Please cite this papar as:
Mitra, E., Bhattacharjee, B., Pal, P., Ghosh, A., Mishra, S., Chattopadhyay, A. and Bandyopadhyay, D. 2019. Melatonin protects against cadmium-induced oxidative damage in different tissues of rat: a mechanistic insight. Melatonin Research. 2, 2 (June 2019), 1-21. DOI:https://doi.org/https://doi.org/10.32794/mr11250018
Research Article
Melatonin protects against cadmium-induced oxidative damage in different tissues of rat: a mechanistic insight
Elina Mitraa^, Bharati Bhattacharjeea^, Palash Kumar Pala^, Arnab Kumar Ghosha, Sanatan Mishraab, Aindrila Chattopadhyayb, Debasish Bandyopadhyaya*
aOxidative Stress and Free Radical Biology Laboratory, Department of Physiology, University of Calcutta, 92, APC Road, Kolkata-700009
bDepartment of Physiology, Vidyasagar College,39, Sankar Ghosh Lane, Kolkata-700006
^Authors have equal contribution
*Correspondence: debasish63@gmail.com, Tel: +91-9433072066
Running title: Melatonin prevents against cadmium-induced tissue damage
Received: February 13, 2019; Accepted: April 11, 2019
ABSTRACT
Cadmium (Cd) is a notorious environmental pollutant known for its wide range of toxicities to organisms. Thus, the present study is designed to examine whether melatonin, a potent antioxidant, protects against Cd-induced oxidative damage in the heart, liver and kidney of rats. Cd treatment at a dose of 0.44 mg/kg for 15 days caused severe damage in all these organs. These included significantly increased activities of SGPT, SGOT, lactate dehydrogenase- 1 and 5 and ALP and levels of total lactate, creatinine, lipid peroxidation, protein carbonyl content and reduced glutathione while the activities of superoxide dismutases, catalase, glutathione peroxidase, glutathione reductase and glutathione-S-transferase along with mitochondrial pyruvate dehydrogenase, isocitrate dehydrogenase, α-keto glutarate dehydrogenase, succinate dehydrogenase, NADH-cytochrome-c-oxidoreductase and cytochrome-c-oxidase were significantly reduced by Cd. However, if melatonin was given orally 30 min before Cd injection, all these alterations induced by Cd were significantly preserved by melatonin. Histological observations also demonstrated that Cd exposure caused cellular lesions, promoting necrotic or apoptotic changes. Notably, all these changes were significantly protected by melatonin. The results suggest that melatonin is a beneficial molecule to ameliorate Cd-induced oxidative damage in the heart, liver and kidney tissues of rats with its powerful antioxidant capacity, heavy metal chelating activity and competition of binding sites with Cd to the GSH and catalase.
Keywords: cadmium, oxidative stress, tissue damage, heart, liver, kidney, melatonin, antioxidant.
___________________________________________________________________________
1. INTRODUCTION
Cadmium (Cd) is a notorious toxic heavy metal that enters the environment from occupational (mining, smelting operations and electronics manufacturing) and non-occupational exposure (1). Humans are exposed to Cd from a variety of sources including cigarette smoke, consumption of contaminated sea food, shellfish, animal liver, water, cereals as well as from occupational and industrial exposure (2). Cd is classified by IARC as a human carcinogen. The half-life of Cd in human body is more than 20 years and it is toxic even at low doses; thus, Cd toxicity in an affected person will last probably for lifetime (3). Epidemiological and clinical data revealed that exposure to toxic doses of Cd primarily causes liver damage, pulmonary edema, renal dysfunction, anemia, osteoporosis and bone fractures (4). Inspite of numerous researching works on Cd have been done, the mechanisms of responsible for its toxicity are not clearly defined yet.
Cd is not a redox-active metal and its oxidant role is not clear. It has been shown that Cd is mainly stored in soft tissues, especially in the liver and kidneys (5, 6) and it induces lipid peroxidation in liver, kidney, brain, lung, heart, and testes of rats (7-11). The mechanisms that are responsible for Cd-induced toxicity may be multifactorial. These factors cause cellular apoptosis (12) and finally lead to death of the organisms (13). Some studies report that Cd-induced damages are mainly due to the generation of reactive oxygen species (ROS) (14). Notably, organisms have equipped antioxidant defense systems to stabilize the ROS level. When these systems fail to function, an imbalance of ROS production and detoxification will result in oxidative stress in body and cause adverse effects leading to chronic diseases (15). Many efforts have been made to find a suitable method to counteract such an imbalance. In this regard, antioxidants and chelators have been used for this purpose; however, none of them were promising for long term usage. Evidence has emerged to show that melatonin, a well-known antioxidant, may be such a molecule. Melatonin (N-acetyl-5-methoxytryptamine) is pleiotropic molecule and it is ubiquitously present in primitive photosynthetic bacteria, fungi, plants, and animals including humans (16). Thus, it can be found in our daily supplies including vegetables, fruits, cereals, wine, beer, meat, eggs and in other edible food products (16). Melatonin is known to influence various physiological activities such as neuroendocrine function, seasonal reproductive regulation, immune- as well as thermoregulations (17). In addition, melatonin is a potent antioxidant in protecting cells, tissues and organs from oxidative damage induced by ROS. Melatonin is particularly effective to scavenger hydroxyl radical (•OH) which is the most reactive species to target DNA, proteins, and lipids and causes severe tissue/organ injury (18). Currently, melatonin is classified as a mitochondria-targeted antioxidant. Notably, melatonin reduces oxidative stress via a variety of mechanisms: direct detoxification of ROS and reactive nitrogen species (RNS) and indirectly by stimulating antioxidant enzymes while suppressing the activity of pro-oxidant enzymes (19). A differential intracellular distribution of melatonin, including its high concentration in mitochondria, probably enhances its capacity to resist oxidative stress and cellular apoptosis. Recent evidence showed that mitochondrial membranes possessed transporters that aided in the rapid uptake of melatonin by these organelles against a melatonin gradient (18). Melatonin also chelates transition metals, which are involved in the Fenton/Haber–Weiss reactions; thereby reducing the formation of devastatingly toxic •OH (19). Additionally, it promoted electron transport in the electron transport chain (ETC) and, therefore, likely reduced electron leakage and the ROS generation. This is referred as radical avoidance of melatonin (20).
Based on these observations, in the current study, we have systematically investigated whether melatonin provides protective effects on the Cd-induced oxidative stress in the in vivo study of rats. If so, what are the mechanisms. The heart, liver and kidney were selected as the indexing organs to reflect the Cd toxicity, since Cd mainly attacks these organs as we mentioned above. The results are reported following.
2. METHODS AND MATERIALS
2.1. Animals.
Male Wistar rats, weighing 150–180 g, were procured from (Saha Enterprise; Kolkata, West Bengal, India; Regd. No. 1828/PO/BT/S15/ CPCSEA) and allowed to acclimate for at least 7 days. The rats were housed in animal facility of (University of Calcutta) with a light/dark cycle of 12/12h and temperature (20-26ºC) fed with standard diet and water ad libitum throughout the investigation (21). Rats were handled as per the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment and Forest, Government of India, with the experimental protocol (IAEC-IV/Proposal/DB-06/2014/Dt.13/03/2014) approved by the Institutional Animal Ethics Committee (IAEC) of Department of Physiology, University of Calcutta.
2.2. Chemicals and reagents.
Cadmium chloride (CdCl2) and melatonin were purchased from Sisco Research Laboratories (SRL), Mumbai, India. All the other chemicals used in this study including the solvents were of analytical grade obtained from Sisco Research Laboratories (SRL), Mumbai, India; Qualigens (Germany); SD fine chemicals (India) and Merck Limited, Delhi, India. Different primary [SOD 1 (ab 16831); SOD 2 (ab13534), catalase (ab16731)] and secondary [Goat anti-rabbit IgG ALP-conjugated (ab97048)] antibodies were procured from Abcam Biotechnology Company (Abcam, USA), while β-actin (sc-130657) primary antibody was purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA, USA).
2.3. Experimental design.
Male Wistar rats (n=48) were used for the present study. In the first experiment, 24 rats were equally divided into four groups. In the control group (n=6), rats were subcutaneously (s.c.) injected with vehicle [normal saline (0.9%)]. In the melatonin alone group (n=6), animals were treated (orally) with melatonin (10 mg/kg body weight) at every other day. In the Cd group (n=6), animals were subcutaneously (s.c.) injected with CdCl2 solution (0.44 mg/kg body weight) (22) at every other day. In the treatment group (n=6), rats were treated (orally) with melatonin (10 mg/kg body weight) 30 min prior to CdCl2 injection (0.44 mg/kg body weight s.c.) for every other day. The experiment was carried out for 15 days. Then, the animals were sacrificed by cervical dislocation following mild ether anesthesia and the organs were collected. Prior to cervical dislocation, blood was collected from the anesthezed animals by cardiac puncture, and the serum was prepared and stored at -80°C for further analyses. Immediately after organ collection, a small piece of each tissue (cardiac, hepatic and renal) was fixed in appropriate fixative solution for microscopic studies. The remaining tissues were stored at -80°C for further biochemical analyses. While, another 24 animals were further divided into four groups (n=6/group) as mentioned above and subjected for the estimation of their endogenous •OH (for details see supplementary file 1).
2.4. Preparation of tissue homogenates.
Ten percent (10%) heart, liver and kidney tissue homogenates were prepared freshly in 50 mM potassium phosphate buffer (pH 7.4), using a Potter–Elvehjem glass homogenizer (23). The required portion of the homogenates was used for the measurement of enzyme activities and Western blot analysis.
2.5. Measurement of activities of serum enzymes associated with functions of heart, liver and kidney.
The activities of enzymes of SGPT and SGOT were measured following the method of Reitman and Frankel (1957) (24). The enzyme activities were expressed as IU/L.
The activities of lactate dehydrogenase-1 (LDH1), lactate dehydrogenase-5 (LDH5) and total lactate dehydrogenase (TLDH) in serum were estimated following the method described by Strittmatter et al.(1965) (25) with some modifications (26). The enzyme activities were expressed as IU/L.
The serum-specific marker alkaline phosphatase (ALP) was measured following the method of Kind and King’s (27). The levels were expressed as KA units.
Blood creatinine level was estimated by the method of Folin and Wu (28) with minor modifications. The results were expressed as mg (%). The blank and standard samples were prepared using distilled water. Creatinine was dissolved in acid solution.
2.6. Measurement of the biomarkers of oxidative stress in heart, liver and kidney.
The reduced glutathione (GSH) contents in the same tissues were estimated following the method of Sedlac and Lindsey (1968) (29) with some modifications (30). The values were expressed as nmoles/mg tissue protein.
Lipid peroxidation (LPO) level was determined following the method of Buege and Aust (1978) (31) with some modifications as adopted by Bandyopadhyay et al. (2004) (30). Levels were expressed as nmoles of TBARS/mg of tissue protein.
The protein carbonyl (PCO) content in the same tissues was estimated by DNPH assay following the method of Levine et al. (1994) (32). The absorbance was recorded at 370 nm using a UV/VIS spectrophotometer. The values were expressed as nmoles/mg tissue protein.
2.7. Measurement of activities of antioxidant enzymes.
The activity of Cu-Zn-SOD and mitochondrial Mn-SOD were measured by pyrogallol autoxidation method of Marklund and Marklund (1974) (33). Change in absorbance/min was recorded at 420 nm and the enzyme activity was expressed as units/mg of tissue protein.
The activity of catalase (CAT) was determined following the method described by Beers and Sizer (34) with some modifications as adopted by Chattopadhyay et al. (2003) (35). The enzyme activity was measured at 240 nm and expressed as nmoles H2O2 consumed/mg of tissue protein.
2.8. Determination of the protein expression of antioxidant enzymes with Western Blot analysis.
Heart, liver and kidney tissue homogenates were prepared for western blot analysis of Cu-Zn-SOD, Mn-SOD, CAT and β-actin proteins following the method described earlier by Bandyopadhyay et al. [30]. In brief, sixty microgram of proteins were loaded in each well and subjected to SDS–PAGE (10%) following the method of Laemmli (36). After transferring the proteins to PVDF membrane (Immobilon P, Sigma), it was incubated in blocking solution for 1 h. Following the proper washes, the immunoblots were incubated overnight in respective primary antibody (dilution 1:2000) at 4°C. After completion of incubation, the membrane was washed 3 times and the blot was incubated in secondary antibody (dilution 1: 3000) for 2 h at room temperature. The membrane was subjected to incubation in the presence of the substrate (BCIP/NBT) after proper washing. Finally, intensity of individual band of each immunoblot was normalized by the intensity of β-actin (internal control) and the relative density of the individual bands was quantified by densitometry using ImageJ software (NIH, Bethesda, MD, USA).
2.9. Measurement of activities of different glutathione-dependent enzymes in rat heart, liver and kidney.
The activity of glutathione peroxidase (GPx) was determined following the method as described by Paglia and Valentine (1967) (37) with some modifications as adopted by Chattopadhyay et al. (35). The enzyme activity was measured at 340 nm and expressed as nmoles/mg tissue protein.
The activity of glutathione reductase (GR) was measured according to the method of Krohne-Ehrichet al. (38). The change in absorbance was recorded at 340 nm using a UV/VIS spectrophotometer and the specific activity of the enzyme was expressed as nmoles/mg tissue protein.
The glutathione-S-transferase (GST) activity was measured spectrophotometrically at 340nm following the method of Habig et al. (39). The enzyme activity was expressed as Units/mg tissue protein.
2.10. Determination of the activities of glucose metabolic enzymes in heart, liver and kidney tissue homogenates.
The cardiac, hepatic and renal tissues were homogenized (10%) in chilled phosphate buffer (50 mM, pH 7.4) with a Potter Elvenjem glass homogenizer (Belco Glass, Inc., Vineland, NJ, USA). The homogenates were centrifuged at 5000 x g for 10 min and the supernatant was further centrifuged at 12000 x g for 15 min to obtain the mitochondrial fraction. The pellet was resuspended in appropriate buffer and used for measuring the activities of following enzymes. The activity of pyruvate dehydrogenase (PDH) was determined spectrophotometrically at 340 nm following the method of Chretien et al. (1995) (40) with some modifications (41). The activity of isocitrate dehydrogenase (ICDH) and α-ketoglutarate dehydrogenase (α-KGDH) were determined following the method as described by Duncan et al. (42). The method of Veeger et al. (43) was used to measure the activity of succinate dehydrogenase (SDH). The enzyme activities were recorded at 420 nm and were expressed as units/mg tissue protein.
2. 11. Determination of the activities of mitochondrial respiratory chain enzymes in heart, liver and kidney.
The activities of NADH-Cytochrome c oxidoreductase and cytochrome c oxidase were determined following the method of Goyal and Srivastava (44). The activities were recorded spectrophotometrically at 565 and 550 nm, respectively for both enzymes. The activities of the enzymes were expressed as units/mg tissue protein.
2.12. Determination of the activities of xanthine oxidase (XO) and xanthine dehydrogenase (XDH).
The method of Greenlee and Handler (1964) (45) along with some modifications (46) was used to determine the activity of XO. The activity of XDH was also measured spectrophotometrically following the method of Strittmatter et al. (25). The enzyme activities were expressed as milliUnits/mg tissue protein.
2.13. Measurement of endogenous •OH.
The •OH generated in cardiac, hepatic and renal tissues in the in vivo condition was measured following the method of Bandyopadhyay et al. (30) by using dimethyl sulfoxide (DMSO) as a specific •OH radical scavenger. Change in absorbance was recorded at 425 nm using benzene sulfinic acid as the standard. The values obtained were expressed as nM •OH/g tissue.
2.14. Histological studies.
2.14.1. Hematoxylin and eosin (H & E) assay.
A portion of the extirpated heart, liver and kidney tissues were fixed, respectively in 10% formalin and embedded in paraffin following routine histological procedure. The tissue sections (5 μm thick) were prepared and stained with H&E. The stained samples were examined under a Leica microscope and the images were captured with a digital camera attached to it (47).
2.14.2. Quantification of tissue fibrosis by confocal microscopy.
The tissue sections (5 μm thick) were stained with Sirius red (Direct Red 80; Sigma Chemical Co, Louis, MO, USA) according to the method of Roy et al. (47) and imaged with a laser scanning confocal system (Zeiss LSM 510 META, Germany) and the stacked images through multiple slices were captured. Four slides of each tissue from an individual rat in different groups were prepared. The digitized images were analyzed using an image analysis system (Image J, NIH Software, Bethesda, MI) and the total collagen area fraction was measured and expressed as the % collagen volume.
2.15. Morphological analysis with scanning electron microscopy (SEM).
Immediately after dissection, the heart, liver and kidney tissues and the mitochondria isolated from each tissue were fixed in 2.5% cold glutaraldehyde for 24-48 h, then the SEM analysis was performed following the method of Watanabe et al. (48) with some modifications (49). Briefly, the tissue sections, as well as the mitochondrial smear, were washed 3-4 times in phosphate buffer (50 mM, pH 7.2), then, the samples were dehydrated through ascending grades of alcohol treatment (30, 50, 70, 90, and 100%; each for 10 min). The dehydrated samples were embedded in iso-amyl alcohol and then placed at 4ºC until the iso-amyl alcohol is solidified. The frozen samples were then dried under vacuum and evaluated by SEM (Zeiss Evo 18 model EDS 8100).
2.16. Estimation of Cd content in cardiac, hepatic and renal tissues with atomic absorption spectrophotometry (AAS).
The cardiac, hepatic and renal tissue samples were processed, respectively and the Cd content was measured as per the protocol mentioned in the cookbook of the Sophisticated Analytical Instrument Facilities’ (SAIF), “Thermo Scientific iCE 3000 Series Atomic Absorption Spectrometer” at the Bose Institute, Kolkata. The samples were prepared with nitric acid (65%) for total dissolution as described by Mitra et al. (50). The Cd content was expressed as μg/g tissue.
2.17. Binding analysis of GSH and CAT with Cd and melatonin in a cell-free/chemical system through isothermal titration calorimetry (ITC).
The binding analyses of GSH or CAT with Cd and melatonin were analyzed by ITC in a cell-free/chemical system using Microcal ITC-200, Malvern, UK. For this assay, in the sample cell, 0.3 ml of CAT (4 ×10−9mM) or GSH (4mM) was titrated, respectively with 0.06 ml of 0.1 mM CdCl2 or/and melatonin (40μM for GSH and 80 μM for catalase, respectively) as ligands in specific combinations as follows: (i) CdCl2 alone (ii) melatonin alone (iii) combination of CdCl2 and melatonin. Melatonin binding with Cd was also examined. In brief, 0.3 ml of melatonin (1 × 10−3 mM) in the sample cell was titrated against 0.06 mL of 0.2mM CdCl2. For a single run, titration was conducted with 20 injections of each ligand (2 μl each) with 150 sec of interval between two successive injections for approximately 1 h at 37°C. Following the titration, data analysis was performed using Origin 7.0 software (Microcal LLC).
2.18. Estimation of protein content.
The concentration of proteins in heart, liver and kidney tissue homogenates were estimated, respectively by the method of Lowry et al. (51) using the bovine serum albumin as the standard.
2.19. Statistical evaluation.
Each experiment was repeated thrice with different rats. The data for various biochemical parameters were expressed as means ± S.E.M. The statistical significance of the data has been determined using one-way analysis of variance (ANOVA) after ascertaining the homogeneity of variances between the treatments and significant difference between the groups were evaluated by Scheffes’ test. The results were considered statistical significance at p<0.05. All statistical analyses were made using Microcal Origin version 6.0 for Windows.
3. RESULTS
3.1. Effects of melatonin on the activities of enzymes associated with functions of heart, liver and kidney in rats treated with/without Cd.
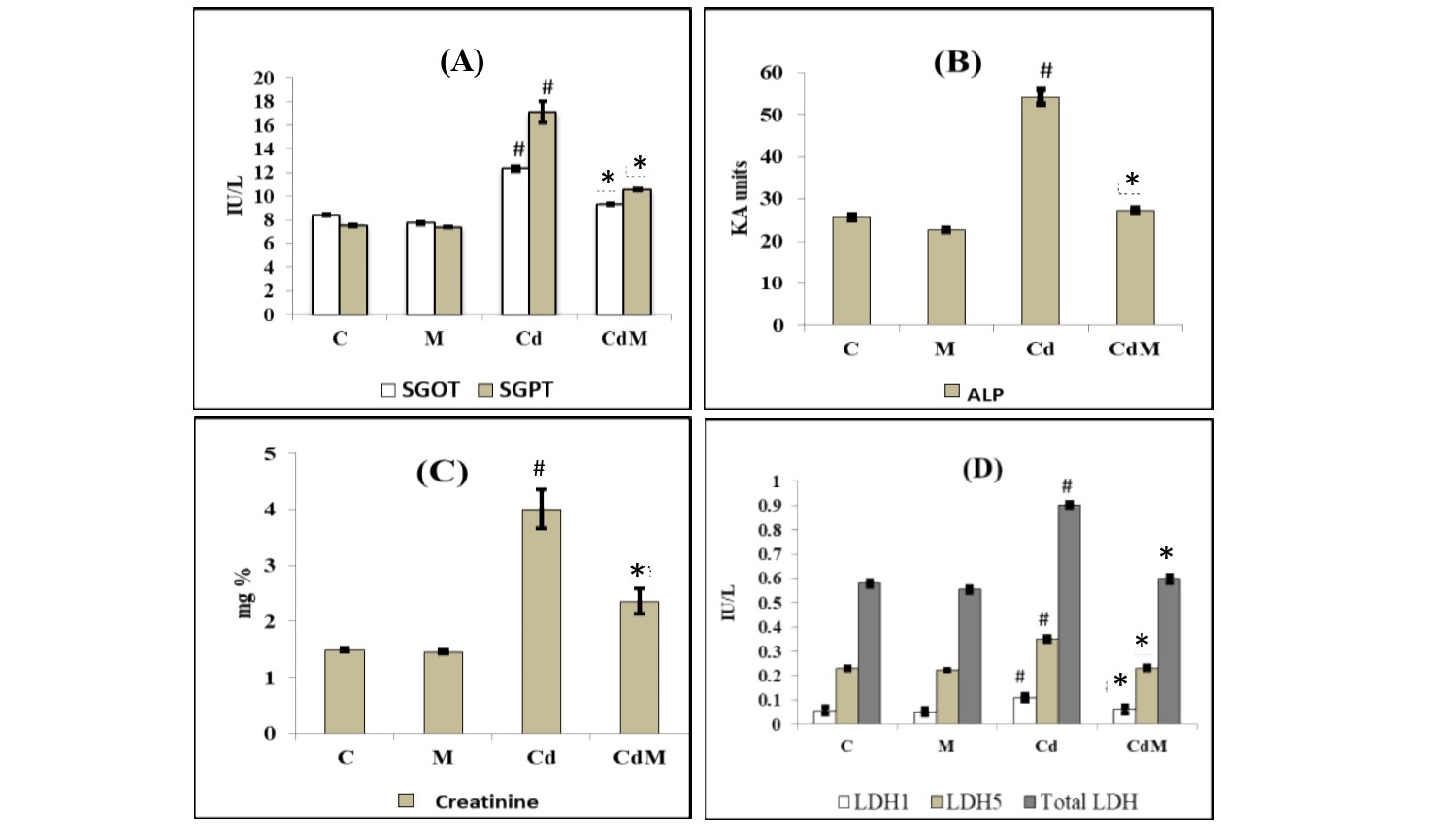
Cd treatment in rats significantly elevated the activities of their serum SGOT, SGPT, LDH1, LDH5, total LDH and ALP as well as the levels of creatinine, compared to the control animals (Fig. 1). Melatonin treatment alone did not modify these parameters compared to controls. However, melatonin pre-treatment before Cd injection significantly suppressed the elevation of activities in these serum enzymes induced by the Cd. In most of the cases, melatonin rescued the activities of these enzymes in Cd treated animals back to the levels similar to that of the control group (Fig. 1).

Figure 1: Effects of melatonin on the activities of serum enzymes and the level of creatinine in rat treated with/without Cd.
Data were expressed as mean ± S.E.M. (n=6). (A) SGOT and SGPT, (B) ALP, (C) creatinine and (D) LDH1, LDH5 and total LDH in the serum of rats treated with CdCl2 (0.44 mg/kg) and/or, melatonin (10 mg/kg). # p<0.001 vs. Control group; * p<0.001 vs. Cd group.
3.2. Effects of melatonin on the biomarkers of oxidative stress in cardiac, hepatic and renal tissues of rats treated with/without Cd.
The levels of LPO and PCO in the cardiac, hepatic and renal tissues were significantly (p<0.001) elevated in Cd-treated rats compared to their controls (Fig. 2). In contrast, the content of GSH was significantly reduced by Cd treatment compared to the control group. Melatonin treatment alone did not cause obvious alterations on these parameters. However, melatonin pre-treatment before the Cd injection significantly reversed the alterations of these oxidative biomarkers induced by Cd (Fig. 2).
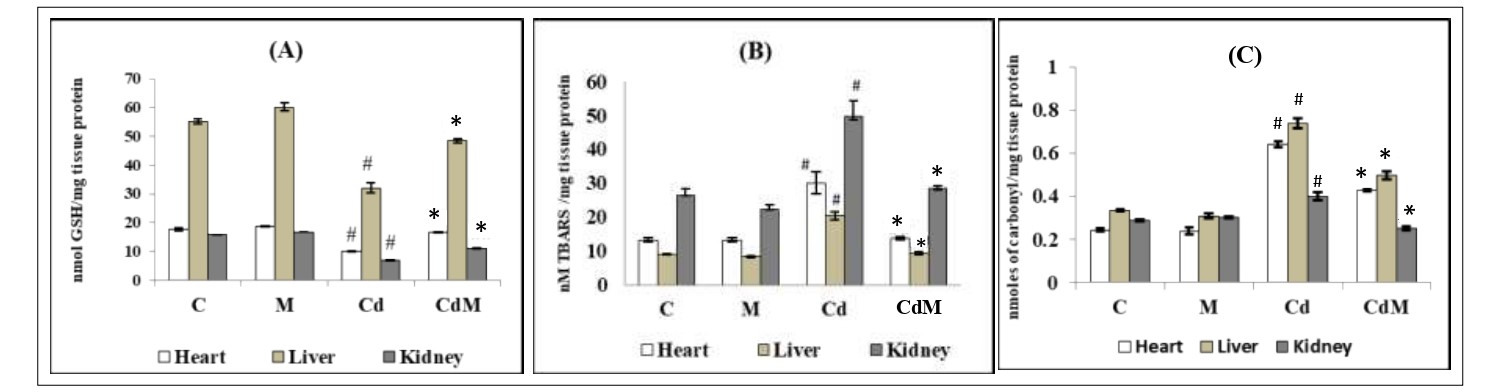
Figure 2: Effects of melatonin on the oxidative biomarkers in cardiac, hepatic and renal tissues of rats treated with/without Cd.
Data were expressed as mean ± S.E.M (n=6). (A) content of GSH, (B) LPO and (C)PCO Rats were treated with CdCl2 (0.44 mg/kg) and/or, melatonin (10 mg/kg). # p<0.001 vs. Control group; * p<0.001 vs. Cd-treated group.
3.3. Effects of melatonin on the activities of antioxidant enzymes as well as their gene expressions in heart, liver and kidney tissues of rats treated with/without Cd.
Cd-treatment significantly (p<0.001) decreased the activities of Cu-Zn-SOD in the cardiac and renal tissues, but increased its activity in hepatic tissue. Melatonin pre-treatment before Cd injection preserved the activities of Cu-Zn-SOD to their respective control values in all the studied tissues (Fig. 3A). For the activity of Mn-SOD, a significant decrease in cardiac tissue and increases in hepatic and renal tissues was observed after Cd treatment. Melatonin treatment almost completely reversed the alterations of Mn-SOD activity induced by the Cd (Fig. 3B). On the other hand, CAT activities were significantly (p<0.001) decreased in the hepatic and renal tissues, but increased in the cardiac tissue after Cd treatment compared to the control. Melatonin treatment reversed these alterations caused by Cd to the levels similar to the control (Fig. 3C). The gene expressions (protein content) analyses on these enzymes showed that the upregulation and downregulation of these proteins exhibited the similar patterns as their activities in all tissues treated with Cd alone or with Cd plus melatonin. The results are shown in Fig. 3; D-L.
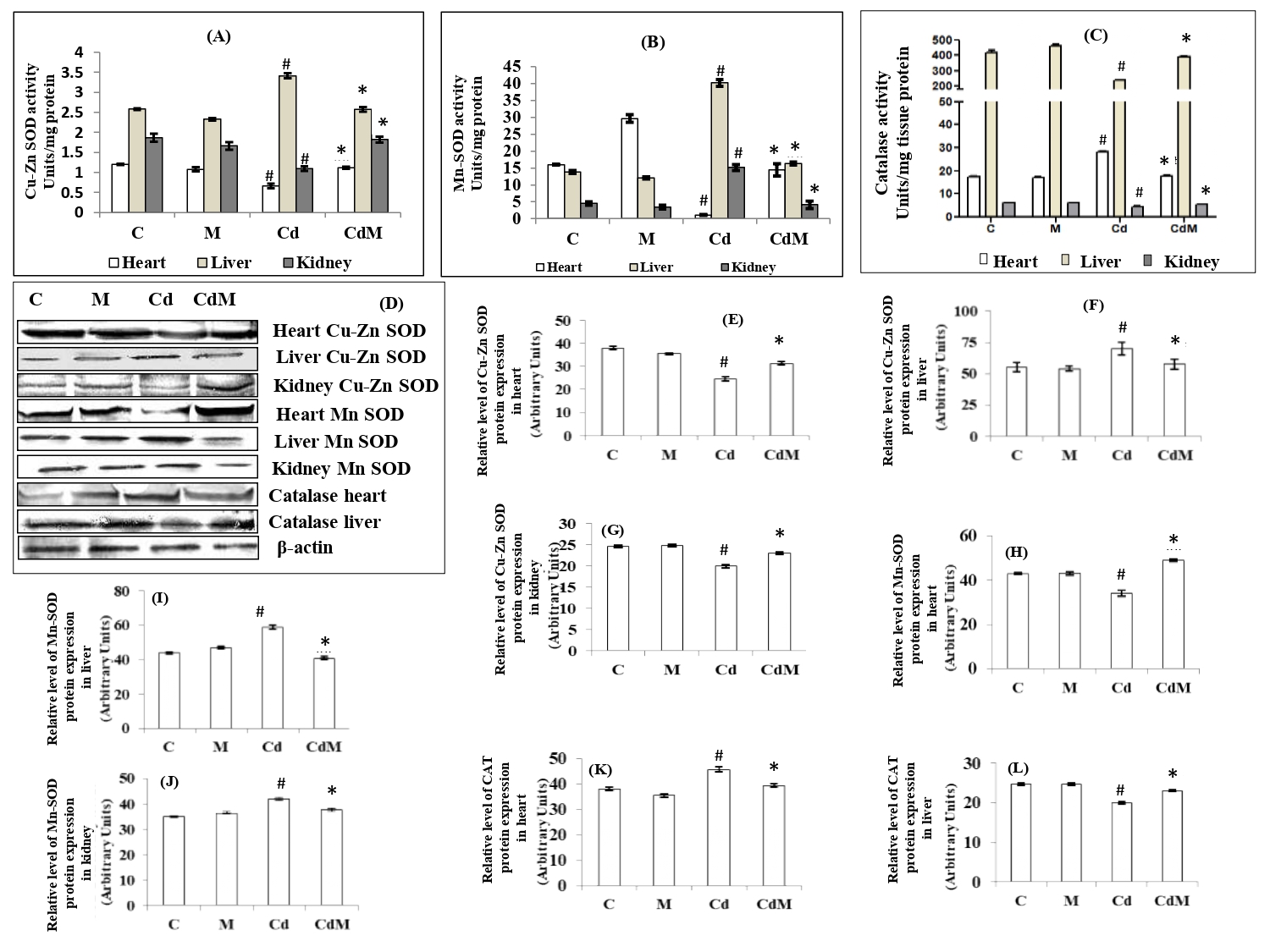
Figure 3: Effects of melatonin on the activities of antioxidant enzymes as well as their gene expressions in rat heart, liver and kidney tissues of rats treated with/without Cd.
Data were expressed as mean ± S.E.M. (n=6) (A) Cu-Zn-SOD, (B) Mn-SOD and (C) CAT activities. (D) the representative images of protein expressions of Cu-Zn-SOD, Mn-SOD and CAT and (E-L) their relative protein levels in different tissues of rat treated with CdCl2 (0.44 mg/kg ) and/or, melatonin (10 mg/kg). # p<0.001versus C; * p<0.001 versus Cd.
3.4. Effects of melatonin on the activities of glutathione-synthetic enzymes in heart, liver and kidney tissues of rats treated with/without Cd.
Cd Treatment significantly (p<0.001) suppressed the activity of GR in the cardiac, hepatic and renal tissues compared to the control (Fig. 4). Pre-treatment with melatonin (10 mg/kg) elevated GR activity which was suppressed by Cd to the level of control in all the studied tissues. On the contrary, the different responses were noted in the activities of GPx and GST. Cd treatment caused a significant increase in the activities of GPx and GST in the cardiac, hepatic and renal tissues compared to their respective controls while melatonin pre-treatment before Cd injection preserved their activities to the similar levels of controls in all tissues (Fig. 4). Melatonin alone did not alter the activities of these enzymes compared to control.
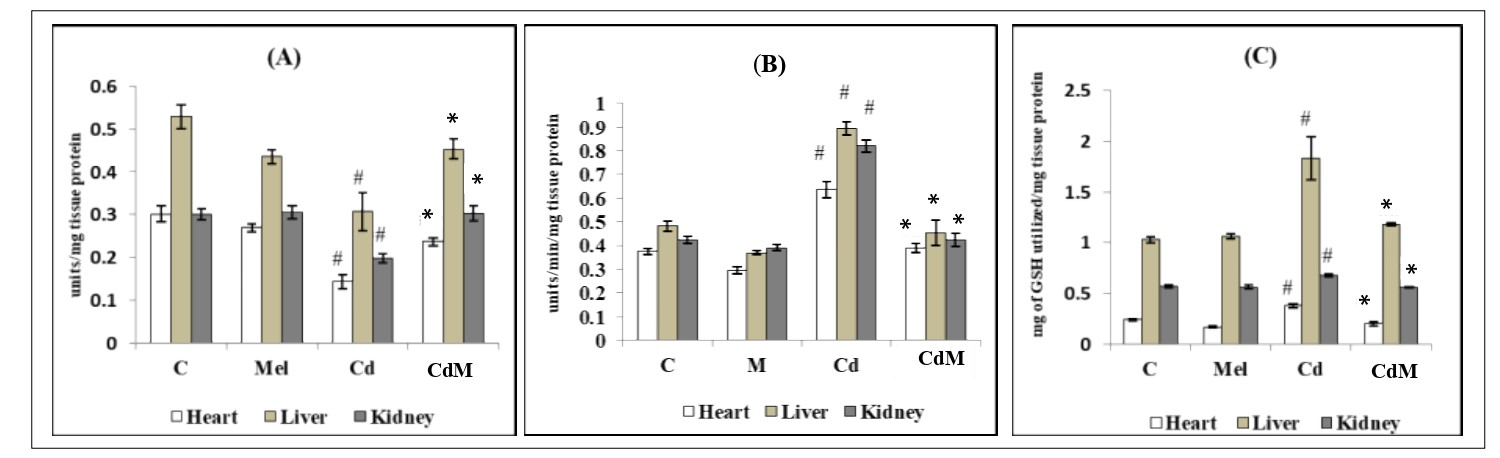
Figure 4: Effects of melatonin on the activities of glutathione synthetic enzymes in heart, liver and kidney tissues of rats treated with/without Cd.
Data were expressed as mean ± S.E.M. (N=6). (A) GR, (B) GPx, (C) GST in the cardiac, hepatic and renal tissues of rats treated with CdCl2 (0.44 mg/kg) and/or, melatonin (10 mg/kg). # p<0.001 vs. Control group; * p<0.001 vs. Cd-treated group.
3.5. Effects of melatonin on the activities of Krebs cycle as well as mitochondrial respiratory chain enzymes in heart, liver and kidney tissues of rats treated with/without Cd.
The activities of PDH, ICDH, α-KGDH) and SDH in the heart, liver as well as kidney significantly reduced by Cd treatment. However, the reduced activities of these enzymes caused by Cd were almost completely recovered to their control levels with melatonin pre-treatment (Fig. 5. A-D).
On the other hand, Cd at a dose of 0.44 mg/kg also significantly decreased the activities of mitochondrial respiratory chain enzymes including NADH cytochrome c reductase and cytochrome oxidase (Fig. 5. E-F) while melatonin pre-treatment blocked these alterations induced by the Cd in all the tissues studied. Melatonin alone had little effects on the activities of these enzymes.
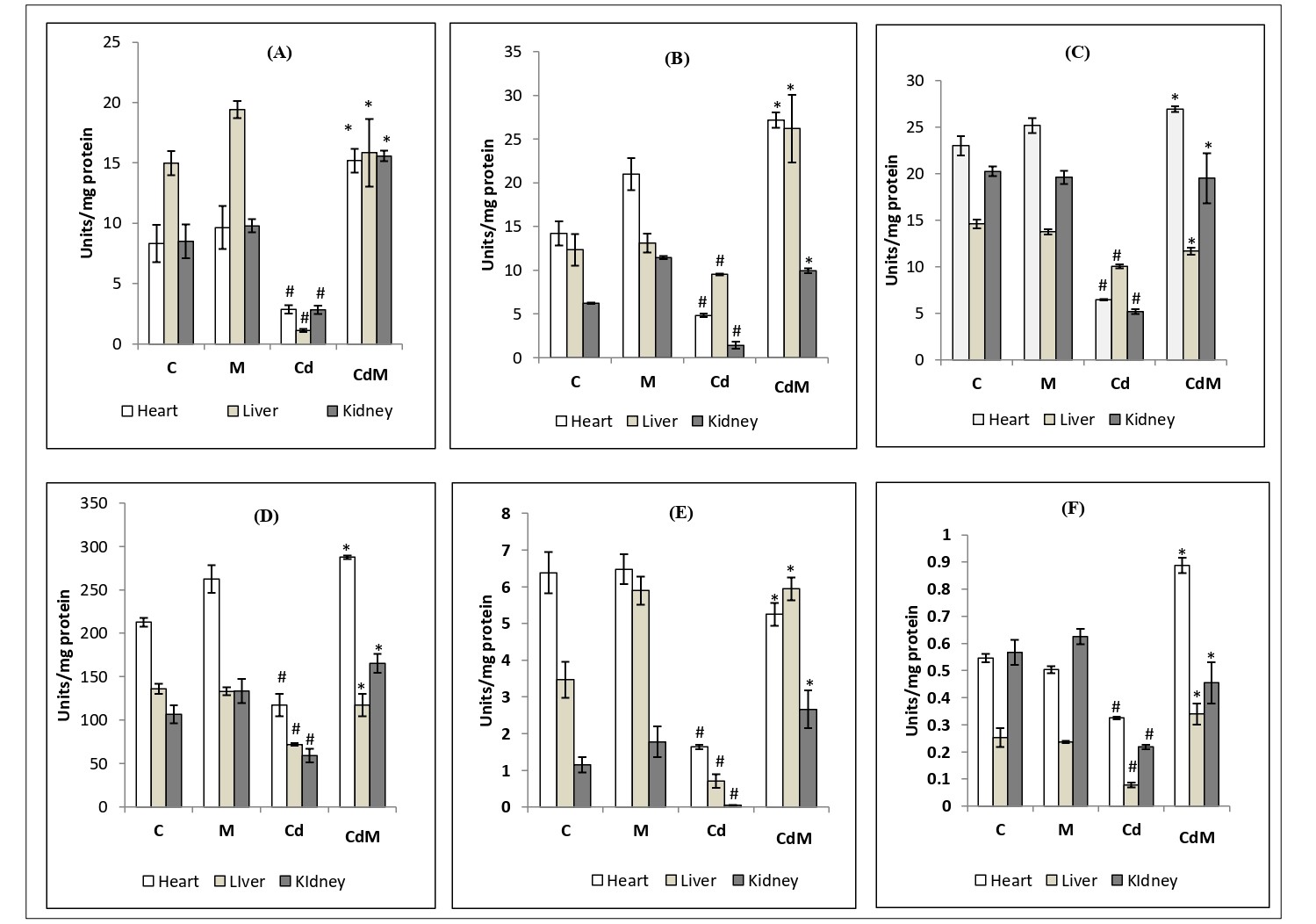
Figure 5: Effects of melatonin on the activities of Krebs cycle as well as mitochondrial respiratory chain enzymes in heart, liver and kidney tissues of rats treated with/without Cd.
Data were expressed as mean ± S.E.M. (n=6). (A) PDH, (B) ICDH, (C) α-KGDH, (D) SDH, (E) NADH cytochrome c reductase activity and (F) cytochrome oxidase in the cardiac, hepatic and renal tissues of rat treated with Cd (0.44 mg/kg) and/or, melatonin (10 mg/kg). # p<0.001 vs. Control group; * p<0.001 vs. Cd-treated group.
3.6. Effects of melatonin on the activities of pro-oxidant enzymes and levels of endogenously generated ·OH in heart, liver and kidney tissues of rats treated with/without Cd.
The activities of XO, XDH, the ratio of XO/XDH, total activity of XO+XDH and the ratio of XO/(XO+XDH) as well as the level of endogenous ·OH were significantly (p<0.001) increased (Fig. 6) in the cardiac, hepatic and renal tissues of rats treated with CdCl2 (0.44 mg/kg) while all of these changes were significantly inhibited with melatonin pre-treatment (Fig. 6). Melatonin alone did not modify there parameters compared to their respective controls.
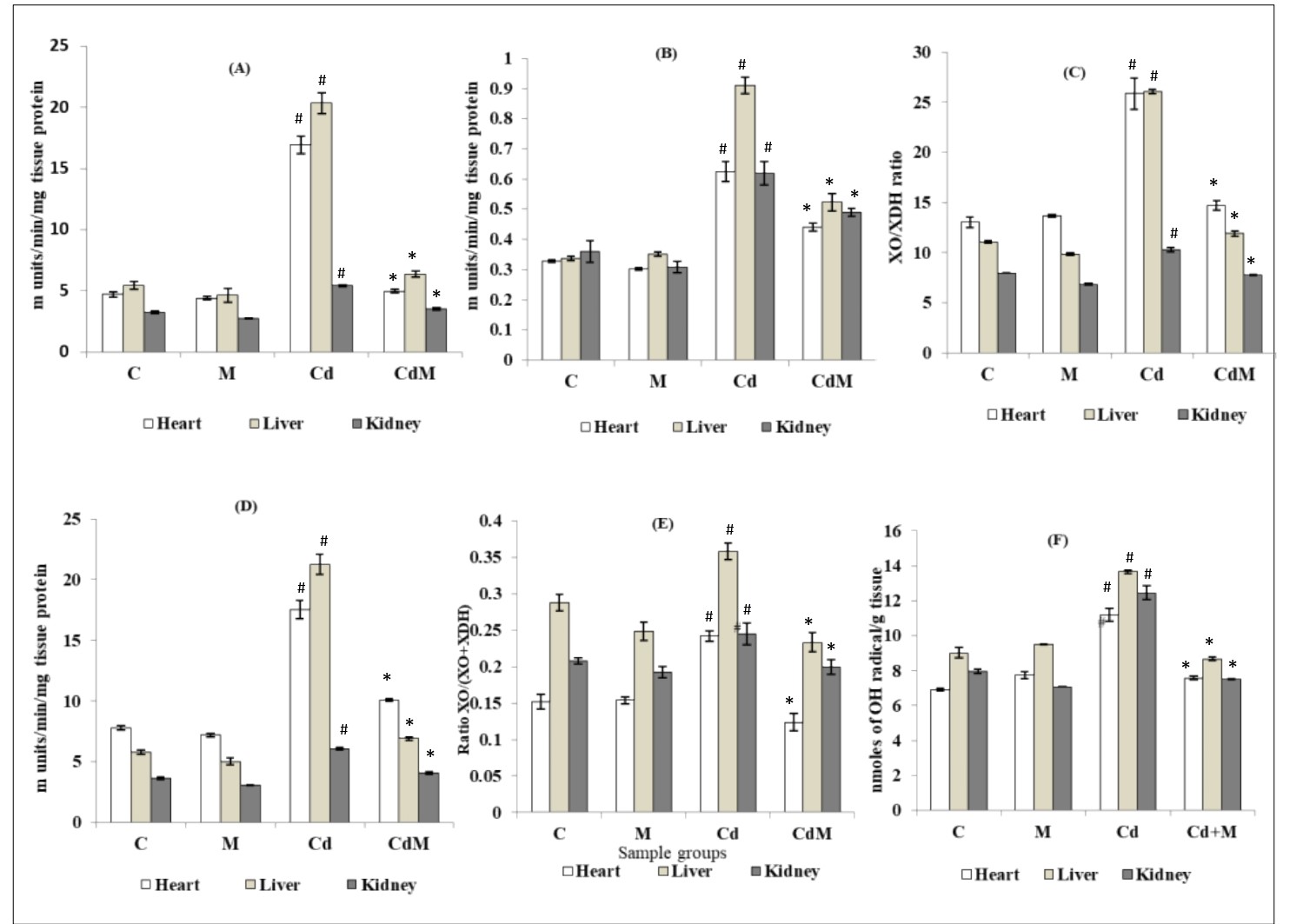
Figure 6: Effects of melatonin on the activities of pro-oxidant enzymes and levels of endogenously generated ·OH in heart, liver and kidney tissues of rats treated with/without Cd.
Data were expressed as mean ± S.E.M. (n=6). (A) XO, (B) XDH, (C) ratio of XO/XDH ratio, (D) total enzyme activity of XO+XDH, (E) ratio of XO/(XO+XDH) and (F) level of .OH in the cardiac, hepatic and renal tissues of rats treated with CDCl2 (0.44 mg/kg) and/or melatonin (10 mg/kg). # p<0.001 vs. Control group; * p<0.001 vs. Cd-treated group.
3.7. Effects of melatonin on the histological alterations in heart, liver and kidney tissues of rats treated with/without Cd.
In liver, H&E staining analysis showed that Cd caused portal and periportal lymphocyte infiltration along with necrosis of periportal hepatocyte. In heart and kidney, Cd resulted in the myocardial fiber necrosis and renal tissue large glomerulus with nodular mesangial expansion, respectively (Fig. 7A). The morphological pathologies caused by Cd were largely provented by melatonin pre-treatment. The acid sirius staining analysis also showed that Cd treatment caused a depletion of collagen content in heart while collagen content almost recovered to the control level when the rats were pre-treated with melatonin (Fig. 7. B-E). In the liver and kidney, a deposition of collagen especially around the central vein of the hepatic lobule and renal malphigian capsule was observed and this indicated tissue fibrosis. Again, melatonin pre-treatment significantly reduced these abnormalities caused by Cd (Fig. 7. B-E). Melatonin alone did not modify the morphological appearances of these tissues compared to controls.
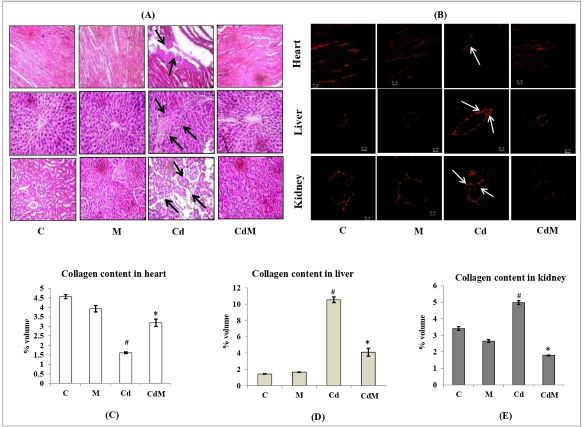
Figure 7: Effects of melatonin on the histological alterations in heart, liver and kidney tissues of rats treated with/without Cd.
Representative images (40X magnification) of (A) H&E staining, (B) acid Sirius staining and (C-E) collagen contents in heart, liver and kidney tissues, respectively. C: control, M: melatonin alone, Cd: cadmium alone, CdM: Cadmium plus melatonin. Arrows indicated Cd-induced abnormalities in the architecture of the respective tissue. Values were expressed as mean ± S.E.M. (n =6). # p<0.001 vs. C; * p < 0.001 vs. Cd.
3.8. Effects of melatonin on the surface architectural alterations of heart, liver and kidney tissue/mitochondria in rats treated with/without Cd.
In this study, SEM was used to analysis the detailed surface architectural alterations of the tissues. In heart, the irregularities in the branching pattern of the cardiac muscle fibers were observed after Cd treatment and these myofibrillar collagen fibers were cross-linked to each other to form a complicated matrix network (Fig. 8A). In hepatic tissue, the dilated central veins, distortion in the polyhedral arrangement of hepatocytes with profound tissue necrosis were identified in Cd-treated group (Fig. 8A). In renal tissue, shrunken glomerular tufts accompanied by loss of structural cohesion were the cases in Cd-treated group (Fig. 8A). In addition, mitochondria isolated from heart and liver exhibited the small membrane blebs with disrupted cristae, vacuolation and the rough and ruptured surface (Fig. 8B). Similarly, the mitochondria isolated from renal tissues also displayed perforated surface and convoluted membranes with the surface covering blebs due to markedly contracted cells (Fig. 8B). All of these abnormalities induced by Cd were significantly prevented with melatonin pre-treatment.
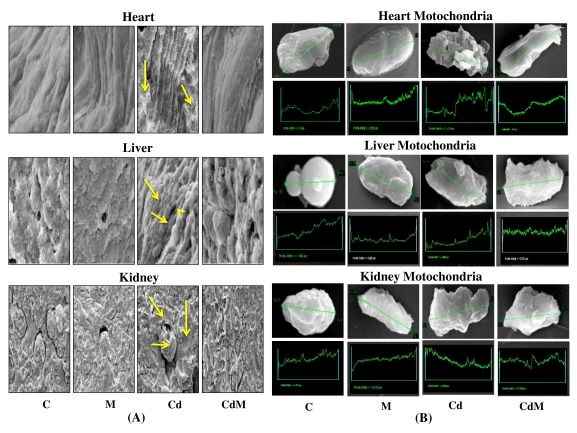
Figure 8: Effects of melatonin on the surface architectural alterations of heart, liver and kidney tissue/mitochondria in rats treated with/without Cd.
Representative images of the SEM study of (A) heart, liver and kidney tissues and (B) isolated mitochondria from their respective tissues. C: control, M: melatonin alone, Cd: cadmium alone, CdM: Cadmium plus melatonin. Arrows indicated Cd-induced irregular and abnormal alterations in the architecture of respective tissue. The green traces indicated area studied for surface morphology.
3.9. Effect of melatonin on the concentrations of Cd in heart, liver and kidney tissues.
The concentrations of Cd in the cardiac, hepatic and renal tissues were significantly (p<0.001) increased in the rats treated with CdCl2 (Fig. 9). Pre-treatment with melatonin (10 mg/kg BW.) significantly reduced the Cd loads in heart, liver and renal tissues by 56.44%, 31.84% and 44.54%, respectively (Fig. 9).
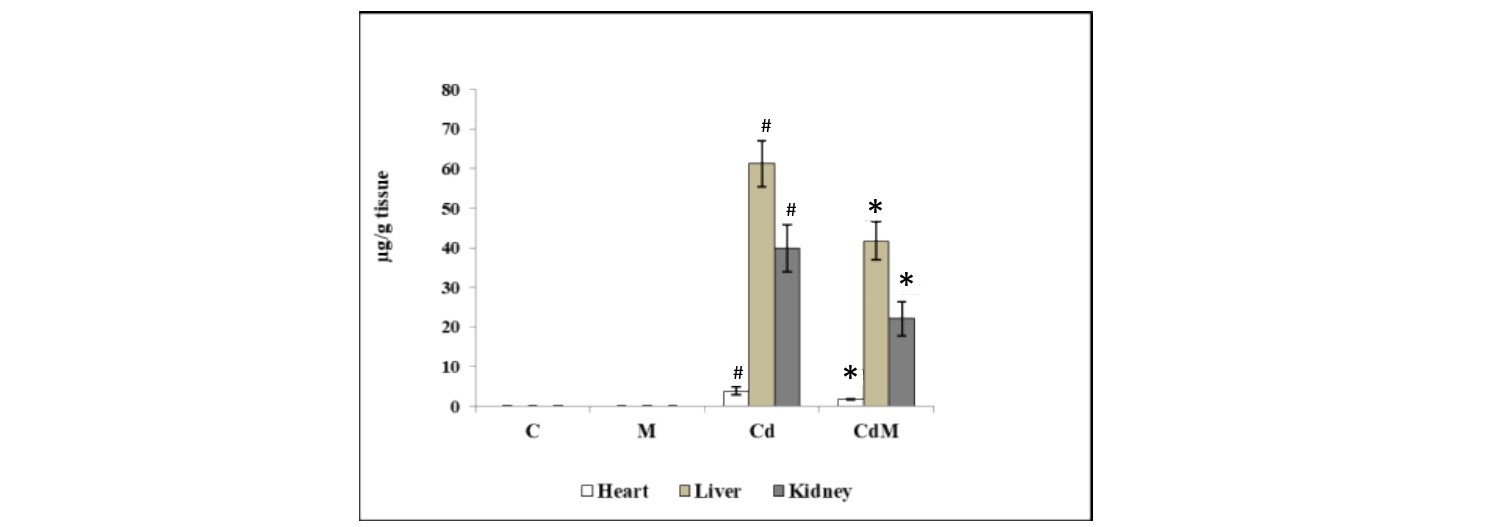
Figure 9: Effect of melatonin on the concentrations of Cd in heart, liver and kidney tissues.
Data were expressed as mean ± S.E.M. (n=6). Rats were treated with CdCl2. C: control, M: melatonin alone, Cd: cadmium alone, CdM: Cadmium plus melatonin (0.44 mg/kg) and/or, melatonin (10 mg/kg). # p<0.001 vs. Cd-treated group; * p<0.001 vs. Cd-treated group.3.10. Binding analyses of GSH and CAT with Cd and melatonin in a cell-free/chemical system.
ITC data chart depicts the binding pattern of GSH with Cd and melatonin, respectively. Upon titration of GSH with Cd, an exothermic interaction with steady saturation of heat change was observed (Fig. 10A). However, GSH with melatonin showed an exothermic interaction with the sequential binding pattern which was fitted to five sites sequential binding model with gradual saturation (Fig. 10B). When titration of GSH with a combination of melatonin and Cd, a significant single site binding with significant heat change was observed (area under the curve) as a progressive saturation with time (Fig. 10C).
As to the titration of CAT with Cd injection, a sequential binding pattern with low-intensity binding was observed and this binding was characteristic with gradual saturation of binding energy (Fig. 10D). However, titration of CAT with melatonin displayed a significant one site binding with high initial heat change which slowed down with time (Fig. 10E). Interestingly, upon titration of CAT with a combination of Cd and melatonin, an endothermic reaction was observed and this reaction characterized a competition between Cd and melatonin for the same binding site in CAT until the gradual saturation was reached (Fig. 1)
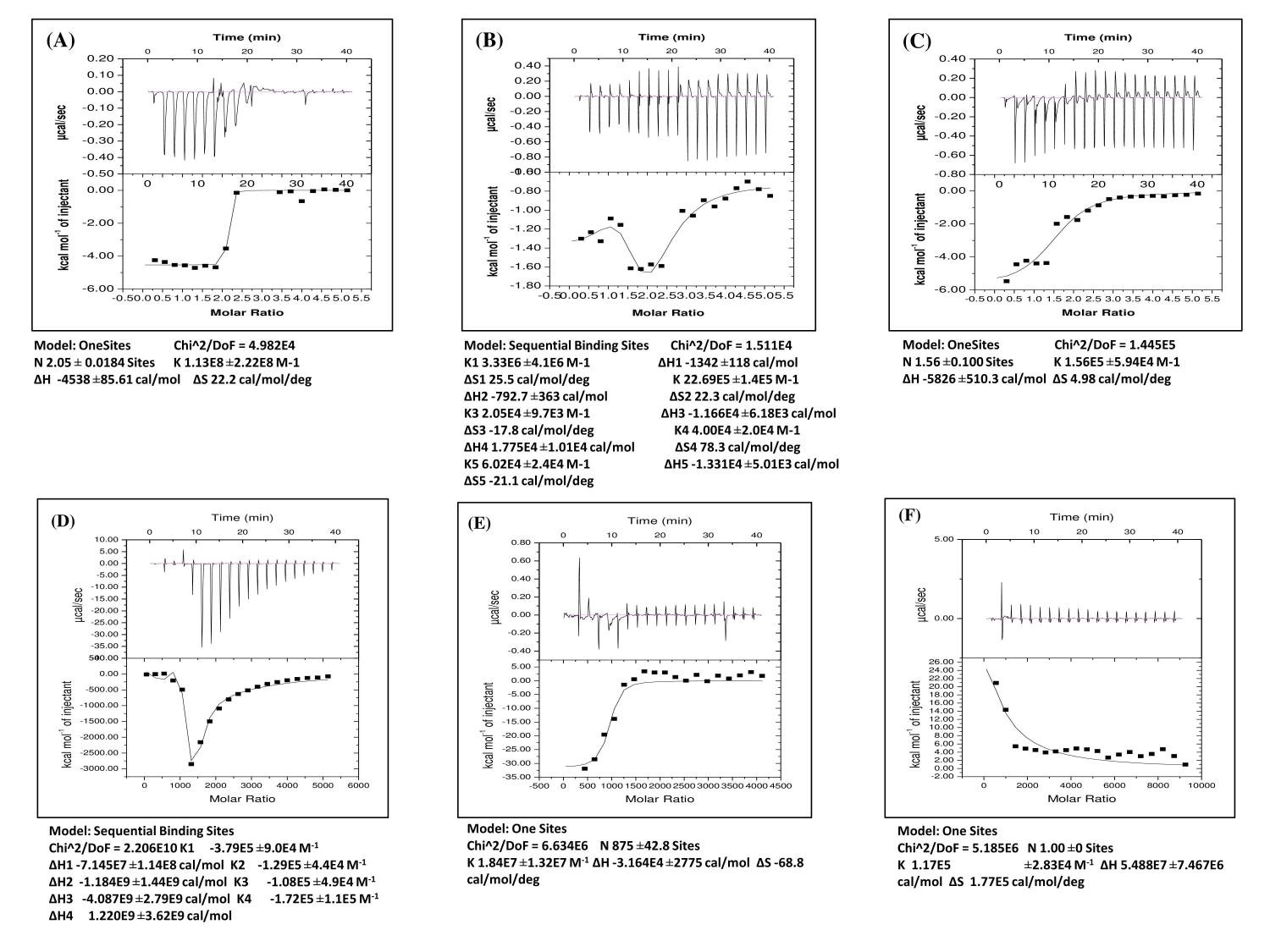
Figure 10: Binding analyses of GSH and CAT with Cd and melatonin in a cell-free/chemical system.
The representative ITC profiles of GSH and CAT with Cd and melatonin. In the heat changes vs. time titration curves, each peak represents an injection of ligand into the sample cell that contains (A) GSH vs Cd as ligand, (B) GSH vs melatonin as ligand, (C) GSH vs Cd and melatonin as ligands, (D) CAT vs Cd as ligand, (E) CAT vs melatonin as ligand and (F) CAT vs Cd and melatonin as ligands. The amount of heat change per second (ΔH) is represented by the area under the curve and the heat change in terms of kcal mol−1 of injectant against molar ratio is shown below curve of each figure, respectively. Values of ΔH, ΔS and no. of sites were expressed in terms of mean ± S.E.
4. DISCUSSION
Cd is a toxic heavy metal with a widely spreading environmental contamination in the industrial or developing countries. This pollutant is classified as a human carcinogen of Group-1 (3). It mainly deposits in the soft tissues of body including the heart, liver and kidneys and initiates oxidative stress in these tissues. Due to its long half-life in biosystem, its low level in body still causes significant damage if it is not properly treated (2). Unfortunately, there is no specific remedy available for Cd toxicity currently (3). The antioxidant treatment seems to be a promising alternative (8). Thus, to identify the effective antioxidants for the Cd toxicity is not only necessary but urgent. Melatonin is a potent free radical scavenger and antioxidant (19). Several studies have documented that melatonin reduces Cd associated oxidative damages in organisms including animals and even in plants (16, 30, 52, 53). However, the underling mechanisms remain to be clarified.
In the current study, the effects of melatonin on the Cd toxicity in heart, liver and kidneys of rats were systemically investigated and the new evidence has emerged to further understand protective mechanisms of melatonin on Cd toxicity.
The study results confirmed that Cd administration to rats resulted in a wide range of injuries in the heart, liver and kidney tissues and this was indicated by the significantly increased serum activities of SGOT, SGPT, LDH1, LDH5, total LDH and ALP and levels of LPO and PCO in rats after Cd treatment. The morphological studies further confirmed the biochemical results, that is, the gross-and ultra- structures of these tissues were significantly disturbed by Cd toxicity. These multiple tissue injuries could be largely attributed to the oxidative stress since Cd treatment significantly increased the ROS, particularly the ·OH in the respective tissues tested. This imbalance of ROS production probably was a result of that Cd suppressed activities of several important antioxidant enzymes including Cu-Zn SOD, Mn-SOD, CAT, GR, GPx and GST. The Cu-Zn-SOD and Mn-SOD participates in dismutation of superoxide anion to H2O2 in cytosol and also in mitochondria, respectively; then, the CAT converts the H2O2 to harmless water and oxygen. The GR, GPx and GST collectively produce GSH, one of the most important intracellular antioxidants. Without the orchestral of these antioxidant enzymes the oxidative stress in Cd distributed tissues is inevitable.
The results showed that all damages in the tested tissues were partially or completely protected by melatonin pre-treatment. This is mainly due to the potent antioxidant capacity of melatonin and also its other functions which will discussed later. Melatonin not only directly scavengers a broad spectrum of ROS but also stimulates the activities of a variety of antioxidant enzymes (46) as well as suppresses the activities of pro-oxidant enzymes [46] as we observed in the study. We also observed that melatonin significantly upregulated the gene expressions of Cu-Zn-SOD, Mn-SOD, CAT which could further contributed to increase in their activities.
As we know that the mitochondria are the major source of the ROS (54, 55). Thus, the effects of Cd and melatonin on mitochondrial metabolisms and mitochondrial morphology were specifically investigated. Cd caused the disturbance of mitochondrial metabolism and deformed their morphology. These alterations were preserved by melatonin. Melatonin not only increased the activities of Krebs cycle enzymes but also the enzymes of ETC which would promote the electron transportation and reduce the electron leakage from the ETC, thus, reduce the ROS formation. It has been well documented that melatonin is a mitochondrial targeted antioxidant [55] and it is generated, metabolized and functioned in mitochondria in all cells (56). The exogenously administrated melatonin in rats can be concentrated in the mitochondria of heart, liver and kidneys by a specific transporter located in the mitochondrial membrane (19) and functions as a mitochondrial antioxidant. These antioxidant activities of melatonin mentioned above are not limited to itself and its many metabolites also possess the similar activities as melatonin (57). This feature of melatonin magnifies its antioxidative capacity compared to other antioxidants.
In addition, the novel aspects of melatonin to protect against Cd toxicity have also be uncovered in the current study. It was reported that Cd could bind to GSH (58) and the interaction of Cd with protein-bound sulfhydryl group of GSH may be the primary cause for Cd toxicity in organisms (59). The results from the ITC study revealed that melatonin also potentially possessed the thermodynamically favorable binding sites with Cd indicated by an increase of entropy upon their binding. Interestingly, when both melatonin and Cd were incubated with GSH, a steady as well as thermodynamically favored binding among them throughout the reaction was favorable to melatonin, thus to protect the GSH from Cd induced oxidation. The similar reaction was also found in the CAT binding study. Wang et al. (60) reported that the misfolding and alterations in the activity of CAT might be due to the interaction of Cd with this enzyme. Our result showed that melatonin and Cd competed for the same binding site in CAT. It is likely that melatonin discharges the Cd in the CAT binding site by its metal chelating property (57, 61). This speculation was also confirmed by the Cd content analysis. A profound accumulation of Cd content in heart, liver and kidney tissues was noted in Cd-treated groups compared to control, which might be due to its excessive accumulation and long half-life in soft tissues (27). Pre-treatment with melatonin significantly reduced this Cd accumulation in all tissues tested. The heavy metal chelating activity of melatonin can plausibly explain this observation.
Collectively, all these actions of melatonin mentioned above are attributed its profound protective effects on Cd associated tissue injuries. The potentially underlying mechanisms were illustrated in the figure 11.
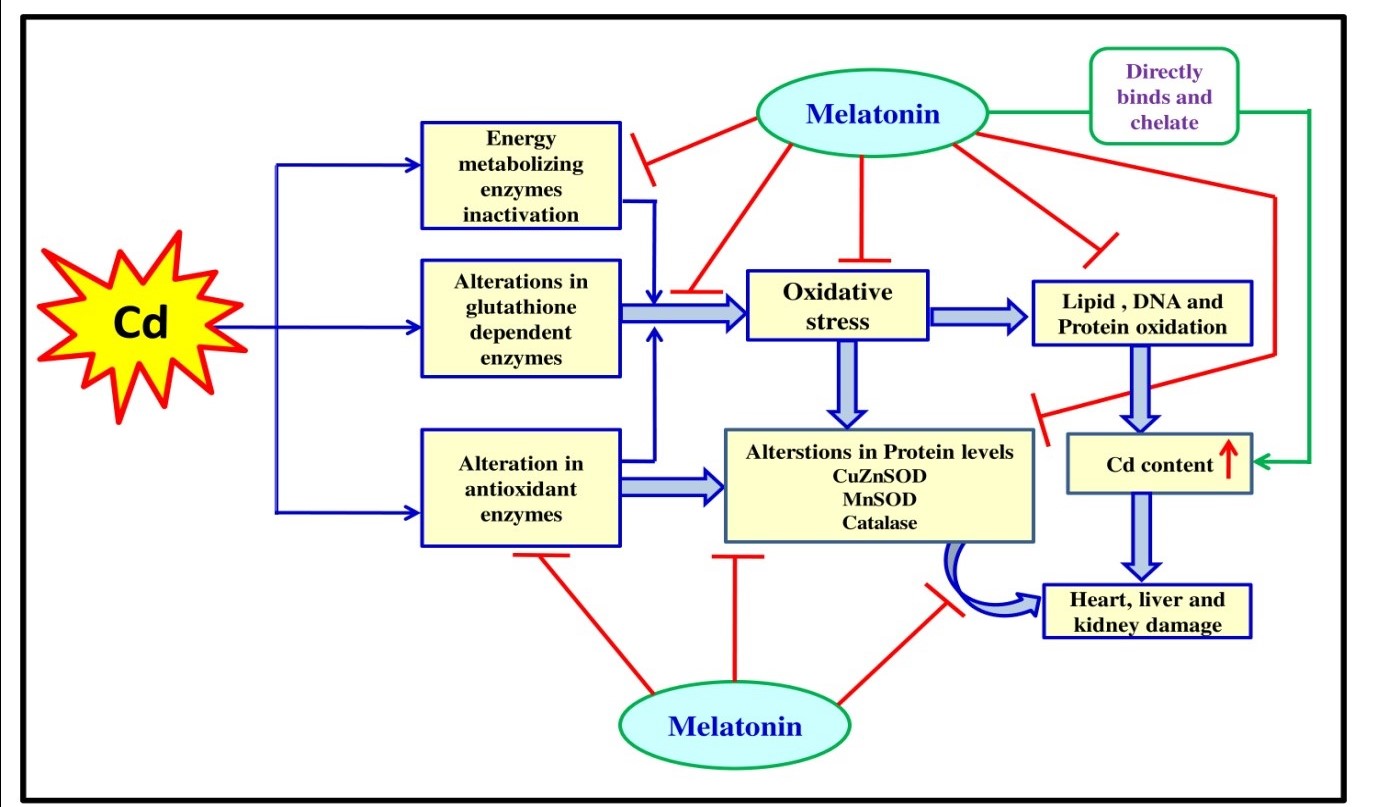
Figure 11: Schematic representation of the possible protective mechanism of melatonin against cadmium induced oxidative stress mediated tissue damage in male Wistar rat.
Based on the results reported here and also reports of others, we believe that melatonin may be a suitable candidate to be selected for treatment of acute or chronic Cd toxicity clinically, especially considering its safety margin and its low cost.
ACKNOWLEDGEMENTS
Dr. Elina Mitra thankfully acknowledges the receipt of a UPE Project Fellowship of UGC, under University of Calcutta. A Senior Research Fellowship (SRF) under DST-INSPIRE program (IF140691), Govt. of India to Bharati Bhattacharjee is also thankfully acknowledged. Dr. Palash Kumar Pal is a Dr. D. S. Kothari Post Doctoral Fellow (BL/16-17/0502)of University Grant Commission (UGC), Govt. of India and a financial assistance as CSIR-RA [09/028(0952)/2015EMR-I] fellowship, Govt. of India to Dr. Arnab Kumar Ghosh is thankfully acknowledged. Dr. Aindrila Chattopadhyay is supported by funds available to her from Department of Science and Technology, Govt. of West Bengal. Prof. DB also extends his grateful thanks to University Grants Commission, Govt. of India, for the award of a Research Project under Center with Potential for Excellence in a Particular Area (CPEPA). Prof. DB is also supported from departmental BI grant of University of Calcutta. DB also gratefully acknowledges the support he received from DST-PURSE Program awarded to University of Calcutta. Additionally, we especially extend our grateful thanks to Dr. Dun Xian Tan for critically editing the manuscript which has made the manuscript more important and increased its scientific and readership quality.
AUTHORSHIP
Dr. DB and Dr. AC contributed to conception, revised the manuscript critically and approved it. Dr. EM executed the experiment, analyzed the data, prepared figures, drafted the manuscript and edited it. BB contributed to executing the experiment, analyzed the data, prepared figures, drafted the manuscript and edited it. Dr. PKP contributed in preparing the figures, drafted the manuscript and edited it. Dr. AKG contributed to executing the experiment. SM contributed to executing the experiment.
CONFLICT OF INTEREST
Authors declare no conflict of interest.
REFERENCES
Pan J, Huang X, Li Y, Li M, Yao N, Zhou Z, Li X (2017) Zinc protects against cadmium-induced toxicity by regulating oxidative stress, ions homeostasis and protein synthesis. Chemosphere 188: 265-273. doi: 10.1016/j.chemosphere.2017.08.106
Goering PL, Waalkes MP, Klaassen CD (1964) Toxicology of cadmium. In: Goyer RA, Cherian MG, eds. Handbook of experimental pharmacology: toxicology of metals, Springer-Verlag, New York, 115: 189-214.
International Agency for Research on Cancer (IARC) (1993) IARC cancer monographs on the evaluation of the carcinogenic risks to humans, vol. 58. IARC, Lyon, France. 119238.
Nair AR, Degheselle O, Smeets K, Van Kerkhove E, Cuypers A (2013) Cadmium-induced pathologies: where is the oxidative balance lost (or not)? Int. J. Mol. Sci. 14: 6116-6143. doi: 10.3390/ijms14036116.
Chwełatiuk E, Włostowski T, Krasowska A, Bonda E (2005) Melatonin increases tissue accumulation and toxicity of cadmium in the bank vole (Clethrionomysglareolus). BioMetals 18: 283–291. DOI 10.1007/s10534-005-1720-7.
Chwełatiuk E, Włostowski T, Krasowska A, Bonda E (2006) The effect of orally administered melatonin on tissue accumulation and toxicity of cadmium in mice. J. Trace Elem. Med. Biol. 19: 259–265. doi:10.1016/j.jtemb.2005.10.006.
Valko M1, Jomova K, Rhodes CJ, Kuča K, Musílek K (2016) Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch. Toxicol. 90: 1-37. doi: 10.1007/s00204-015-1579-5.
El-Sokkary GH, Nafady AA, Shabash EH (2009) Melatonin ameliorates cadmium-induced oxidative damage and morphological changes in the kidney of rat. Open Neuroendocrinol. J. 2: 1-9.
El-Sokkary GH, Nafady AA, Shabash EH (2010) Melatonin administration ameliorates cadmium-induced oxidative stress and morphological changes in the liver of rat. Ecotox. Environ. Safe 73: 456–463. doi:10.1016/j.ecoenv.2009.09.014.
Romero A, Caride A, Pereiro N, Lafuente A (2011) Modulatory effects of melatonin on cadmium-induced changes in biogenic amines in rat hypothalamus. Neurotox. Res. 20: 240-249. doi: 10.1007/s12640-010-9237-4.
Jiménez-Ortega V, Barquilla PC, Fernández-Mateos P, Cardinali DP, Esquifino A (2012) Cadmium as an endocrine disruptor: Correlation with anterior pituitary redox and circadian clock mechanisms and prevention by melatonin. Free Radic. Biol. Med. 53: 2287–2297. http://dx.doi.org/10.1016/j.freeradbiomed.2012.10.533
Li Y, Wang H, Meng C, Zhao XF, Zhang C, Zhang Y, Zhao M, Chen YH, Meng XH, Xu DX (2012) Melatonin alleviates cadmium-induced cellular stress and germ cell apoptosis in testes. J. Pineal Res. 52: 71–79. Doi:10.1111/j.1600-079X.2011.00921.x.
Miura N, Yanagiba Y, Ohtani K, Mita M, Togawa M, Hasegawa T (2012) Diurnal variation of cadmium-induced mortality in mice. J. Toxicol. Sci. 37: 191-196.
Cuypers A, Plusquin M, Remans T, Jozefczak M, Keunen E, Gielen H, Opdenakker K, Nair AR, Munters E, Artois TJ, Nawrot T, Vangronsveld J, Smeets K (2010) Cadmium stress: an oxidative challenge. Biometals 23: 927-940. DOI: 10.1007/s10534-010-9329-x
Birben E1, Sahiner UM, Sackesen C, Erzurum S, Kalayci O (2012) Oxidative stress and antioxidant defense. World Allergy Organ J. 5: 9. doi: 10.1097/WOX. 0b013e31 82439613.
Tan DX, Manchester LC, Esteban-Zubero E, Zhou Z, Reiter RJ (2015) Melatonin as a potent and inducible endogenous antioxidant: synthesis and metabolism. Molecules. 20: 18886-18906. doi: 10.3390/molecules201018886.
Tan DX, Zheng X, Kong J, Manchester LC, Hardeland R, Kim SJ, Xu X, Reiter RJ (2014) Fundamental issues related to the origin of melatonin and melatonin isomers during evolution: relation to their biological functions. Int. J. Mol. Sci. 15:15858-15890. doi: 10.3390/ijms150915858.
Tan DX, Manchester LC, Qin L, Reiter RJ (2016) Melatonin: A Mitochondrial targeting molecule involving mitochondrial protection and dynamics. Int. J. Mol. Sci. 17: 2124. doi: 10.3390/ijms17122124.
Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre JM, Qin L (2016) Melatonin as an antioxidant: under promises but over delivers. J. Pineal Res. 61: 253-278. doi: 10.1111/jpi.12360.Hardeland R (2017) Melatonin and the electron transport chain. Cell Mol. Life Sci. 74: 3883-3896. doi: 10.1007/s00018-017-2615-9.
Hardeland R (2017) Melatonin and the electron transport chain. Cell Mol. Life Sci. 74: 3883-3896. doi: 10.1007/s00018-017-2615-9.
Mitra E, Ghosh AK, Ghosh D, Mukherjee D, Chattopadhyay A, Dutta S, Pattari SK, Bandyopadhyay D (2012) Protective effect of aqueous Curry leaf (Murrayakoenigii) extract against cadmium-induced oxidative stress in rat heart. Food Chem. Toxicol. 50: 1340-53. doi: 10.1016/j.fct.2012.01.048.
Mitra E, Ghosh AK, Ghosh D, Firdaus SB, Mukherjee D, Chattopadhyay A, Pattari SK, Datta S, Bandyopadhyay D (2014) Ameliorative effect of aqueous tulsi leaf (Ocimum sanctum) extract against cadmium-induced oxidative stress in rat liver. Int. J. Pharm. Pharm. Sci. 5: 557-568.
Bhattacharjee B, Ghosh AK, Mishra S, Das J, Chattopadhyay A, Bandyopadhyay D (2016) Terminalia arjuna aqueous bark extract protects against cadmium acetate-induced injury to rat liver and heart through antioxidant mechanisms: a dose response study. J. Pharma. Res.10: 771-792.
Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 28: 56-63.
Strittmatter CF (1965) Studies on avian xanthine dehydrogenases: Properties and patterns of appearance during development. J. Biol. Chem. 240: 2557–2564.
Varcoe JS (2001) Clinical Biochemistry: Techniques and Instrumentation-A practical approach, first ed. World Scientific Publishing Company, pp. 40-43.
Folin O, WU H (1919) A system of blood analysis. J. Biol. Chem. 38: 81.
Kind PRN, King EJ (1954) Estimation of plasma phosphatase by determination of hydrolysed phenol with anti-pyrine. J. Clin. Pathol. 7: 322–326. doi:10.1136/jcp.7.4.322.
Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound, nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 25: 192-205. doi:org/10.1016/ 0003-2697 (68)90092-4.
Bandyopadhyay D, Ghosh G, Bandyopadhyay A, Reiter RJ (2004) Melatonin protects against piroxicam-induced gastric ulceration. J. Pineal Res. 36: 195–203. doi:org/ 10.1111/j.1600-079X.2004.00118.x
Buege JA, Aust SG (1978) Microsomal lipid peroxidation. Methods Enzymol. 52: 302-310. doi: org/10.1016/S0076-6879(78)52032-6.
Levine RL, Williams JA, Stadtman ER, Shacter E (1994) Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol. 233: 346-357. doi:org/10.1016/S0076-6879(94)33040-9
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyragallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 47: 469-474. doi:org/10.1111/j.1432-1033.1974.tb03714.x.
Beers Jr. RF, Sizer IW (1952) A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 195: 133-140.
Chattopadhyay A, Biswas S, Bandyopadhyay D, Sarkar C, Datta AG (2003) Effect of isoproterenol on lipid peroxidation and antioxidant enzymes of myocardial tissue of mice and protection by quinidine. Mol. Cell. Biochem. 245: 43-49. doi: 10.1023/A: 1022808224917.
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 27: 680–685. doi: 10.1038/227680a0.
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 70: 158-169.
Krohne-Ehrich G, Schirmer RH, Untucht-Grau R (1977) Glutathione reductase from human erythrocytes. Isolation of the enzyme and sequence analysis of the redox-active peptide. Eur. J. Biochem. 80: 65-71. doi:org/10.1111/j.1432-1033.1977.tb11856.x
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione -S- transferases, the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 249: 7130 -7139.
Chretien D, Pourrier M, Bourgeron T, Séné M, Rötig A, Munnich A, Rustin P (1995) An improved spectrophotometric assay of pyruvate dehydrogenase in lactate dehydrogenase contaminated mitochondrial preparations from human skeletal muscles. Clin. Chim. Acta 240: 129-136. doi:org/10.1016/0009-8981(95)06145-6.
Mukherjee D, Ghosh AK, Dutta M, Mitra E, Mallick S, Saha B, Reiter RJ, Bandyopadhyay D (2015) Mechanism of isoproterenol induced cardiac mitochondrial damages: protective action of melatonin. J. Pineal Res. 58: 275-290. doi: 10.1111/ jpi.12213
Duncan MJ, Fraenkel DG (1979) Alpha-ketoglutarate dehydrogenase mutant of Rhizobium meliloti. J. Bacteriol. 137: 415-419.
Veeger C, DerVartanian DV, Zeylemaker WP (1969) Succinate dehydrogenase. Methods Enzymol. 13:81-90. doi:org/10.1016/0076-6879 (69)13020-7.
Goyal N, Srivastava VM (1995) Oxidation and reduction of cytochrome c by mitochondrial enzymes of Setariacervi. J. Helminthol. 69: 13-17.
Greenlee L, Handler P (1964) Xanthine oxidase. IV. Influence of pH on substrate specificity. J. Biol. Chem. 239: 1090-1095.
Mukherjee D, Ghosh Roy S, Bandopadhyay A, Chattopadhyay A, Basu A, Mitra E, Ghosh AK, Reiter RJ, Bandhyopadhyay D (2010) Melatonin protects against isoproterenol induced myocardial injury in the rat: antioxidative mechanism. J. Pineal. Res. 48: 251-262. doi: 10.1111/j.1600-079X.2010.00749.x.
Roy SG, De P, Mukherjee D, Chander V, Konar A, Bandyopadhyay D, Bandyopadhyay A (2009) Excess of glucocorticoid induces cardiac dysfunction via activating angiotensin II pathway. Cell. Physiol. Biochem. 24: 1–10.
Watanabe I, Ogawa K, Yamada E (1988) Taste buds of rabbits foliate papillae. A scanning electron microscopy study. Cienc. Cult. 40: 787–790.
Bhattacharjee B, Pal PK, Ghosh AK, Mishra S, Chattopadhyay A, Bandyopadhyay D (2019) Aqueous bark extract of Terminalia arjuna protects against cadmium-induced hepatic and cardiac injuries in male Wistar rats through antioxidative mechanisms. Food Chem. Toxicol. 124: 249-264. doi: 10.1016/j.fct.2018.12.008.
Mitra E, Basu A, Ghosh D, Ghosh AK, Chattopadhyay A, Pattari SK, Datta S, Bandyopadhyay D (2013) Ameliorative effect of aqueous tulsi leaf (Ocimum sanctum) extract against cadmium-induced oxidative stress in rat liver. Int. J. Pharm. Pharm. Sci. 5: 557-568.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193: 265-275.
Ni J, Wang Q, Shah FA, Liu W, Wang D, Huang S, Fu S, Wu L (2018) Exogenous melatonin confers cadmium tolerance by counterbalancing the hydrogen peroxide homeostasis in wheat seedlings. Molecules 23: pii: E799. doi: 10.3390/ molecules 23040799.
Gu Q, Chen Z, Yu X, Cui W, Pan J, Zhao G, Xu S, Wang R, Shen W (2017) Melatonin confers plant tolerance against cadmium stress via the decrease of cadmium accumulation and reestablishment of microRNA-mediated redox homeostasis. Plant Sci. 261: 28-37. doi: 10.1016/j.plantsci.2017.05.001.
Cuypers A, Plusquin M, Remans T, Jozefczak M, Keunen E, Gielen H, Opdenakker K, Nair AR, Munters E, Artois TJ, NawrotT, Vangronsveld J, Smeets K (2010) Cadmium stress: an oxidative challenge. Biometals 23: 927-940. doi: 10.1007/s10534-010-9329-x.
Zhang HM, Zhang Y, Zhang BX (2011) The role of mitochondrial complex III in melatonin-induced ROS production in cultured mesangial cells. J. Pineal Res. 50: 78-82. doi: 10.1111/j.1600-079X.2010.00815.x.
Tan DX, Reiter RJ (2019) Mitochondria: the birth place, battle ground and the site of melatonin metabolism in cells. Melatonin Res. 2: 44-66. doi: https://doi.org/https:// doi.org/10.32794/mr11250011.
Galano A, Reiter RJ (2018) Melatonin and its metabolites vs oxidative stress: From individual actions to collective protection. J. Pineal. Res. 65: e12514. doi: 10.1111/jpi.12514.
Chekmeneva E, Prohens R, Díaz-Cruz JM, Ariño C, Esteban M (2008) Thermodynamics of Cd2+ and Zn2+ binding by the phytochelatin (gamma-Glu-Cys) 4-Gly and its precursor glutathione. Anal. Biochem. 375: 82-89. doi: 10.1016/j.ab.2008.01.008.
Wang Y, Fang J, Leonard SS, Rao KM (2004) Cadmium inhibits the electron transfer chain and induces reactive oxygen species. Free Radic. Biol. Med. 36: 1434–1443.
Wang J, Zhang H, Zhang T, Zhang R, Liu R, Chen Y (2015) Molecular mechanism on cadmium-induced activity changes of catalase and superoxide dismutase. Int. J. Biol. Macromol. 77: 59-67. doi: 10.1016/j.ijbiomac.2015.02.037.
Limson J, Nyokong T, Daya S (1998) The interaction of melatonin and its precursors with aluminium, cadmium, copper, iron, lead, and zinc: An adsorptive voltammetric study. J. Pineal Res. 24: 15-21.
This work is licensed under a Creative Commons Attribution 4.0 International License