED BY NSAIDS.
The GI injury caused by NSAIDs is a complicated and multistage process. During this process, the specific biochemical alterations and subcellular organelle damages are triggered by a series of tissue inflammatory reactions (39). The increased intestinal permeability (39), microvascular damage (40), gastric hypermotility (41), prostaglandin depletion (42), etc. have been implicated in the development of intestinal pathogenesis caused by NSAIDs.
NSAIDs cause intracellular energy depletion and cellular damages which finally increases intestinal permeability- a potent inducer of intestinal enteropathy (39). Patients receiving long term treatment of certain NSAIDs, particularly diclofenac, have frequently developed small intestinal enteropathy (38). The intestinal permeability is considered as one of the major therapeutic markers to evaluate the potential gastrointestinal damages caused by NSAIDs, especially in case of enteropathy (43).
Mucosal microvascular response is important for the mucosal defence since microvascular damage plays the initial and critical roles during the gastric ulcer development induced by NSAID (40). This contention is confirmed by the observation that the gastric ulcers caused by NSAIDs usually are present in the gastric antrum region where gastric mucosal blood flow level is lowest, possibly due to focal ischaemia (44). A significant correlation between decreasing mucosal blood flow and increasing injuries in the gastric antral region supports the argument mentioned above (44). Notably, the superficial erosions induced by NSAIDs occur mainly in the corpus region while the deep ulcers are in the antral region of the stomach (44).
A disruption of epithelial cell barrier in the gastric mucosa causes the topical injury with initial mucosal erosions while depletion of intracellular prostaglandin promotes the development of gastric and duodenal ulcers with use of NSAIDs (42). This assumption was based on the observation that inhibition of cyclooxygenase-1 (COX-1) by NSAID leads to the development of gastric hypermotility and microvascular disturbances in GI tract of rats with reduced levels of tissue prostaglandins (45).
Numerous studies have confirmed that gastric hypermotility is highly associated with the initiation and progression of gastric ulcers (41). The exact molecular mechanism of NSAIDs induced injury is currently unknown. It appears that high levels of NSAIDs are extracted in the GI tract and this temporarily restricts the blood supply to the gastric mucosa. The low blood supply and high tissue NSAIDs concentration synergistically promote microvascular damage and gastric hypermotility. If the process is prolonged, it enhances permeability of the mucosal layer and activity of myeloperoxidase, finally resulting in gastric lesions (41, 45). The unique pharmacokinetic and pharmacodynamic properties of NSAIDs add additional factors to promote the gastric inflammation and ulcer formation. The interactions between a particular NSAID and its pre-existing intracellular risk factors also contribute to the development of gastric ulcers (40). For example, a combination of indomethacin and bile is cytotoxic to the epithelial cells of the gastric mucosa. Making things worse is that NSAID-bile mixture repeatedly passes GI tract via enterohepatic circulation, thereby, increasing the risk of gastric injury (46). In addition, neutrophils are also known to be potent inducers of NSAIDs mediated gastric enteropathy (47), although the underlying mechanism is far from being understood. The potential pathways are illustrated in figure 1.

Figure 1: The illustration of potential pathways involved in NSAID-induced gastrointestinal damage.
4. NSAIDs AND PROSTAGLANDINS- A VIS-A-VIS INTERACTION.
The therapeutic actions of NSAIDs are principally based on antagonizing/blocking the synthesis of particular prostaglandins (PGs) through the inhibition of cyclooxygenases including cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2) (3, 14, 15). It is well known that prostaglandin E2 (PGE2) and prostacyclin (PGI2) are required to maintain the integrity of gastric mucosa. The COX-1 and COX-2 are responsible for the production of prostaglandin endoperoxide (PGG2) and prostanoids from membrane bound arachidonic acid (AA) (48, 49). PGG2 is the precursor of PGE2 and PGI2 which are compelling vasodilators and are solely responsible for regulating mucosal defense and healing of the gastric tissues. Although the functions of them are correlated, the function of PGE2 is mainly mediated by four G-protein coupled receptors (GPCRs)- EP1, EP2, EP3 and EP4, using diverse signaling pathways while function of PGI2 is solely mediated through IP receptors (49). For example, when PGE2 mediates its signals through EP1 and EP2⁄EP4 receptors, it increases intra-cellular levels of calcium ion and cAMP, respectively, while cAMP level is reduced when the signal is transmitted via EP3 (49). The different effects of PGE2 may uncover the potential mechanism related to gastric injuries induced by NSAIDs. The syntheses of PGE2 and PGI2 are triggered by several events including feeding cues, levels of histamine or gastrin in the gastrointestinal tract. Under physiological condition, both PGE2 and PGI2 inhibit gastric acid secretion. Hence, PGs maintain the gastric mucosal integrity, promote healing and protect the gastric epithelium (50). NSAIDs significantly suppress prostaglandin synthesis and lead to systemic complications in the gastric mucosa. NSAIDs are weak acids and the ionization of NSAIDs in acidic gastric medium is inhibited, therefore, their absorption rate within the GI tract is high. In addition, the intracellular pH of gastric tissue lumen causes extensive ionization of NSAIDs; thus, the NSAIDs molecules are trapped. The extremely high levels of NSAIDs in GI tract potentiate their anti-prostaglandin activities and result in severe mucosal injury (51, 52).
Being a bifunctional enzyme, cyclooxygenase (COX) displays both COX and peroxidase activities. COX can add two O2 molecules to AA to generate PGG2 (an unstable cyclic hydroperoxide) which will be reduced by the peroxidase activity of COX to form PGH2 (an endoperoxide) (48). Finally, PGH2 is converted to biologically active and stable prostanoids (PGE2, and thromboxane A2) with several enzymatic processes. The peroxidase activity of COX is known to generate NAD+ and NADP+ radicals that lead to the production of O2 (53). COX-1 is constitutively expressed in several tissues including the gastric mucosa and also regulates platelet hemostasis; whereas COX-2 is up-regulated only at the sites of any inflammation, tissue injury and tumorigenesis (54). COX-1 regulates the synthesis of prostaglandins and thromboxane A2 that primarily controls the mucosal defense in GI tract and numerous other physiological aspects. On the other hand, COX-2 controls the production of PGs that are associated with pain and inflammation (3).
Evidence from animal studies suggested that COX-1 dependent PGE2 depletion decreased blood flow in the gastric tissue while COX-2 inhibition induced leucocyte adherence (55). The inhibition of both COX-1 and COX-2 by NSAIDs causes severe gastric lesions (55, 56). At physiological condition, COX-1 is profoundly expressed in the gastric mucosa while COX-2 expression is low.However, COX-2 levels will be significantly up-regulated when COX-1 expression is inhibited during tissue injury or in pre-existing ulcers. COX-2 derived prostaglandins are the prime regulators for the healing of gastric ulcer, while during their shortage the COX-1 derived prostaglandins will take over and play the similar protective roles (57).
The anti-inflammatory, antipyretic and analgesic functions of most NSAIDS are mediated through the inhibition of COX-2 and some also inhibit the activity of COX1. Inhibition of both enzymes inevitably promotes gastric toxicity (3). Noteworthy, different NSAIDs possess varied specificity for COX-1 and/or COX-2 (58). For example, indomethacin is a non-selective COX inhibitor. Thus, the dose required to suppress COX-2 activity at an inflammatory site will also suppress COX-1. On the contrary, NSAIDs, such as naburnetone and etodolac, are highly selective COX-2 inhibitors and therefore, the ulcerogenic potentiality of these drugs is considerably reduced due to their low specificity toward COX 1 (58, 59). Even though these NSAIDs can selectively inhibit COX-2, for example coxibs, they still have the potential to induce the gastric ulceration due to inhibition of the production of cyto-protective prostaglandins (3). It was reported that the inhibition of platelet COX-1 expression by non-specific NSAIDs decreases thromboxane production and increases bleeding tendency in the concerned tissue. This is a major cause of gastric bleeding complications related to NSAIDs (such as piroxicam) (40, 60, 61). Thus, no matter, whether it is selective or non-specific inhibition of either COX-1 or COX-2 by NSAIDs, it will cause gastric mucosal injury by different degrees (56, 62). For example, expressions of both COX-1 and COX-2 are found to be up-regulated at gastric ulcer margins in humans, whereas under similar patho-physiological condition only COX-2 is up-regulated in rats (63, 64). Selective inhibition of COX-1 caused gastric hypermotility and increased microvascular complications in rat (45). Inhibition of both COX-1 and COX-2 caused more damage during ulcer healing than that treated with selective inhibitor of COX-2 in mice. Interestingly, no obvious effect on ulcer healing was found following selective inhibition of COX-1 (57). In contrary, COX-1 derived PGE2 is involved in early stage protection on gastric injury (65). The NSAIDs with both selective and non-selective COX inhibitory properties, such as naproxen, aspirin and celecoxib, delay healing of gastric ulcers in humans and animals (57, 66,67). In order to understand the underlying molecular interaction between NSAIDs and COX enzymes, aspirin is a better example since it has been investigated thoroughly. In brief, aspirin covalently binds and acetylates the serine residue (Ser 530) present in the active site of the COX enzymes. Such binding to COX-1 promotes conformational change of the enzyme structure to interrupt formation of oxidizing arachidonic acid, thereby inhibiting the activity of OX-1 (68). However, binding of aspirin to serine residue (Ser 516) in COX-2 is unable to inhibit formation of oxidizing arachidonic acid, thus it continues to produce 15-R-hydroxyeicosatetraenoic acid (15-R-HETE). 15-R-HETE, then is further metabolized by lipooxygenases to a potent neutrophil inhibitor, known as 15-epi-Lipoxin A4 (15 epi-LXA4) (68).
5. OTHER FACTORS IN GASTRIC DAMAGES INDUCED BY NSAIDs.
Although the gastrointestinal injury induced by NSAIDs is primarily due to blocking of prostaglandin synthesis through inhibition of the COX enzymes, this is not the only underlying mechanism but other prostaglandin independent pathways are also involved (40). Many autacoids along with prostaglandins co-orchestrate to maintain the integrity of the gastric mucosa and promote ulcer healing. These autacoids include nitric oxide and hydrogen sulphide (H2S) as the gaseous mediators, neuropeptides (such as calcitonin gene related peptide [CGRP] and substance P), hormones (such as melatonin and gastrin), matrix metalloproteinases, growth factors, polyamines and stress proteins (40). As mentioned above, NSAIDs can accumulate in the gastric epithelial cell due to ‘ion trapping’. This accumulation interferes in mitochondrial oxidative phosphorylation and finally disrupts the electron transport chain. Therefore, this activity depletes intracellular level of ATP, induces Ca2+ toxicity and eventually enhances the generation of ROS. Overproduction of ROS is detrimental to cell since they oxidize proteins, lipids or nucleic acids and impede important intracellular signalling pathways, even lead to cellular necrosis and apoptosis (69). NSAIDs, such as indomethacin and aspirin, disrupt the hydrophobic barrier of the epithelial lining by establishing chemical association with extracellular phospholipids lying on and within gastric mucosa (70-72). Acid back-diffusion via damaged gastric mucosa plays a key role in promoting superficial lesions to serious mucosal ulcers (73-74). Induction of different growth factors, various cytokines (IL-1β and IL-6) and hormones (such as gastrin) at the ulcer margins by PGE2 is also involved in the abatement of complex pathogenesis induced by NSAIDs in the GI tract (75). Therefore, maintenance of an appropriate level of cyto-protective PGE2 in the gastric mucosa is very important. Depletion of this cyto-protective prostaglandin causes gastric mucosal injury. Leukocyte accumulation induced by NSAID is another culprit in the development of gastric mucosal injury either by enhancement of tissue ROS and proteases, or by reduction of gastric blood flow through formation of capillary obstacle (76).
6. EFFECTS OF NSAIDS ON FREE RADICAL GENERATION IN GASTRIC TISSUES.
Oxidative stress is a physio-pathological condition with excessive production of ROS which disrupts intracellular antioxidant defense system (25). Re-establishment of normal cellular oxidative balance depends on the adaptability and repair-replacement capability of the cells/tissues (77). The role of ROS in the gastric ulcerations induced by NSAIDs has been well documented in animal studies and also in clinical trials (78, 79). Numerous NSAIDs, such as aspirin, indomethacin, ibuprofen, etc., are known to induce oxidative stress in the gastric tissues by inhibiting prostaglandin synthesis, an essential gastro-protective molecule (80). NSAIDs also inhibit cell proliferation and deplete cellular energy supply, thus leading to increased levels of H2O2 and hydroxyl radical (•OH) in the gastric mucosa which results in oxidative mucosal damage (7, 78). A gene expression study in the rat stomach has also confirmed this observation (19). For example, indomethacin treatment irreversibly inactivated gastric peroxidase in the rat gastric mucosa, and caused oxidative stress in this tissue (17). Piroxicam administration elevated the level of •OH in the gastric tissue of rats and caused depletion of antioxidants, lipid peroxidation, protein oxidation and gastric ulceration. (61). NSAIDs also target mitochondria by disrupting its trans-membrane potential and increasing permeability transition pore with cytochrome c release (81). Mitochondrial damage inevitably promotes ROS production, induces caspase cascade and membrane lipid peroxidation and cellular apoptosis (39). Consequently, mitochondrial damage, in turn, increases intestinal permeability, mucosal erosion and intestinal injuries as well (39).
In addition to ROS, the RNS is also involved in NSAID induced gastric ulcerogenesis (18). NSAIDs enhance the activity of endothelin-converting enzyme-1 (ECE-1) to increase intracellular endothelin-1 (ET-1) which suppresses the expression of cNOS to reduce endothelial nitric oxide and ultimately distorts mucosal integrity (18). Indomethacin increased serum levels of NO by up-regulation of iNOS expression in activated circulating neutrophils while at the same time g astric NO levels are decreased since the gastric mucosal cNOS expression was down-regulated (82). Interestingly, up-regulation of iNOS mRNA expression is reported to involve in gastric ulcerogenesis in arthritic rats (83). An increase in gastric tissue iNOS activity is also observed in human volunteers when treated with ibuprofen (84). The mechanisms by how that NOS regulates the gastric physiopathology were reported by Souza et al. (85-86). For example, indomethacin caused significantly less gastric mucosal damage in animals pre-treated with either non-specific NOS inhibitor (NG-nitro-L-arginine methyl ester) or specific iNOS inhibitor (85, 86). The levels of both iNOS and eNOS derived NO are increased in NSAID-induced gastric ulcer healing process indicating that NO participates in the healing process under pathological conditions rather than in the normal healing scenario (57).
7. IMPACTS OF NSAIDs ON CELLULAR STRESS RESPONSIVE PROTEINS AND APOPTOSIS.
A number of in vitro and in vivo studies have shown the capability of NSAIDs in up-regulating several stress responsive proteins, such as heat shock protein 72 (HSP-72), glucose-regulated protein 78 (GRP-78), and HO-1 (also known as HSP-32) (87). Stress responsive proteins play an important role as potent cyto-protectors of gastric mucosa from NSAID-induced cellular apoptosis, particularly when synthesis of prostaglandin is hampered by NSAIDs. Notably, resistance to NSAID-induced gastric erosions and ulcers was reported in transgenic mice with a particular phenotype which was over expression of human HSP27 (88). Moreover, NSAIDs (such as sodium diclofenac, flurbiprofen, zaltoprofen, etodolac, etc.) are reported to induce DNA fragmentation associated to cellular apoptosis and enhances COX-2 mRNA expression in isolated AGS cells derived from human gastric epithelium (89).
8. DEVELOPMENT OF PREVENTIVE STRATEGIES.
Considering multiple side effects of NSAIDs on gastric tissue, it is necessary to develop their alternatives with no or less side effects to treat inflammation. To achieve this goal, a better understanding of the normal physiology and gastric mucosal defence mechanisms is essential (90). Although many attempts have been made to try overcoming the pro-inflammatory and topical irritant properties of NSAIDs, most of them have failed to reduce gastric ulcerogenesis, tissue perforation and bleeding complications (91). The major cause behind such failure was that the structural modifications for the new NSAIDs indeed improved their topical irritancy but were unable to modify their inhibitory effect on the synthesis of gastric prostaglandins. Some of the attempts are discussed herein. Firstly, incorporation of a nitric oxide (NO) generating moiety into the NSAID molecule was desired to suppress its side effects. Since, NO is well known for its potent vasodilation property and neutrophil associated functions, the design was predicted to counteract the harmful effects of COX suppression in the gastric microcirculation and to reduce mucosal injuries (92). The rational to select NO as a tag to NSAIDs was based on the notion that inhibition of NO synthesis might associate with NSAIDs induced gastric damages (93). Moreover, NO and NO donors were found to reduce the clinical severity of gastric injuries in experimental animal models (68, 94). It was observed that rats with pre-existing colitis treated with NO-NSAIDs on daily basis, caused serious ulceration in the small intestine, leading to gastric perforation and ultimately death (95). In addition, the derivatives of NO-NSAIDs also possessed adverse effects since they shared same property to suppress gastric prostaglandin synthesis as their parent NSAIDs (95). Secondly, co-administration of a prostaglandin analogue and NSAIDs was suggested as an alternate strategy in reducing ulcerogenic characteristics. Thus, administration of PGE1 analogue, misoprostol, was found to markedly decrease the ulcerogenic incidences caused by any NSAID following long term treatment (96). However, other severe side effects, such as diarrhea, as well as the cost effectiveness issues impeded this strategy (97). Latter discovery of two isoforms (COX-1 and 2) of cyclooxygenase has restricted in designing novel anti-inflammatory drugs/agents that were assumed to spare the gastrointestinal tract.
In addition, several other strategies were also used in an attempt to reduce NASIDs associated side effects. These included enteric coating technology to reduce gastric absorption, parenteral administration, designing several proto-drugs that requires metabolism in the hepatic cells for their desired effects on cyclooxygenase activity, synthesis of basic molecules instead of acidic properties, co-administration of exogenous prostaglandins or acid secretion inhibitors or COX-2 inhibitors (52,98). However, none of these attempts were successful due to their adverse reactions and severe ulcerogenic properties again in the GI tract (51). Thus, chronic use of NSAIDs was not recommended by the Beers Criteria of The American Geriatric Society in 2015.due to their high risk of gastric bleeding. Interestingly, administration of synthesized cysteamine (a well-accepted antioxidant) tagged amide derivatives of some NSAIDs, such as diclofenac acid, tolfenamic acid, ibuprofen and indomethacin, was found to significantly reduce gastric ulcerogenesis in animal models (13). Such observation requires further careful investigation before its clinical use. It seems that if NSAIDs possess both anti-inflammatory and antioxidant activities along with reduced acidic property, the novel NSAIDs, will potentiate their anti-inflammatory action while they will also cause none or less side effects in the GI tract (13).
9. MELATONIN- A MULTI TASKING MOLECULE IN THE GASTROINTESTINAL TRACT.
Melatonin (N-acetyl-5-methoxytryptamine) is not only synthesized in the pineal gland, but its production has also been identified in several extra-pineal tissues/organs in vertebrates (99), including the gastrointestinal tract (100). Presence of melatonin in the GI tract was first reported in rat (101). Subsequently, melatonin was immuno-histologically detected in the enterochromaffin cells (EC) of the gastric mucosa in rat (102-105) and its presence in the gastrointestinal tract was confirmed by radioimmunoassay (RIA) and high performance liquid chromatography (HPLC) (106-107). Using autoradiography, the maximum binding of melatonin was detected in the mucosa and intestinal villi (104, 108). At the sub-cellular level, the highest melatonin binding was found in the nuclear fraction, followed by the microsomal, mitochondrial, and cytosolic fractions (109). Notably, arylalkylamine-N-acetyltransferase (AANAT) as the rate-limiting enzyme in melatonin biosynthesis was identified in GI tract (110). Not only melatonin but melatonin receptors are also found abundant in the GI tract (111). As a signal molecule, melatonin regulates seasonal and circadian rhythms, reproduction, day/night activity, sleep behaviour and many other physiological events in vertebrates (99). In addition, melatonin also participates in the protection of gastric tissues from oxidative and inflammatory injuries (79, 112).
10. RELEVANCE OF MELATONIN RECEPTORS IN GASTRIC TISSUE.
Melatonin receptors and/or binding sites have been identified in the GI tract of vertebrates including human (108,113, 111). The GI derived melatonin also releases into the peripheral circulation (79,114, 115) All of these clearly suggest the paracrine function of GI melatonin. It is widely accepted that most of the physiological effects of melatonin in the GI tract are mediated by activation of its membrane receptors. Two melatonin membrane receptors have been reported, that is MT1 and MT2. These receptors belong to the G-protein receptor family. A third receptor for melatonin, MT3, is also under consideration though it is classified to be a quinine reductase 2 (116). Current knowledge indicates that melatonin is also likely to act through its nuclear receptors (or binding sites) which belong to the RZR/ROR orphan receptor family including three subtypes (α, β, γ) and four splicing variants of the α subtype (117). Melatonin acts on these specific receptors to exert its physiological and pharmacological effects on the target cell/tissue/organ. For example, melatonin acts on MT1 receptor to stimulate G proteins (Giα2, Giα3, Gαq) and to inhibit the cAMP signalling pathways (118); whereas its action on MT2 receptors is associated with phosphoinositide signal transduction pathways and inhibits the adenylyl cyclase and guanylyl cyclase pathways (119).
11. MELATONIN AS AN ANTIOXIDANT IN GASTRIC TISSUE.
Melatonin, being an amphiphilic molecule, can easily pass through any biological membrane, thus possesses a free access to any cell/tissue/organ (120). Such an advantage renders this indolamine with diverse antioxidant properties that not only protects cells from oxidative damage induced by ROS but also from RNS (120-122). Melatonin is a broad spectrum antioxidant with a direct and indirect free radical scavenging property (121-122). It is especially effective in scavenging the most reactive and harmful •OH, a property of melatonin first reported by Tan et al. (22). Despite of the notable presence of various other endogenous antioxidants, melatonin is found to be unique due to its diverse actions on different cells/tissues. It not only directly scavenges free radicals (22), but also reduces the production of reactive species by inhibiting the activities and/or expression of pro-oxidative enzymes. Thus, evaluation of the functional characterization of melatonin and its physiological relevance in regulation of oxidative status in diverse cells/organs, especially gastrointestinal tract (61, 39, 79, 112) became the prime focus of the researchers from the last decade (120, 122).
Several lines of evidence were in favour of melatonin acting as a potent detoxifier of the highly toxic peroxynitrite anion, hydroxyl-, peroxynitrite-, and peroxyl radicals and singlet oxygen (23, 122-126) to protect against oxidative membrane damage (125) under diverse gastric pathophysiological conditions (39, 125, 127). Available data have unequivocally demonstrated the antioxidant capacity of melatonin in GI tract of vertebrates (125, 127-130). Not only melatonin but its metabolites are also the potent antioxidants (127, 131) to scavenge highly toxic hydroxyl radical, peroxynitrite anion as well as peroxyl radical and to quenche singlet oxygen (124). They cooperate with other intracellular antioxidants to maintain membrane integrity and protect the cells from the deleterious ROS (127). Basically, under oxidative stress, melatonin executes its antioxidant action on the target cells via two pathways One is receptor-independent, that is, melatonin directly scavenges harmful free radicals. The other is its receptor dependent and indirect pathway. In this pathway, melatonin regulates the levels/activities of numerous intracellular enzymatic or non-enzymatic antioxidants (14, 23, 132). It was reported that melatonin up-regulated the levels of mRNA and activities of different endogenous antioxidant enzymes including SOD, CAT, GPx, GR, glucose-6-phosphate dehydrogenase (G6PD) and gamma-glutamylcycteine synthase under oxidative stress (23, 122, 133).
Currently, the attention has been given to the effects of melatonin on the mitochondrial physiology.Melatonin is able to reduce electron leakage from the mitochondrial electron transport chain (134), increases mitochondrial respiration and ATP synthesis by enhancement of activities of complex I and IV (134-136). Notably, mitochondrial matrix is the primary site for free radical generation during electron transfer to molecular oxygen. On the other hand, melatonin inhibits NOS activity, ultimately reducing NO production in the cell/tissue (126). All these activities render melatonin to effectively prevent proteins, lipids, as well as DNA from oxidative damage (122, 137-138). Therefore, due to the potent antioxidant and free radical scavenging properties, melatonin is suggested as a potential therapeutic agent in the inhibition and healing of gastric injury induced by any sort of oxidative damage, particularly induced by NSAIDs (139-140).
12. MELATONIN- A POTENT THERAPEUTIC AGENT AGAINST NSAIDs-INDUCED GASTRIC INJURY.
It was well documented that melatonin effectively protected against gastric injuries caused by a variety of experimentally induced stress in the GI tract (79,112, 141-142) and this suggested the potentially beneficial effects of melatonin against NSAID-induced oxidative stress and gastric tissue injury. Since melatonin treatment could suppress ROS in circulatory system and prevent neutrophil accumulation locally (79, 112), it clearly indicates its protective potential against any pathophysiological condition in the GI tract. It was observed that, prostaglandins enhanced nocturnal melatonin synthesis in the gastric tissues (79) while NSAIDs, such as aspirin and ibuprofen, inhibited the production of PGs and reduce the synthesis of gastric melatonin; thus, an important factor of GI tract damage induced by NSAIDs may be related to the reduced melatonin levels in the GI tract of human (143).
12.1. Melatonin increases mucosal blood flow and promotes ulcer healing:
Melatonin has been reported to exhibit dose-dependently protective effects on indomethacin as well as piroxicam- induced gastric damages in rat (61). Likewise, aspirin induced mucosal damages in the GI tract of human is also significantly reduced following oral administration of melatonin as well as its precursor L-Tryptophan (144-145). Interestingly, an increase in circulating levels of melatonin was observed in aspirin-induced acute GI injuries and this indicated that the synthesis of melatonin in the gastric tissue was induced and probably the tissues try to combat against the injury (144). The same study also depicted that exogenous melatonin and L-tryptophan increased the levels of mRNA expression of MT2 and two major enzymes of the melatonin biosynthetic pathway,AANAT and hydroxyindole-O-methyltransferase (HIOMT) along with an enhanced blood flow in the gastric mucosa. These results suggest the potential ulcer healing property of melatonin in the gastric tissue (144). Melatonin and L-Tryptophan are reported to increase the mucosal blood flow and release of NO in the lumen of GI tract in rats that were under indomethacin or NOS (L-NAME) administration (16, 146).
12.2. Melatonin scavenges free radicals and inhibits development of gastric ulcer.
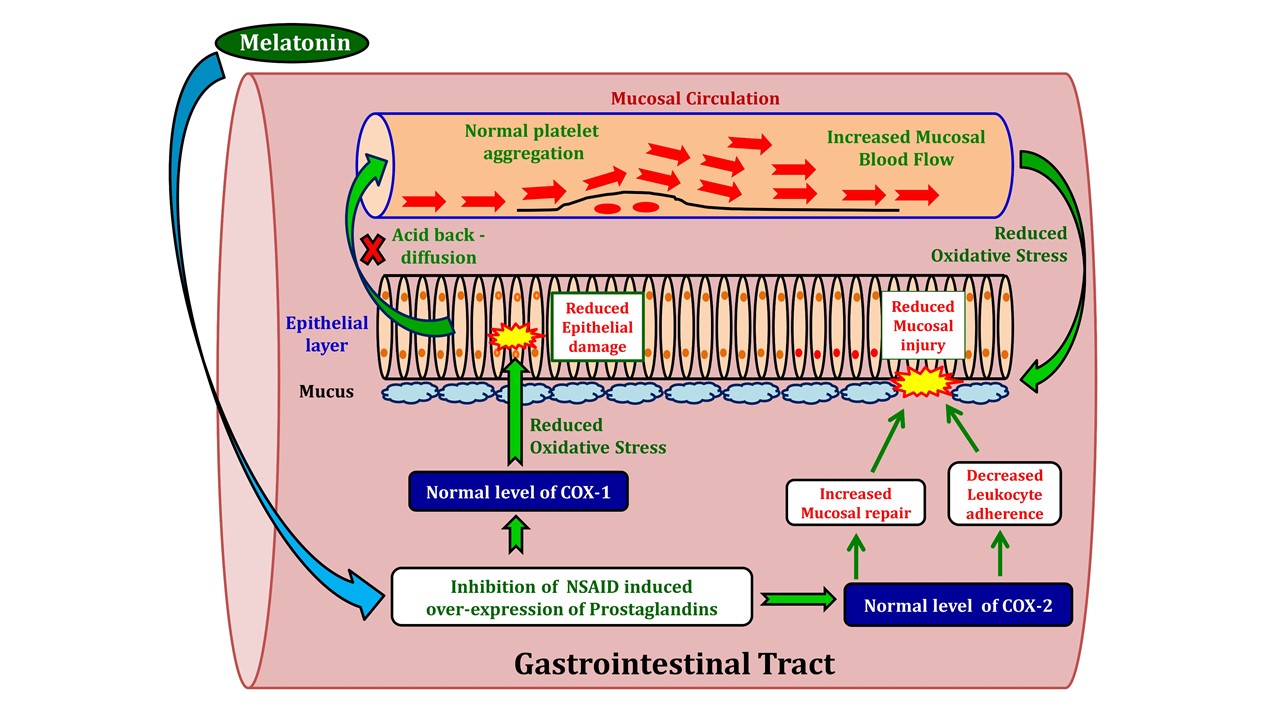
Melatonin acting as a direct as well as indirect antioxidant was observed to improve the intestinal permeability and restore mucosal architecture at ultrastructural level which were distorted by diclofenac treatment (147-148). The mechanism might involve in altering the ion channels (148) to re-establish membrane potential and restore normal energy metabolism in the mitochondria and thus, preventing cellular death (150). Additional studies clearly demonstrated the capability of melatonin in preventing ROS mediated gastric ulceration induced by NSAIDs, such as piroxicam (61). This was achieved by the melatonin’s capacity to restore the activities of gastric peroxidise, superoxide dismutase, catalase and to lower tissue levels of .OH, thus providing direct evidence regarding antioxidant and free radical scavenging properties of melatonin against gastric injuries induced by NSAIDs (61). Mechanistic exploration indicated that protective effects of melatonin on indomethacin-induced gastric injury and its healing process were primarily mediated by its MT2 receptors (151). MT2 receptor activation increases MMP-2 expression, attenuates the MMP-9 activity and TIMP-2 expression and finally lowers the ROS in the gastric tissue (152-153). Numerous studies in mammals, have shown that melatonin dose-dependently inhibits secretion of gastric acid, reduces neutrophil infiltration and cytotoxicity by, enhances gastric mucosal blood flow at ulcer bed, induces secretion of bicarbonate in the duodenum, stimulates diverse intracellular defence systems associated with the cycloxygenase-prostaglandins and NOS-nitric oxide as well as CGRP (145,154-155) (Fig. 2). Finally, potent anti-apoptotic action of melatonin should also be added to its protective effects on the gastric mucosal injury induced by NSAIDs (139).

Figure 2: The illustration of the potentially protective roles of melatonin against NSAID-induced gastrointestinal damage.
12.3. Melatonin as an alternative to NSAIDs.
The beneficial roles of melatonin against NSAID-induced gastric injury have been listed in the table 1. In addition to the protective effects of melatonin on gastric injury induced by the NSAIDs, melatonin per se can also decrease inflammatory pain, by inhibiting NO generation and regulating the signalling pathways of NO‑cyclic GMP (156). The analgesic action of melatonin involves several components and diverse pathways including β‑endorphins, GABA receptor, opioid l‑receptors and the nitric oxide (NO)‑ arginine pathway (157-158). Interestingly, due to steric and electronic complementarity, melatonin can directly bind to the active site of COX-1 and COX-2 acting as a natural inhibitor of COX to suppress the inflammatory reaction (158-159). Its application not only reduces the GI tract injury induced by the NSAIDs but also improves the anti-inflammatory effects of NASID.
Table 1: Beneficial roles of melatonin against NSAID-induced gastric injury.
|
NSAID |
Adverse effects on the GI tract |
Affected cellular agents |
Effect of melatonin |
Applied doses of melatonin |
References |
|
Indomethacin |
(i) Disrupts epithelial lining (ii) Develops gastric ulcer |
Inhibits synthesis of prostaglandins (PGs) |
(i) Enhances ulcer healing process (ii) Increases mucosal cell proliferation (iii) Promotes mucosal repair |
20 mg/kg |
17, 58, 72, 147-148, 160 |
|
Piroxicam |
(i) Increases gastric bleeding complications (ii) Develops gastric ulcer |
Inhibits gastric COX-1 level |
(i) Restores gastric COX-1 level (ii) Decreases gastric ulceration |
60 mg/kg |
40, 60-61 |
|
Naproxen |
Delays gastric ulcer healing |
Acts as selective and non-selective inhibitors of COX |
No data available |
No data available |
57, 67 |
|
Aspirin |
Delays gastric ulcer healing |
(i) Acts as selective and non-selective inhibitors of COX (ii) Decreases synthesis of gastric melatonin |
Enhances gastric ulcer healing process |
2.5-10 mg/kg |
57, 67, 70-72, 79 |
|
Celecoxib |
Delays gastric ulcer healing |
Acts as selective and non-selective inhibitors of COX |
No data available |
No data available |
57, 66, 67 |
|
Diclofenac |
Increases mucosal permeability |
(i) Damages DNA (ii) Increases gastric COX-2 expression |
(i) Decreases intestinal injuries (ii) Restores intestinal permeability |
10 mg/kg |
39, 89, 149 |
|
Ibuprofen |
Increases gastric disease severity |
(i) Inhibits PGs (ii) Reduces gastric melatonin synthesis |
No data available |
No data available |
144 |
13. FUTURE PERSPECTIVES.
There is no doubt that unregulated application of NSAIDs is a potent threat to cause diverse gastrointestinal complications leading to gastric bleeding, ulceration and even causing death of the individual. Reduced levels of mucosal prostaglandins and increased ROS seem to convey the most adverse effects of NSAIDs in the GI tissues. The low levels of PGs also cause low melatonin production in the gastric tissue which leads to the vicious cycle of oxidative stress and gastric injury in GI tract. Therefore, elaborate studies regarding the structural complementarity of a potent antioxidant- melatonin with prostaglandins related enzymes (COX-1 and 2) may evolve new strategy to overcome the side effects of NSAIDs in the gastric tissues. Numerous NSAIDs and existence of variety of functional pathways of them make the situation much more complicated and challenging to develop novel therapeutic agent with no or least gastric toxicity. It is our opinion that the novel NSAIDs must be re-designed in such a way that they possess both anti-inflammatory and antioxidant activity along with ability to reduce acid secretion. Overwhelming evidences on the multi-functional property and numerous beneficial characteristics of melatonin in animal studies and clinical trials clearly indicated this indolamine as an effective alternative against the gastric toxicity induced by different NSAIDs, although further careful investigations are required to reveal the exact underlying molecular mechanism(s). Here, we suggest that melatonin can be used along with NASIDs clinically to improve the anti-inflammatory effects of the NASIDs and to reduce their gastric side effects. The clinical trials for this combination definitely deserve to be tried.
AUTHORSHIP
Dr. DB and Dr. AC contributed to conception, revised the manuscript critically and approved it. Dr. PKP contributed to conception, prepared figures, drafted the manuscript and edited it. BB contributed in drafting of manuscript.
ACKNOWLEDGEMENTS
Dr. Palash Kumar Pal gratefully acknowledges the receipt of UGC Dr. D. S. Kothari Post Doctoral Fellowship (BL/16-17/0502), Govt. of India. A financial assistance as Senior Research Fellowship (SRF) under DST-INSPIRE program (IF140691), Govt. of India to Bharati Bhattacharjeeis also thankfully acknowledged. Dr. Aindrila Chattopadhyay is supported by funds available to her from Department of Science and Technology, Govt. of West Bengal. Prof. DB also extends his grateful thanks to University Grants Commission, Govt. of India, for the award of a Research Project under Center with Potential for Excellence in a Particular Area (CPEPA). Prof. DB is also supported from departmental BI grant of University of Calcutta. Prof. DB also gratefully acknowledges the support he received from DST-PURSE Program awarded to University of Calcutta, and also, financial assistance from UPE-II Scheme awarded to University of Calcutta by UGC, Govt. of India. Prof. DB gratefully acknowledges the contribution of the Editor-In-Chief of Melatonin Research in critically reading and editing of the manuscript which has definitely improved the scientific and readership quality of the article.
CONFLICT OF INTEREST
Authors declare no conflict of interest.
REFERENCES
Siu SS, Yeung JH, Lau TK (2000) A study on placental transfer of diclofenac in first trimester of human pregnancy. Hum. Reprod. 15: 2423-2425.
Kudo C, Kori M, Matsuzaki K, Yamai K, Nakajima A, Shibuya A, Niwa H, Kamisaki Y, Wada K (2003) Diclofenac inhibits proliferation and differentiation of neural stem cells. Biochem.Pharmacol. 66: 289-295. https://doi.org/10.1016/S0006-2952(03)00235-1.
Wongrakpanich1 S, Wongrakpanich A, Melhado K, Rangaswami J (2018) A Comprehensive Review of Non-Steroidal Anti-Inflammatory Drug Use in The Elderly. Aging Dis. 9: 143-150. http://dx.doi.org/10.14336/AD.2017.0306
Ericson A, Källén BA (2001) Nonsteroidal anti-inflammatory drugs in early pregnancy. Reprod. Toxicol. 15: 371-375. DOI: 10.2174/138920012800166607.
Aygün D, Kaplan S, Odaci E, Mehmet EO,Muhammad EA (2012) Toxicity of non-steroidal anti-inflammatory drugs: a review of melatonin and diclofenac sodium association. Histol. Histopathol. 27: 417-436. DOI: 10.14670/HH-27.417.
Tenenbaum, J (1999) The epidemiology of non-steroidal anti-inflammatory drugs. Can. J. Gastroenterol. 13: 119–122.
Banerjee RK (1990) Nonsteroidal anti-inflammatory drugs inhibit gastric peroxidase activity. Biochim. Biophys. Acta. 1034: 275-280.
Wormsley KG (1988) Is Chronic Long-Term Inhibition of Gastric Secretion Really Dangerous? Scand. J. Gastroenterol. 146 (Suppl): 166-174.
Lambersts R, Crentzfeldt W, Struber HG (1993) Long-term omeprazole therapy in peptic ulcer disease: gastrin, endocrine cell growth, and gastritis. Life Sci. 60: 169-174.
Koelz HR (1986) Scand. J. Gastroenterol. 21: 156-164.
Wormsley KG (1984) Assessing the safety of drugs for the long-term treatment of peptic ulcers. Gut. 25: 1416-1423.
Hansten PD (1994) In: A Pharmacologic Approach to Gastrointestinal Disorders, Lewis, J.H., Ed., Academic Press, New York, pp. 535-540.
Kourounakis PN, Tsiakitzis K, Kourounakis AP, Galanakis D (2000) Reduction of gastrointestinal toxicity of NSAIDs via molecular modifications leading to antioxidant anti-inflammatory drugs. Toxicol. 144: 205–210. https://doi.org/10.1016/S0300-483X(99)00208-5.
Bandyopadhyay D, Biswas K, Bhattacharyya M, Reiter RJ, Banerjee RK (2001) Gastric toxicity and mucosal ulceration induced by oxygen derived reactive species: protection by melatonin. Curr. Mol. Med.1: 501-513.DOI: 10.2174/1566524013363483.
Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE (2014) Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 94: 329–354. DOI: 10.1152/physrev.00040.2012.
Hassan A, Martin E, Puig-Parellada P (1998) Role of antioxidants in gastric mucosal damage induced by indomethacin in rats. Methods Find. Exp. Clin. Pharmacol. 20: 849–854. DOI: 10.1358/mf.1998.20.10.487540.
Chattopadhyay I, Bandyopadhyay U, Biswas K, Maity P, Banerjee RK (2006) Indomethacin inactivates gastric peroxidase to induce reactive-oxygen-mediated gastric mucosal injury and curcumin protects it by preventing peroxidise inactivation and scavenging reactive oxygen. Free Radic. Biol. Med. 40: 1397–1408. DOI: 10.1016/j.freeradbiomed.2005.12.016.
Slomiany BL, Slomiany A (2000) Role of endothelin-converting enzyme-1 in the suppression of constitutive nitric oxide synthase in rat gastric mucosal injury by indomethacin. Scand. J. Gastroenterol. 35: 1131–1136.
Naito Y, Yoshikawa T (2006) Oxidative stress Involvement and gene expression in indomethacin-induced gastropathy. Redox Rep. 11: 243–253. DOI: 10.1179/135100006X155021.
Kotler M, Rodriguez C, Sáinz RM, Antolín I, and Menédez-Peláez A (1998) Melatonin increases gene expression for antioxidant enzymes in rat brain cortex. J. Pineal Res. 24: 83–89. https://doi.org/10.1111/j.1600-079X.1998.tb00371.x.
Lerner AB, Case JD, Lee TH, Mori W (1958) Isolation of melatonin, the pineal factor that lightens melanocytes. J. Am. Chem. Soc. 80: 2587–2587.
Tan DX, Chen LD, Poeggeler B, Manchester LC, Reiter RJ (1993) Melatonin: a potent, endogenous hydroxyl radical scavenger. Endocr. J. 1: 57–60.
Rodriguez C, Mayo JC, Sainz RM, Antolin I, Herrera F, Martin V, Reiter RJ (2004) Regulation of antioxidant enzymes: A significant role for melatonin. J. Pineal Res. 36: 1–9. https://doi.org/10.1046/j.1600-079X.2003.00092.x.
Jan JE, Hamilton D, Seward N, Fast DK, Freeman RD, Laudon, M (2000) Clinical trials of controlled-release melatonin in children with sleep–wake cycle disorders. J. Pineal Res. 29: 34-39. https://doi.org/10.1034/j.1600-079X.2000.290105.x.
Bandyopadhyay D, Biswas K, Bandyopadhyay U, Banerjee, RK (2000) Melatonin protects against stress-induced gastric lesions by scavenging the hydroxyl radical. J. Pineal Res. 29: 143–151. https://doi.org/10.1034/j.1600-079X.2000.290303.x.
Ennis ZN, Dideriksen D, Vaegter HB, Handberg G, Pottegard A (2016) Acetaminophen for chronic pain: a systematic review on efficacy. Basic Clin. Pharmacol. Toxicol. 118: 184-189. DOI: 10.1111/bcpt.12527.
Costa D, Gomes A, Reis S, Lima JL, Fernandes E (2005) Hydrogen peroxide scavenging activity by non-steroidal anti-inflammatory drugs. Life Sci. 76: 2841-2848. DOI: 10.1016/j.lfs.2004.10.052.
Garner A (1992) Adaptation in the pharmaceutical industry, with particular reference to gastrointestinal damages and diseases. Scand. J. Gastroenterol. 27 (Sqpl. 193): 83 - 89.
Ragbetli MC, Ozyurt B, Aslan H, Odaci E, Gokcimen A, Sahin B, Kaplan S (2007) Effect of prenatal exposure to diclofenac sodium on Purkinje cell numbers in rat cerebellum: a stereological study. Brain Res. 1174: 130-135. DOI: 10.1016/j.brainres.2007.08.025.
Murphy PJ, Myers BL, Badia P (1996) Nonsteroidal anti-inflammatory drugs alter body temperature and suppress melatonin in humans. Physiol. Behav. 59: 133-139. https://doi.org/10.1016/0031-9384(95)02036-5.
Kang JH, Grodstein F (2003) Regular use of nonsteroidal anti-inflammatory drugs and cognitive function in aging women. Neurology 60: 1591-1597. DOI: https://doi.org/10.1212/01.WNL.0000065980.33594.B7.
Yakushiji T, Shirasaki T, Akaike N (1992) Non-competitive inhibition of GABAA responses by a new class of quinolones and non-steroidal anti-inflammatories in dissociated frog sensory neurones. Br. J. Pharmacol. 105: 13-18.
Vidal AC, Howard LE, Moreira DM, Castro-Santamaria R, Andriole GL, Freedland SJ (2015) Aspirin, NSAIDs, and risk of prostate cancer: results from the REDUCE study. Clin. Cancer Res. 21: 756-762. DOI: 10.1158/1078-0432.CCR-14-2235.
Verdoodt F, Friis S, Dehlendorff C, Albieri V, Kjaer SK (2016) Non-steroidal anti-inflammatory drug use and risk of endometrial cancer: A systematic review and meta-analysis of observational studies. Gynecol. Oncol. 140: 352-358. DOI: 10.1016/j.ygyno.2015.12.009.
Macfarlane TV, Lefevre K, Watson MC (2014) Aspirin and non-steroidal anti-inflammatory drug use and the risk of upper digestive tract cancer. Br. J. Cancer. 111: 1852-1859. doi: 10.1038/bjc.2014.473.
García Rodríguez LA, Jick H (1994) Risk of upper gastrointestinal bleeding and perforation associated with individual non-steroidal anti-inflammatory drugs. Lancet 343: 769-772. https://doi.org/10.1016/S0140-6736(94)91843-0.
Awtry EH, Loscalzo J (2000) Cardiovascular Drugs. Aspirin. Circulation 101: 1206-1218.
Bjarnason I, Scarpignato C, Takeuchi K, Rainsford KD (2007) Determinants of the short-term gastric damage caused by NSAIDs in man. Aliment. Pharmacol. Ther. 26: 95–106. DOI: 10.1111/j.1365-2036.2007.03348.x.
Mei Q, Diao L, Xu J, Liu X, Jin J (2011) A protective effect of melatonin on intestinal permeability is induced by diclofenac via regulation of mitochondrial function in mice. Acta Pharmacol. Sin. 32: 495–502. DOI: 10.1038/aps.2010.225.
Musumba C, Pritchard DM, Pirmohamed M (2011) Review article: cellular and molecular mechanisms of NSAID-induced peptic ulcers. Aliment. Pharmacol. Ther. 30: 517–531. DOI: 10.1111/j.1365-2036.2009.04086.x.
Tanaka A, Matsumoto M, Hayashi Y, Takeuchi K (2005) Functional mechanism underlying cyclooxygenase-2 expression in rat small intestine following administration of indomethacin: relation to intestinal hypermotility. J. Gastroenterol. Hepatol. 20: 38–45. DOI: 10.1111/j.1440-1746.2004.03520.x.
Tomisato W, Tsutsumi S, Hoshino T, Hwang HJ, Mio M, Tsuchiya T, Mizushima T (2004) Role of direct cytotoxic effects of NSAIDs in the induction of gastric lesions. Biochem. Pharmacol. 67: 575–85.DOI: 10.1016/j.bcp.2003.09.020.
Bjarnason I, Takeuchi K (2009) Intestinal permeability in the pathogenesis of NSAID-induced enteropathy. J. Gastroenterol. 44: 23–29. DOI: 10.1007/s00535-008-2266-6.
Kim HK, Kim JI, Kim JK, Han JY, Park SH, Choi KY, Chung IS (2007) Preventive effects of rebamipideon NSAID-induced gastric mucosal injury and reduction of gastric mucosal blood flow in healthy volunteers. Dig. Dis. Sci. 52: 1776–1782. DOI: 10.1007/s10620-006-9367-y.
Tanaka A, Araki H, Hase S, Komoike Y, Takeuchi K (2002) Up-regulation of COX-2 by inhibition of COX-1 in the rat: a key to NSAID-induced gastric injury. Aliment. Pharmacol. Ther. 16 (Suppl 2): 90–101.
Yamada T, Deitch E, Specian RB, Perry MA, Sartor RB, Grisham MB (1993) Mechanisms of acute and chronic intestinal inflammation induced by indomethacin. Inflammation 17: 641 -662.
Miura S, Suematsu M, Tanaka S, Nagata H, Houzawa S, Suzuki M, Kurose I, Serizawa H, Tsuchiya M (1991) Microcirculatory disturbance in indomethacin-induced intestinal ulcer. Am. J. Physioi. 261: G213 -G219. DOI: 10.1152/ajpgi.1991.261.2. G213.
Simmons DL, Botting RM, Hla T (2004) Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol. Rev. 56: 387-437. DOI: 10.1124/pr.56.3.3.
Dey I, Lejeune M, Chadee K (2006) Prostaglandin E2 receptor distribution and function in the gastrointestinal tract. Br. J. Pharmacol. 149: 611–623. doi: 10.1038/sj.bjp.0706923.
Cambell, NB, Halushka PV (1996) Lipid derived autocoids. Eicosanoides and platelet activating factor. In: Hardman JG, Limbird AE, Molinoff PB, Ruddon RW, Goodman Gilman A (Eds.) Goodman & Gilman’s The Pharmacological Basis of Therapeutics. McGraw-Hill, New York, pp. 601–616.
Wallace JL, Cirino G (1994) The development of gastrointestinal-sparing non-steroidal anti-inflammatory drugs. Trends Pharmacol. Sci. 15: 405–406.
Gavalas A, Hadjipetrou L, Kourounakis P (1998) Synthesis of novel derivatives of aroyl-aminoalcohols and 3-amino-substituted 1-phenylpropanols with potential anti-inflammatory and immunomodulating activity. J. Pharm. Pharmacol. 50: 583–591. https://doi.org/10.1111/j.2042-7158.1998.tb06891.x.
Kukreja RC, Kontos HA, Hess ML, Ellis EF (1986) PGH synthase and lipoxygenase generate superoxide in the presence of NADH or NADPH. Circ. Res. 59: 612–619.
Marnett LJ (2009) The COXIB experience: a look in the rear view mirror. Annu. Rev. Pharmacol. Toxicol. 49: 265–290. DOI: 10.1146/annurev.pharmtox.011008.145638.
Wallace JL, McKnight W, Reuter BK, Vergnolle N (2000) NSAID-induced gastric damage in rats: requirement for inhibition of both cyclooxygenase 1 and 2. Gastroenterology 119: 706–714. https://doi.org/10.1053/gast.2000.16510.
Tanaka A, Araki H, Komoike Y, Hase S, Takeuchi K (2001) Inhibition of both COX-1 and COX-2 is required for development of gastric damage in response to nonsteroidal anti-inflammatory drugs. J. Physiol. Paris 95: 21–27. https://doi.org/10.1016/S0928-4257(01)00005-5.
Schmassmann A, Zoidl G, Peskar BM, Waser B, Schmassmann-Suhijar D, Gebbers J, Reubi JC (2006) Role of the different isoforms of cyclooxygenase and nitric oxide synthase during gastric ulcer healing in cyclooxygenase-1 and -2 knockout mice. Am. J. Physiol. Gastrointest. Liver Physiol. 290: G747–G756.
Mitchell JA, Akarasereenont P, Thiemermann C, Flower RJ, Vane JR (1993) Selectivity of nonsteroidal anti-inflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proc. Natl. Acad. Sci. U.S.A. 91: 11693-11697. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC48050.
Melarange R, Gentry C, Durie M, O'Connell C, and Blower PR (1994) Gastrointestinal irritancy, anti-inflammatory activity, and prostanoid inhibition in the rat: differentiation of effects between nabumetone and etdolac. Dig. Dis. Sci. 39: 601-608. https://doi.org/10.1007/BF02088349.
Wolfe MM, Lichenstein DR, Singh G (1999) Gastrointestinal toxicity of non-steroidal anti-inflammatory drugs. N. Engl. J. Med. 340: 1888–1899. DOI: 10.1056/NEJM199906173402407.
Bandyopadhyay D, Ghosh G, Bandyopadhyay A, Reiter RJ (2004) Melatonin protects against piroxicam-induced gastric ulceration. J. Pineal Res. 36: 195–203. https://doi.org/10.1111/j.1600-079X.2004.00118.x.
Ehrlich K, Sicking C, Respondek M, Peskar BM (2004) Interaction of cyclooxygenase isoenzymes, nitricoxide, and afferent neurons in gastric mucosal defense in rats. J. Pharmacol. Exp. Ther. 308: 277–283. DOI: 10.1124/jpet.103.057752.
To KF, Chan FK, Cheng AS, Lee TL, Ng YP, Sung JJ (2001) Up-regulation of cyclooxygenase-1 and-2 in human gastric ulcer. Aliment. Pharmacol. Ther. 15: 25–34. https://doi.org/10.1046/j.1365-2036.2001.00889.x.Bhandari P, Bateman AC, Mehta RL, Patel P (2005) Mucosal expression of cyclooxygenase isoforms1 and 2 is increased with worsening damage to the gastric mucosa. Histopathology 46: 280–286. DOI: 10.1111/j.1365-2559.2005.02053.x.
Starodub OT, Demitrack ES, Baumgartner HK, Montrose MH (2008) Disruption of the Cox-1 genes lows repair of microscopic lesions in the mouse gastric epithelium. Am. J. Physiol. Cell. Physiol. 294: C223–C232. DOI: 10.1152/ajpcell.00395.2006.
Chan FK, Wong VW, Suen BY, et al. (2005) Effect of Celecoxib on the Healing of Complicated Gastric Ulcers: a Prospective, Double Rounded Randomised Trial (Abstract). Gasroenterology 128: A24.
Dikman A, Sanyal S, Von AC, Whitson M, Desai J, Bodian C, Brooks A, Bamji N, Cohen L, Miller K, Aisenberg J (2009) A randomized, placebo-controlled study of the effects of naproxen, aspirin, celecoxib or clopidogrel on gastroduodenal mucosal healing. Aliment. Pharmacol. Ther. 29: 781–91. DOI: 10.1111/j.1365-2036.2009.03928.x.
Wallace JL (2006) Nitricoxide, aspirin-triggered lipoxins and NO-aspirinin gastric protection. Inflamm. Allergy Drug Targets. 5: 133–137. DOI: 10.2174/187152806776383116.
Orrenius S (2007) Reactive oxygen species in mitochondria-mediated cell death. Drug Metab. Rev. 39: 443–55. DOI: 10.1080/03602530701468516.
Mashita Y, Taniguchi M, Yokota A, Tanaka A, Takeuchi K (2006) Oral but not parenteral aspirin upregulates COX-2 expression in rat stomachs: a relationship between COX-2 expression and PG deficiency. Digestion 73: 124–132. DOI: 10.1159/000094098.
Darling RL, Romero JJ, Dial EJ, Akunda JK, Langenbach R, Lichtenberger LM (2004) The effects of aspirin on gastric mucosal integrity, surface hydrophobicity, and prostaglandin metabolism in cyclooxygenase knockout mice. Gastroenterology 127: 94–104.https://doi.org/10.1053/j.gastro.2004.04.003.
Lichtenberger LM, Zhou Y, Dial EJ, Raphael RM (2006) NSAID injury to the gastrointestinal tract: evidence that NSAIDs interact with phospholipids to weaken the hydrophobic surface barrier and induce the formation of unstable pores in membranes. J. Pharm. Pharmacol. 58: 1421–1428. DOI: 10.1211/jpp.58.10.0001.
Barnett K, Bell CJ, McKnight W, Dicay M, Sharkey KA, Wallace JL (2000) Role of cyclooxygenase-2 in modulating gastric acid secretion in the normal and inflamed rat stomach. Am. J. Physiol. Gastrointest. Liver Physiol. 279: G1292–G1297.DOI: 10.1152/ajpgi.2000.279.6.G1292.
Funatsu T, Chono K, Hirata T, Keto Y, Kimoto A, Sasamata M (2007) Mucosal acid causes gastric mucosal microcirculatory disturbance in nonsteroidal anti-inflammatory drug-treated rats. Eur. J. Pharmacol. 554: 53–59. DOI: 10.1016/j.ejphar.2006.10.023.
Koike T, Shimada T, Fujii Y, Chen G, Tabei K, Namatame T, Yamagata M, Tajima A, Yoneda M, Terano A, Hiraishi H (2007) Upregulation of TFF1 (pS2) expression by TNF-alpha in gastric epithelial cells. J. Gastroenterol. Hepatol. 22: 936–942. DOI: 10.1111/j.1440-1746.2007.04861.x.
Wallace JL, Granger DN (1992) The pathogenesis of NSAIB gastropathy- are neutrophils the culprits? Trends Pharmacol. Sci. 13: 129-131.
Finkel T, Holbrook NJ (2000) Oxidants, oxidative stress and the biology of ageing. Nature 408: 239-247. DOI: 10.1038/35041687.
Yoshikawa T, Naito Y, Kishi A, Tomii T, Kaneko T, Jinuma S, Ichikawa H, Yasuda M, Takahashi S, Kondo M (1993) Role of active oxygen, lipid peroxidation, and antioxidants in the pathogenesis of gastric mucosal injury induced by indomethacin in rats. Gut 34: 732-737. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1374252.
Brzozowski T, Konturek PC, Konturek SJ, Pajdo R, Bielanski W, Brzozowska I, Stachura J, Han EG (1997) The role of melatonin and L-tryptophan in prevention of acute gastric lesions induced by stress, ethanol, ischemia, and aspirin. J. Pineal Res. 23: 79-89. https://doi.org/10.1111/j.1600-079X.1997.tb00339.x.
Das D, Bandyopadhyay D, Bhattacharjee M, Banerjee RK (1997) Hydroxyl radical is the major causative factor in stress-induced gastric ulceration. Free Radical Biol. Med. 23: 8-18.https://doi.org/10.1016/S0891-5849(96)00547-3.
Jou MJ, Peng TI, Hsu LF, Jou SB, Reiter RJ, Yang CM, Chiao CC, Lin YF, Chen CC (2010) Visualization of melatonin’s multiple mitochondrial levels of protection against mitochondrial Ca2+- mediated permeability transition and beyond in rat brain astrocytes. J. Pineal Res. 48: 20–38. DOI: 10.1111/j.1600-079X.2009.00721.x.
Motawi TK, bdElgawad HM, Shahin NN (2007) Modulation of indomethacin-induced gastric injury by spermine and taurine in rats. J. Biochem. Mol. Toxicol. 21: 280–288. DOI: 10.1002/jbt.20194.
Nagai N, Fukuhata T, Ito Y, Usui S, Hirano K (2009) Involvement of interleukin 18 in indomethacin-induced lesions of the gastric mucosa in adjuvant-induced arthritis rat. Toxicol. 255: 124–130.DOI: 10.1016/j.tox.2008.10.005.
Gallego-Sandin S, Novalbos J, Rosado A, Gisbert JP, Gálvez-Múgica MA, García AG, Pajares JM, Abad-Santos F (2004) Effect of ibuprofen on cyclooxygenase and nitric oxide synthase of gastric mucosa: correlation with endoscopic lesions and adverse reactions. Dig. Dis. Sci. 49: 1538–1544.
Souza SMB, Oliveira ON, Scarpa MV, Oliveira AG (2004) Study of the diclofenac/phospholipid interactions with liposomes and monolayers. Colloids Surf. B Biointerfaces 36: 13-17. DOI: 10.1016/j.colsurfb.2004.05.001.
Souza MH, Mota JM, Oliveira RB, Cunha FQ (2008) Gastric damage induced by different doses of indomethacin in rats is variably affected by inhibiting iNOS or leukocyte infiltration. Inflamm. Res. 57: 28–33. DOI: 10.1007/s00011-007-7089-z.
Mizushima T (2007) Various stress proteins protect gastric mucosal cells against non-steroidal anti-inflammatory drugs. Inflammopharmacol. 15: 67–73. DOI: 10.1007/s10787-006-1560-2.
Ebert MP, Schafer C, Chen J, Hoffmann J, Gu P, Kubisch C, Carl-McGrath S, Treiber G, Malfertheiner P, Röcken C (2005) Protective role of heat shock protein 27 in gastric mucosal injury. J. Pathol. 207: 177–184.DOI: 10.1002/path.1815.
Kusuhura H, Komatsu H, Sumichika H, Sugahara K (1999) Reactive oxygen species are involved in the apoptosis induced by nonsteroidal anti-inflammatory drugs in cultured gastric cells. Eur. J. Pharmacol. 383: 331–337.https://doi.org/10.1016/S0014-2999(99)00599-3.
Wilhelmsen M, Amirian I, Reiter RJ, Rosenberg J, Gögenur I (2011) Analgesic effects of melatonin: A review of current evidence from experimental and clinical studies. J. Pineal Res. 51: 270–277.DOI: 10.1111/j.1600-079X.2011.00895.x.
Graham DY (1990) The relationship between nonsteroidal anti-inflammatory drug use and peptic ulcer disease. Gastroenterol. Clinics. North Am. 19: 171–182.
May GR, Crook P, Moore PM, Page CP (1991) The role of nitric oxide as an endogenous regulator of platelet and neutrophil activation within the pulmonary circulation of the rabbit. Br. J. Pharmacol. 102: 759–763.
Whittle BJR (1990) Role of endogenous nitric oxide in the mechanisms underlying gastric mucosal integrity. In: Nitric oxide from L-arginine: abioregulatory system. Edited by Moncada S and Higgs EA. Elsevier Science Publishers, Amsterdam. pp. 365–371.
Lopez-Belmonte J, Whittle BJW, Moncada S (1993) The actions of nitric oxide donors in the prevention or induction of injury to the rat gastric mucosa. Br. J. Phamacol. 108: 73–78.
Reuter BK, Cirino G, Walkce JL (1994) Markedly reduced intestinal toxicity of a diclofenac derivative. Life Sci. 55: PL1-PL8.https://doi.org/10.1016/0024-3205(94)90083-3.
Graham DY, Agrawal NM, Roth SH (1988) Prevention of NSAID-induced gastric ulcer with misoprostol: multicentre, doubleblind, placebo-controI1d trial. Lancet 2: 1277–1288. https://doi.org/10.1016/S0140-6736(88)92892-9.
Soll AH, Weinstein WM, Kurata J, McCarthy D (1991) Nonsteroidal anti-inflammatory drugs and peptic ulcer disease. Ann. Intern. Med. 114: 387–319. DOI: 10.18773/austprescr.2017.037.
Farkouh ME, Kirshner H, Harrington RA, Ruland S, Verheugt FW, Schnitzer TJ, Burmester GR, Mysler E, Hochberg MC, Doherty M, Ehrsam E, Gitton X, Krammer G, Mellein B, Gimona A, Matchaba P, Hawkey CJ, Chesebro JH (2004) Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), cardiovascular outcomes: randomised controlled trial. Lancet 364: 675–684.DOI: 10.1016/S0140-6736(04)16894-3.
Maitra SK, Pal PK (2017) Melatonin rhythms in the pineal and non-pineal tissues and their physiological implications in subtropical fish. Biol. Rhythm Res. 48: 757–776.https://doi.org/10.1080/09291016.2017.1345453.
Pal PK, Bhattacharjee B, Ghosh A, Chattopadhyay A, Bandyopadhyay D (2018) Adrenaline induced disruption of endogenous melatonergic system, antioxidant and inflammatory responses in the gastrointestinal tissues of male Wistar rat: an in vitro study. Melatonin Research 1: 109–131. doi: 10.32794/mr11250007.
Raikhlin NT, Kvetnoy IM (1974) Lightening effect of the extract of human appendix mucosa on frog skin melanophores. Bull. Exp. Biol. Med. 8: 114–116.
Raikhlin NT, Kvetnoy IM (1976) Melatonin and enterochromaffin cells. Acta Histochem. 55: 19–25.
Rakhlin NT, Kvetnoy IM, Tolkachev VN (1975) Melatonin may be synthesized in enterochromafinne cells. Nature 255: 344–345.
Lee PPN, Shiu SYU, Chow PH, Pang SF (1995) Regional and diurnal studies on melatonin binding sites in the duck gastrointestinal tract. Biol. Signals 4: 212–224.
Bubenik GA (1999) Localization and physiological significance of gastrointestinal melatonin. In: R Watson (eds.) Melatonin in Health Promotion. CRC press, Boca Raton, Florida, pp. 21–39.
Vakkuri O, Rintamaki H, Leppaluoto J (1985a) Presence of immunoreactive melatonin in different tissues of the pigeon. Gen. Comp. Endocrinol. 58: 69–75.
Vakkuri O, Rintamaki H, Leppaluoto J (1985b) Plasma and tissue concentrations of melatonin after midnight light exposure and pinealectomy in the pigeon. J. Endocrinol. 105: 263–268.
Lee PP, Pang SF (1993) Melatonin and its receptors in the gastrointestinal tract. Biol. Signals 2: 181–193.
Chow PH, Lee PN, Poon AMS, Shiu SYW, Pang SF (1996) The gastrointestinal system: A site of melatonin paracrine action. In: Tang PL, Pang SF, Reiter RJ (eds.) Melatonin: A universal photoperiodic signal with diverse action. Front. Horm. Res. Basel, Karger 21: 123–132.
Klein D (2007) Arylalkylamine N-acetyltransferase: “the Timezyme”. J. Biol. Chem. 282: 4233–4237.DOI: 10.1074/jbc.R600036200.
Dobocovich M, Mankowsha M (2005) Functional MP1 and MP2 melatonin receptors in mammals. Endocrine 27: 101–110. DOI: 10.1385/ENDO:27:2:101.
Konturek PC, Konturek SJ, Majka J, Zembala M, Hahn EG (1997) Melatonin affords protection against gastric lesions induced by ischemia-reperfusion possibly due to its antioxidant and mucosal microcirculatory effects. Eur. J. Pharmacol. 322: 73–77. https://doi.org/10.1016/S0014-2999(97)00051-4.
Bubenik GA, Niles LP, Pang SF, Pentney PJ (1993) Diurnal variation and binding characteristics of melatonin in the mouse brain and gastrointestinal tissues. Comp. Biochem. Physiol. 104: 221–224. https://doi.org/10.1016/0742-8413(93)90027-I.
Bubenik GA, Ayles HL, Ball RO, Friendship RM, Brown GM (1998) Relationship between melatonin levels in plasma and gastrointestinal tissues and the incidence and severity of gastric ulcers in pigs. J. Pineal Res. 24:62–66. https://doi.org/10.1111/j.1600-079X.1998.tb00367.x.
Ercan F, Cetinel S, Contuk G, Cikler E, Sener G (2004) Role of melatonin in reducing water avoidance stress induced degeneration of the gastrointestinal mucosa. J. Pineal Res. 37: 113–121. DOI: 10.1111/j.1600-079X.2004.00143.x.
Srinivasan V, Lauterbach EC, Ho KY, Acuña-Castroviejo D, Zakaria R, Brzezinski A (2012) Melatonin in antinociception: Its therapeutic applications. Curr. Neuropharmacol. 10: 167–178. DOI: 10.2174/157015912800604489.
Becker-Andre M, Wiesenberg I, Schaeren-Wiemers N, Andre E, Missbach M, Saurat JH, Carlberg C (1994) Pineal gland hormone melatonin binds and activates an orphan of the nuclear receptor superfamily. J. Biol. Chem. 269: 28531–28534.
Witt-Enderby PA, Radio NM, Doctor JS, Davis VL (2006) Therapeutic treatments potentially mediated by melatonin receptors: potential clinical uses in the prevention of osteoporosis, cancer and as an adjuvant therapy. J. Pineal Res. 41: 297–305. DOI: 10.1111/j.1600-079X.2006.00369.x.
Boutin JA, Audinot V, Ferry G, Delagrange P (2005) Molecular tools to study melatonin pathways and actions. Trends Pharmacol. Sci. 26: 412–419. DOI: 10.1016/j.tips.2005.06.006.
Manchester LC, Coto-Montes A, Boga JA, Andersen LP, Zhou Z, Galano A, Vriend J, Tan DX, Reiter RJ (2015)Melatonin: an ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res.59:403–419. DOI: 10.1111/jpi.12267.
Galano A, Tan DX, Reiter RJ (2018) Melatonin: a versatile protector against oxidative DNA damage.Molecules.27:23. pii: E530. DOI: 10.3390/molecules23030530.
Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre-Jimenez M, Qin L (2016) Melatonin as an antioxidant: under promises but over delivers.J. Pineal Res. 61:253–278. DOI: 10.1111/jpi.12360.
Galano A, Medina ME, Tan DX, Reiter RJ (2015) Melatonin and its metabolites as copper chelating agents and their role in inhibiting oxidative stress: a physicochemical analysis.J. Pineal Res. 58:107–116. DOI: 10.1111/jpi.12196.
Reiter RJ, Rosales-Corral S, Tan DX, Jou MJ, Galano A, Xu B (2017) Melatonin as a mitochondria-targeted antioxidant: one of evolution's best ideas. Cell. Mol. Life. Sci. 74: 3863–3881. DOI: 10.1007/s00018-017-2609-7.
Konturek PC, Konturek SJ, Celinski K, Slomka M, Cichoz-Lach H, Bielanski W, Reiter RJ (2010) Role of melatonin in mucosal gastroprotection against aspirin-induced gastric lesions in humans .J. Pineal Res. 48:318–323. https://doi.org/10.1111/j.1600-079X.2010.00755.x
Tan DX, Manchester LC, Qin L, Reiter RJ (2016) Melatonin: a mitochondrial targeting molecule involving mitochondrial protection and dynamics.Int. J. Mol. Sci.17 (12): pii: E2124. DOI: 10.3390/ijms17122124.
Nosál'ová V, Zeman M, Černá S, Navarová J, Zakálová M (2007) Protective effect of melatonin in acetic acid induced colitis in rats. J. Pineal Res. 42: 364–370. DOI: 10.1111/j.1600-079X.2007.00428.x
Torres-Farfan C, Richter HG, Rojas-García P, Vergara M, Forcelledo ML, Valladares LE, Torrealba F, Valenzuela GJ, Seron- Ferre M (2003) mt1 Melatonin receptor in the primate adrenal gland: inhibition of adrenocorticotropin-stimulated cortisol production by melatonin. J. Clin. Endocrinol. Metab. 88: 450–458. DOI: 10.1210/jc.2002-021048
Pal PK, Hasan NK, Maitra SK (2016) Gut melatonin response to microbial infection in carp Catlacatla. Fish Physiol. Biochem. 42: 579–592. DOI: 10.1007/s10695-015-0161-7
Pal PK, Maitra SK (2018) Response of gastrointestinal melatonin, antioxidants, and digestive enzymes to altered feeding conditions in carp (Catlacatla). Fish Physiol. Biochem. 44: 1061–1073. DOI: 10.1007/s10695-018-0494-0
Lopez-Burillo S, Tan DX, Rodriguez-Gallego V, Manchester LC, Mayo JC, Sainz RM, Reiter RJ (2003) Melatonin and its derivatives cyclic 3-hydroxymelatonin, N1-acetyl-N2-formyl-5- methoxykynuramine, and 6-hydroxymelatonin reduced oxidative damage induced by Fenton reagents. J. Pineal Res. 34: 237–256. DOI: 10.1034/j.1600-079X.2003.00025.x
Tomas-Zapico C, Coto-Montes A (2005) A proposed mechanism to explain the stimulatory effect of melatonin on antioxidative enzymes. J. Pineal Res. 39: 99–104. DOI: 10.1111/j.1600-079X.2005.00248.x
El-Sokkary GH, Kamel ES, Reiter RJ (2003) Prophylactic effect of melatonin in reducing lead-induced neurotoxicity in the rat. Cell. Mol. Biol. Lett. 8: 461-470.
León J, Acuña-Castroviejo D, Escames G, Tan DX, Reiter RJ (2005) Melatonin mitigates mitochondrial malfunction. J. Pineal Res. 38: 1–9. DOI: 10.1111/j.1600-079X.2004.00181.x
Martín M, Macías M, León J, Escames G, Khaldy H, Acuña- Castroviejo D (2002) Melatonin increases the activity of the oxidative phosphorylation enzymes and the production of ATP in rat brain and liver mitochondria. Int. J. Biochem. Cell. Biol. 34: 348–357.
Acuña-Castroviejo D, Escames G, León J, Carazo A, Khaldy H (2003) Mitochondrial regulation by melatonin and its metabolites. Adv. Exp. Med. Biol. 527: 549–557.
Hussein MR, Abu-Dief EE, Abd El-Reheem MH, Abd-Elrahman A (2005) Ultrastructural evaluation of the radioprotective effects of melatonin against X-ray-induced skin damage in Albino rats. Int. J. Exp. Pathol. 86: 45–55. DOI: 10.1111/j.0959-9673.2005.00412.x
Sener G, Sert G, Sehirli AO, Arbak S, Gedik N, Ayanoglu-Dülger G (2006) Melatonin protects against pressure ulcer-induced oxidative injury of the skin and remote organs in rats. J. Pineal Res. 40: 280–287. DOI: 10.1111/j.1600-079X.2005.00313.x
Maity P, Bindu S, Dey S, Goyal M, Alam A, Pal C, Reiter R, Bandyopadhyay U (2009) Melatonin reduces indomethacin-induced gastric mucosal cell apoptosis by preventing mitochondrial oxidative stress and the activation of mitochondrial pathway of apoptosis. J. Pineal Res. 46: 314–323.
Konturek PC, Konturek SJ, Ketal C (2004) Role of melatonin in mucosal gastroprotection against aspirin-induced gastric lesions in human. J. Pineal Res. 48: 318–323.
Cabeza J, Alarcón-de-la-Lastra C, Jiménez D, Martín MJ, Motilva V (2003) Melatonin modulates the effects of gastric injury in rats: role of prostaglandins and nitric oxide. Neurosignals 12: 71–77.
Ozturk H, Oztürk H, Yagmur Y, Uzunlar AK (2006) Effects of melatonin administration on intestinal adaptive response after massive bowel resection in rats. Dig. Dis. Sci. 51: 333–337. DOI: 10.1007/s10620-006-3134-y
Voisin P, Van Camp G, Pontoire C, Collin JP (1993) Prostaglandins stimulate serotonin acetylation in chick pineal ceils: involvement of cyclic AMP-dependent and calcium/calmodulin-dependent mechanisms. J. Neurochem. 60: 666–670.
Pandi-Perumal SR, Srinivasan V, Maestroni GJ, Cardinali DP, Poeggeler B, Hardeland R (2006) Melatonin: Nature's most versatile biological signal? FEBS J. 273: 2813–2838. DOI: 10.1111/j.1742-4658.2006.05322.x
Konturek PC, Konturek SJ, Burnat G, Brzozowski T, Brzozowska I, Reiter RJ (2008) Dynamic physiological and molecular changes in gastric ulcer healing achieved by melatonin and its precursor L-tryptophan in rats. J. Pineal Res. 45: 180–190. DOI: 10.1111/j.1600-079X.2008.00574.x
Brzozowska I, Konturek PC, Brzozowski T, KonturekSJ, Kwiecien S, Pajdo R, Drozdowicz D, Pawlik M, Ptak A, Hahn EG (2002) Role of prostaglandins, nitric oxide, sensory nerves and gastrin in acceleration of ulcer healing by melatonin and its precursor, L-tryptophan. J. Pineal Res. 32: 149–162.
Hardeland R, Tan DX, Reiter RJ (2009) Kynuramines, metabolites of melatonin and other indoles:the resurrection of an almost forgotton class of biogenic amines. J. Pineal Res. 47: 109–124. DOI: 10.1111/j.1600-079X.2009.00701.x
Gitto E, Pellegrino S, Gitto P, Barberi I, Reiter RJ (2009) Oxidative stress of the newborn in the pre-and postnatal period and the clinical utility of melatonin. J. Pineal Res. 44: 128–139. DOI: 10.1111/j.1600-079X.2008.00649.x
Odaci E and Kaplan S (2009) Melatonin and nerve regeneration. Int. Rev. Neurobiol. 87: 317–335. DOI: 10.1016/S0074-7742(09)87016-5
Chen LJ, Gao YQ, Li XJ, Shen DH, Sun FY (2005) Melatonin protects against MPTP/MPP+ -induced mitochondrial DNA oxidative damage in vivo and in vitro. J. Pineal Res. 39: 34–42. DOI: 10.1111/j.1600-079X.2005.00209.x
Jaworek J, Brzozowski T, Konturek SJ (2005) Melatonin as an organoprotector in the stomach and the pancreas. J. Pineal Res. 38: 73–83. DOI: 10.1111/j.1600-079X.2004.00179.x
Ganguly K, Maity P, Reiter RJ, Swarnakar S (2005) Effect of melatonin on secreted and induced matrix metalloproteinase -9 and -2 activity during prevention of indomethacin-induced gastric ulcer. J. Pineal Res. 39: 307–315. DOI: 10.1111/j.1600-079X.2005.00250.x
Ganguly K, Kundu P, Banerjee A, Reiter RJ, Swarnakar S (2006) Hydrogenperoxide-mediated down regulation of matrixmetalloprotease-2 in indomethacin-induced acute gastric ulceration is blocked by melatonin and other antioxidants. Free Radic. Biol. Med. 41: 911–925. https://doi.org/10.1016/j.freeradbiomed.2006.04.022
Brzozowska I, Konturek PC, Brzozowski T, Konturek SJ, Kwiecien S, Pajdo R, Drozdowicz D, Pawlik M, Ptak A, Hahn EG (2002) Role of prostaglandins, nitric oxide, sensory nerves and gastrin in acceleration of ulcer healing by melatonin and its precursor, L-tryptophan. J. Pineal Res. 32: 149–162. https://doi.org/10.1034/j.1600-079x.2002.1o811.x
Brzozowski T, Konturek PC, ZwirskaKorczala K, Konturek SJ, Brzozowska I, Drozdowicz D, Sliwowski Z, Pawlik M, Pawlik WW, Hahn EG (2005) Importance of the pineal gland, endogenous prostaglandins and sensory nerves in the gastroprotective actions of central and peripheral melatonin against stress-induced damage. J. Pineal Res. 39: 375–385. https://doi.org/10.1111/j.1600-079X.2005.00264.x
Sjoblom M, Flemstrom G (2003) Melatonin in the duodenal lumen is a potent stimulant of mucosal bicarbonate secretion. J. Pineal Res. 34: 288–293. https://doi.org/10.1034/j.1600-079X.2003.00044.x
Hernández-Pacheco A, Araiza-Saldaña CI, Granados-Soto V and Mixcoatl-Zecuatl T (2008) Possible participation of the nitric oxide-cyclic GMP-protein kinase G-K+ channels pathway in the peripheral antinociception of melatonin. Eur. J. Pharmacol. 596: 70–76. DOI: 10.1016/j.ejphar.2008.07.068.
Marseglia L, D'Angelo G, Manti S, Aversa S, Arrigo T, Reiter RJ, Gitto E (2015) Analgesic, anxiolytic and anaesthetic effects of melatonin: New potential uses in pediatrics. Int. J. Mol. Sci. 16: 1209–1220. DOI: 10.3390/ijms16011209.
Rocha N, Rotelli A, Aguilar CF, Pelzer L (2007) Structural Basis of the Anti-inflammatory. Arzneimittel-Forschung/Drug Research 57: 782–786. DOI: 10.1055/s-0031-1296680.
Maity P, Bindu S, Dey S, Goyal M, Alam A, Pal C, Reiter RJ, Bandyopadhyay U (2009) Melatonin reduces indomethacin‐induced gastric mucosal cell apoptosis by preventing mitochondrial oxidative stress and the activation of mitochondrial pathway of apoptosis. J. Pineal Res. 46: 314–323. DOI: 10.1111/j.1600-079X.2009.00663.x.
This work is licensed under Creative Commons Attribution 4.0 International License