Please cite this paper as:
Molina-Carballo, A., Jerez-Calero, A., Fernández-López, L., Augustin-Morales, M. del C., Muñoz-Hoyos, A. and Agil, A. 2023. The preventive and protective role of melatonin in SARS-CoV-2 infection: a retrospective study. Melatonin Research. 6, 3 (Sep. 2023), 372-396. DOI:https://doi.org/https://doi.org/10.32794/mr112500159.
Research Article
The preventive and protective role of melatonin in SARS-CoV-2 infection: a retrospective study
Antonio Molina-Carballo1,2,5*, Antonio Jerez-Calero1,2,5, María Luisa Fernández-López1,2,5, María del Carmen Augustin-Morales3,5, Antonio Muñoz-Hoyos1,5*, Ahmad Ágil4,5.
1Department of Pediatrics. School of Medicine. University of Granada. Spain
2“Clínico San Cecilio” University Hospital. Granada. Spain
3Health Center “Las Gabias”. Metropolitan District. Granada. Spain
4Department of Pharmacology, Biohealth Institute and Neurosciences Institute, School of Medicine, University of Granada, 18016 Granada, Spain
5Biohealth Research Institute Granada (ibs.GRANADA), University Hospital of Granada, 18016. Granada, Spain
*Correspondence: amolinac@ugr.es (A.M-C), amunozh@ugr.es (A.M-H), Tel: +34-9582407040
Running title: Melatonin protection in COVID-19
Received: May 3, 2023; Accepted: September 19, 2023
ABSTRACT
This study has investigated the protective role of melatonin against SARS-CoV-2 infection. For this purpose, 62 adults were recruited who were in daily relatively high doses of melatonin intaking, with the particularity that they started taking it before the beginning of the COVID-19 pandemic and continued to present. A continuous validation process has been carried out with a series of questionnaires to identify the risk factors, whether they were contacts, were infected, if yes, the level of disease severity, need for treatment, hospitalization, etc. According to the dose of melatonin the individuals took/are taking, they were divided into two groups: a) those taking 20 mg (n = 27) and, b) those taking ≥ 40 mg (n = 32). For statistical analysis, the shi2 test and Fisher's exact test were used. The number of infected subjects with positive PCR was 7 (11.9%). Only one required medication, the rest had a very favorable clinical evolution, mild in three cases and asymptomatic in three others. While in their environment this percentage is 22.05% (chi2 = 2.928; p < 0.087). Melatonin offers a good safety profile, is well tolerated and can play an important role in the different levels of COVID-19 prevention.
Key words: COVID-19, SARS-CoV-2, melatonin, infection, retrospective study
______________________________________________________________________________________________________________________
1. INTRODUCTION
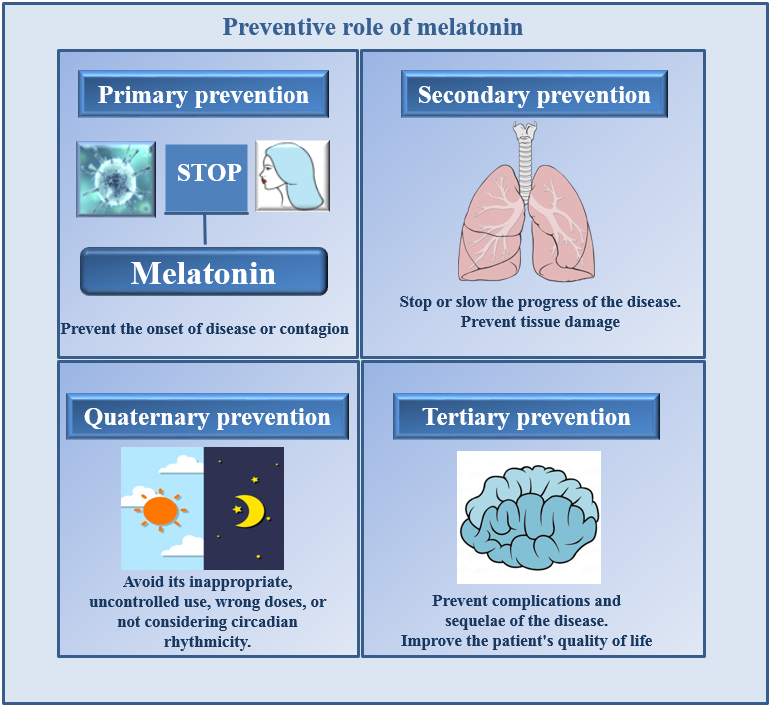
Depending on the point in the natural history of the disease in which the activities are carried out, preventive medicine has been described as four levels of prevention (1-5) (Figure 1): 1) At the primary level, the disease is not present, and focuses are on disease prevention by eliminating causes or increasing people's resistance to disease. In the current COVID-19 pandemic, the following procedures could be used during the primary level: the use of masks, maintaining the appropriate distance between people, the administration of vaccines, and even medications that make it difficult for SARS-CoV-2 to initiate its pathophysiological process, such as the administration of melatonin (6-12). 2) At the secondary level, the disease is diagnosed at an early stage, and measures are applied to stop or delay the progress of the disease that the person already has; important features include early detection, diagnosis, and treatment of the disease. An example of what secondary preventive medicine is the campaign for the early detection of COVID-19. Due to its various actions (Figure 2), including its role as an immune system enhancer, melatonin should receive serious consideration as a treatment for COVID-19 in the early disease stage (13-24). 3) When it is evident that there is a disease, preventive medicine acts at the tertiary level. At this stage the treatments are directed reducing complications, including the risk of disability or death. The properties of melatonin such as; antioxidant, anti-inflammatory, immunomodulatory, etc. once again are relevant to its use at this stage of the disease (25-33). 4) Finally, the main goal of quaternary prevention is to protect people from unnecessary and excessive medical interventions.

Fig. 1. The illustration of the potential preventive effects of melatonin at different levels of disease development.
Patients in the health care system may be at risk of excessive or inappropriate interventions, resulting in unnecesary and/or erroneous diagnostic testing and medical procedures. Relative to the use of melatonin, some issues are unknown, e.g., the optimal doses suitable for each of level of treatment, best routes of administration, consideration of circadian variation, pharmaceutical form, etc. In this sense, various authors have recommended the need to carry out adequate clinical trials to identify essential aspects to provide guidelines that should be used in each case of a patient with COVID-19 (34-38). (Figure 1).
As an antioxidant, melatonin capacity to chemically reduce up to ten free radicals per molecule, while other antioxidants, with high antioxidand power such as vitamins C and E, have the capacity to scavenge a single radical (39). Indirectly, antioxidant efficiency also is related to stimulation of antioxidative enzymes including superoxide dismutase, glutathione peroxidase, reductase and catalase (40, 41). Melatonin stimulates the activities of each of these enzymes and additionally has immunomodulatory, anti-inflammatory, sleep-inducing, chronobiological, oncostatic, etc. (42-51) (Figure 2). Several of these actions would improve melatonin’s ability to protect againts a SARS-CoV-2 infection.

Fig. 2. The proposed physiological effects of melatonin related to its disease protective potentials.
A particularly interesting evolutionary aspect is melatonin’s capacity as a cellular protector. This universal action is attributed to the fact that melatonin is synthesized in the mitochondria of every cell in an organism so it is always available for this purpose (52). Circulating melatonin is derived primarily from the pineal gland and secrets into the systemic circulation as well as into the cerebrospinal fluid. In contrast, extrapineal melatonin generated in mitochondria does not enter the circulation (53). Melatonin in the blood is primarily for modualtion of circadian rhythms (54). Being highly lipophilic, melatonin can penetrate all subcelluar organelles and is in especially high concentrations in the mitochondria (55, 56).
Melatonin exerts its actions through a variety of molecular pathways, some of the best characterized pathway being the activation of two types of specific membrane receptors, the high-affinity MT1 receptors and the low-affinity MT2 receptors (57, 58). Likewise, this molecule also has relevant intracellular actions by binding to cytosolic calmodulin (59) and to two nuclear binding sites of the retinoid Z family of nuclear receptors (60). Melatonin receptors have been found in various peripheral tissues, including the heart, adrenal gland, kidney, lung, liver, gallbladder, small intestine, adipocytes, ovaries, uterus, breast, prostate, and skin and probably exist on all cells (61) . Its actions as a direct radical scavengre are, of course, receptor independent. Given this functional diversity attributed to melatonin, we objectively evaluated its possible preventive aspects on SARS-CoV-2 infection in humans keeping in mind the multiple means by which this molecule acts. The involvement of multiple signaling pathways (i.e., NF-κB, STAT1 and Nrf2) related to anti-inflammatory and antioxidant control and mitochondrial protection in experimental animals have been described (62).
Regarding SARS-CoV-2 infection, due to the continuous appearance of new variants of the virus, together with the considerable number of people who object to receiving the vaccine and the insufficient availability of the vaccine for underdeveloped countries and regions, we believe that other alternatives should be considered to combat SARS-CoV-2 infection. We feel that melatonin could be one of these alternatives, due to the various functions that allow it to slow down or reduce the pathogenic actions of SARS-CoV-2 infection, significantly reducing the mortality of critically ill patients with COVID-19 and the length of stay in hospital Intensive Care Units (ICU) (23, 35). Thus, we propose the following working hypothesis: melatonin plays a preventive and protective role against SARS-CoV-2 infection.
The data that we provide in this survey fundamentally aim at studying the preventative and protective role that melatonin could have at the primary level (avoid contagion/colonization), as well as stopping or reducing the evolution of the infection in its different stages.
2. MATERIALS AND METHODS
2.1. Patients and Data Collection.
A total of 62 adults who were administered melatonin and completed a series of follow-up questionnaires voluntarily participated in this research study. In the process, informed consent and follow-up of the procedure were signed in accordance with the current regulations established in terms of confidentiality and privacy of data for each of the participants included. Cronbach's alpha coeffi-cient (0.87) was calculated to validate the consistency of the questionnaires. The 62 adults had taken melatonin continuously prior to the onset of the COVID-19 pandemic, and were still taking it today. Each of the 62 participants followed the same circadian administration protocol for intake (30 minutes before bedtime), at doses ranging from 20 mg to 500 mg. In addition to the 62 participants involved and considered, other potential participants were excluded for various reasons: doses lower than the minimum considered for this study, having temporarily interrupted the intake of melatonin during the study period, not providing informed consent and/or not agreeing to provide data for the study. All the subjects included were correctly vaccinated according to the protocol and phases in which they corresponded according to the program proposed by the health authorities; the great majority had received the Pfizer vaccine and the others the Astra Seneca or Moderna preparations. The study was conducted in accordance with the Declaration of Helsinki. And informed consent was obtained from all subjects involved in the study.
2.2. Statistical Analysis.
The data included in the survey aim at assessing various aspects related to the possible role that melatonin may have in the prevention and protection against COVID-19, as well as in its evolutionary process. The following data have been included: 1) Affiliation and identification of the person surveyed, 2) Sex, 3) Age, 4) Weight, 5) Fundamental inclusion criteria, taking melatonin continuously since before the pandemic beganing and continuing to do so (it is the way to guarantee its possible protective effect), 6) Identify and/or describe possible adverse or secondary effects after ingestion, 7) Describe beneficial effects as indicated by the collected data, 8) Have or suffer from any co-morbidity risk factor for SARS-CoV-2 (hypertension, obesity, lung disease, heart disease, immunodeficiency, cancer, etc.), 9) If you have had a PCR-confirmed infection by SARS CoV-2, 10). If you have been vaccinated against to SARS CoV-2, 11) What vaccine have you received and how many doses, 12) Your professional activity can be considered a risk for contracting SARS CoV-2 infection (caring for infected patients, serving the public during the pandemic, being a teacher, etc.), 13) Have you had any contact narrow during the pandemic. Close contact is considered when three conditions are met: a) Being close to an infected person less than 1.5 meters away, b) Not wearing a face mask, c) Staying in these circumstances for at least 15 minutes. 14) If it has been close contact, on how many occasions has this occurred, 15) If infected, how was the SARS CoV-2 infection classified: Asymptomatic, Mild. Moderate, Severe. 16) Has required medication during the infection: a) Symptomatic, b) Specific according to the protocol: Corticosteroids, biological therapy, heparin, sedatives, antivirals, antibiotics, etc. 17) You have had to be admitted to the hospital due to COVID-19. 18) How many people in your family environment, with whom you have lived, have suffered from the COVID-19 disease.
For the analysis of the results, we divided the survey sample into two groups, the group that takes 20 mg of melatonin daily (N = 30), and the group that takes ≥ 40 mg of melatonin daily (N = 32). For the statistical study, the chi2 test and Fisher's exact test (when the number of cases was <5) were used. To show an overview of the scope of the surveys carried out. the Table 1 included each of item from the survey.
Table 1. Items included in the survey.
Survey Items |
Surveyed Affiliation and identification data |
Gender |
Age |
Weight |
Fundamental inclusion criteria, taking melatonin continuously since before the pandemic began and continuing to (it is the way to guarantee its possible protective effect) |
Identify and/or describe possible adverse or secondary effects after ingestion |
Describe beneficial effects if appreciated |
Have or suffer from any risk factor to get sick from SARS-CoV-2 (hypertension, obesity, lung disease, heart disease, immunodeficiency, cancer, etc.) |
If you have had a PCR-confirmed infection by SARS CoV-2 |
You have been vaccinated against to SARS CoV-2 |
What vaccine have you received and how many doses. |
Your professional activity can be considered a risk for contracting SARS CoV-2 infection (caring for infected patients, serving the public during the pandemic, being a teacher, etc.). |
Have you had any contact narrow during the pandemic. Close contact is considered when three conditions are met: a) Being close to an infected person less than 1.5 meters away, b) Not wearing a face mask, c) Staying in these circumstances for at least 15 minutes. |
If it has been close contact, on how many occasions has this occurred |
If infected, how was the SARS CoV-2 infection classified: Asymptomatic, Mild. Moderate, Severe |
Has required medication during the infection: a) Symptomatic, b) Specific according to the protocol: Corticosteroids, biological therapy, heparin, sedatives, antivirals, antibiotics, etc. |
You have had to be admitted to the hospital due to COVID-19 |
How many people in your family environment, with whom you have lived, have suffered from the COVID-19 disease |
3. RESULTS
The number of surveys conducted and completed for all items was 62, of which 30 (48.38%) were male and 32 (51.61%) were female. The mean age of the respondents was 58.46 +/- 12.7 years. The mean weight was 71.87+/-15.61 kg. The mean dose of melatonin they had received during the study period was 50.96 +/- 77.16 mg; all respondents had followed the same protocol during the pandemic period, taking the same dose daily half an hour before bedtime with a minimum of 20 mg and a maximum of 500 mg (With this last dose there is only one male respondent). Regarding possible adverse effects with melatonin intake after two consecutive years of use, only one respondent (1/62; 1.61%) reported having gained more weight and this was mainly attributed to the combination of higher intake and lower physical activity during the whole pandemic period rather than to melatonin as many reports claim (65). We emphasize that in the rest of participants no adverse effects or intolerance were found among their answers. On the other hand, the positive or beneficial effects described by the respondents were the following: 1) 58.06% (36/62), had had a more restful sleep and better rest. 2) 3.22% (2/62) of the respondents indicated a better state of health and 3) Another 3.22% (2/62) reported better performance in their daily activities (Table 2).
Table 2. The risk factors that were detected in the surveyed population.
Risk factor´s | Number of cases | Percentage (%) | Infected with SARS-CoV-2 |
Arterial hypertension | 10 | 16.2 | 0 |
Obesity | 7 | 11.29 | 2 |
Cancer | 3 | 4.83 | 1 |
Inflammatory bowel disease | 1 | 1.61 | 0 |
Heart disease | 2 | 3.22 | 0 |
Pneumopathy | 3 | 4.83 | 0 |
Immunosuppression | 3 | 4.83 | 1 |
Bronchial asthma | 2 | 3.22 | 1 |
Multiple sclerosis | 1 | 1.61 | 0 |
Diabetes | 3 | 4.83 | 0 |
The number of respondents who have been infected by SARS CoV-2, with positive PCR were 7/62 (11.9%), and 88.70% (55 respondents) were not infected. Chi2 = 26.54 (p = 2.579e-7). Among those infected with SARS CoV-2, 5 of the 7 people belong to the group that takes 20 mg of melatonin, which represents 71.42% of those infected, 18.51% of the group that takes 20 mg (5/27) and 8.06 % (5/62) of the total sample. While in the group that takes ≥ 40 mg of melatonin only 2 people were infected, which represents 28.5% of those infected (2/7), 5.71% of the group that takes ≥ 40 mg of melatonin (2/35) and 3.22 % of total respondents (2/62) (Figure 3).
If we compare the infected numbers of individuls reported here with those of the background numbers during the similar period occurred in Spain, we find the following data: 1) Total population in Spain were 47,326,687, number of people infected (officially) were 10,439,302 (22.05%), 2) Survey data: Numbers of respondents were 62, numbers of infected were 7. (11.9%), chi2 = 2,928; p < 0.087. With the particularity that the number of infected we know is much higher than the reported due to the impossibility of confirming all cases, among other reasons due to the collapse of the health system. On the other hand, if we analyze the data on the frequency of those infected between the group that takes ≥ 40 mg of melatonin and the general group in the country, we find the following results: chi2 = 4.009 (p < 0.045). Regarding infected and the evolution of the disease, of the seven cases detected, only one required medication, a patient who was taking 20 mg of melatonin, the rest had a very favorable clinical evolution, classified as mild in three cases and asymptomatic in other three cases. In relation to the two cases that took ≥ 40 mg of melatonin, both had the disease without problems, with minor manifestations and without the need for a specific medication, finally classified as mild.

Fig. 3. Percentage of infected individuals who take 20 or ≥ 40 mg of melatonin, respectively.
(A = Relative percentage among those infected) (B = Relative percentage among those in each group 20 and 40 mg) (C = Relative percentage with the total number of respondents).
Among the participants, 38 (61.40%) are considered at risk due to their professional activities, of these only 3 people (7.89%) of this group and (4.83%) of the total number of respondents were infected. While the rest 24 respondents did not have risk activities (38.59%), among these there were two infected with SARS CoV-2. (p = 0.35; N.S). In five of the seven infected, their professional activity is considered risky; they are health workers, some of whom have been treating COVID-19 patients throughout the pandemic, and teachers, in permanent contact with the children and/or adolescent population.
In this representative patients samples, we found 151 contacts as risk antecedents, who met the previously indicated criteria, which represents an average contact/respondent of 2.43 contacts/person surveyed. On the other hand, the number of people who had suffered from the COVID-19 disease and were living with the respondents were 102 individuales, which represents an average of 1.64 patients per respondent. Data that are synthesized in the following, for each person surveyed there have been 2.43 contacts and they have lived with 1.64 patients, an aspect that in some way also reflects a certain protective effect of melatonin, without considering the presence of these data in the general population, clearly inferior. The seven patients who finally became infected had an asymptomatic process in 3 cases, mild without the need for medication in two cases, and mild-moderate with the need for medication in another two cases. Finally, after a few days of treatment there was a favorable evolution, without the need for intensive treatment (no patient required admission to the Intensive Care Unit), the condition evolving favorably and with no recognized sequelae to date.
4. DISCUSSION
a. Melatonin and first level prevention.
The first objective of this work was to assess the possible preventive role that melatonin may have against SARS CoV-2 infection. The literature has already pointed out various possibilities and mechanisms that would justify this initial hypothesis. In this sense, it has been described that melatonin constitutes a first line defense mechanism, without the participation of the immune system (16). In fact, melatonin concentrations in the skin and liver are higher than in the serum, where it reaches picomolar levels because it is synthesized by two different pathways (63, 64). Melatonin synthesized in the skin promotes DNA repair and the expression of antioxidant enzymes, in addition, it regulates mitochondrial function through interaction with cytochrome c and the electron transport chain (63).
In the lung, melatonin has the capacity to protect against particles that arrive in suspension (65, 66). Alveolar macrophages participate in this process (65), which could synthesize melatonin when stimulated by airborne biotic and abiotic particles (67). These macrophages are the first defense barrier against the entry of material through inhaled air and are essential for the resolution of pulmonary inflammation (68). In addition, they reduce the deleterious effects of apoptotic neutrophils (69), attenuate the inflammatory response induced by the inoculation of large numbers of bacteria (70), and phagocytize medium particles and microorganisms (62, 64, 71). The melatonin synthesized by these alveolar macrophages probably coordinates the first line of defense and could even be a factor in distinguishing symptomatic from asymptomatic carriers (72). Therefore, it should be considered that the melatonin of these physiological barriers forms part of the first line of defense against infection, without the involvement of the immune system.
Our results must initially be included in this context, since if, in addition to these considerations, we add continuous melatonin supplementation, it is logical to deduce that it is probably synergizing in enhancing these mechanisms against SARS-CoV-2 infection. Although we have found significant differences between the respondents and the general population, in terms of the proportion/percentage of infection, we cannot ignore two important aspects: 1) Our population must be considered at risk due to the age of a considerable number of people, with a mean age of 58.46 years, of which 61.40% (34 cases) are older than 60 years, and 14.51% (9 cases) are older than 70 years. Persons who have also had 35 risk factors as a whole (Table 2), to which must be added the contacts they have had during the study period (2.43 contacts/person), and 2) The data used for the comparison with the Spanish reference population, are clearly lower than the real ones as there are a significant number of cases that have not been recorded , among other reasons, due to the collapse of the health system.
There is increasing evidence about the role that melatonin can play when administered together with vaccines and/or medications, improving the immune response in the case of vaccines and acting synergistically or minimizing certain side effects of some medications. As an immune enhancer in vaccines, melatonin promotes T-cell immunity and plays an immunoregulatory role by inhibiting inflammatory phenomena. Knowing its anti-inflammatory and immunomodulatory properties (73, 74), Wang et al. (10) confirmed that the supplementation of the vaccine against bovine diarrhea virus (BVDV) with melatonin caused the production of T lymphocytes in BALB/c significantly inhibited the NF-κB signaling pathway both in vitro and in vivo. Concluding that melatonin can be used as an immunopotentiator in recombinant vaccines because it has a direct inhibitory effect against the virus in vitro and exhibits the ability to stimulate hosts to produce adequate T lymphocytes to protect against BVDV infection.
In contrast to downregulation of the overreaction of innate immune response, melatonin can enhance the adaptive immune response, including balancing the ratio of T cell populations (75) and increasing the number of B lymphocytes and their antibody titer after vaccination (76). Gurunathan et al. (77) have suggested that melatonin administration could increase the potency of the immune response and the duration of vaccine-induced immunity.
b. Melatonin and second level prevention.
The use of melatonin in the early stage of the COVID-19 disease, as already indicated in Figure 1, is about analyzing what role melatonin can have in the infected patients, to slow down its evolution and avoid tissue damage. Considering the results that we have provided in this work, and other contributions from the literature, the use of melatonin, before and even in the early stages of the disease, could be a beneficial procedure for the patient. This idea is also supported by its low cost and its tolerance to relatively high doses (78-82). Along with the possible role of melatonin in early viral replication, its use in patients with COVID-19 could curb viral infection (83). In the case of SARS-CoV-2 infection, the reduction of the prolonged inflammatory and oxidative stress of the virus by melatonin allow the patient's own immune system to respond appropriately to the infection and recover more efficiently with a reduced recovery time (84).
Another aspect of interest has been its use together with other antiviral drugs such as ribavirin and acyclovir, demonstrating a synergistic effect and a better response to infection (85-88). In addition, in pharmacological therapy, the joint administration with melatonin has been documented that can also improve their efficacy and reduce certain adverse effects (89). With caution in polymedicated patients due to possible interactions and inhibition of cytochrome P450 since melatonin is mainly metabolized by this enzyme (90-92). For all these reasons, it seems logical to consider the possibility of using melatonin for these purposes against the infection of patients with COVID-19, at least we believe there are reasons for this.
c. Melatonin and third level prevention.
At this level of prevention, the role that melatonin plays through its ability to slow down the progress of the disease, avoiding or reducing the possible complications that COVID-19 may generate is discussed. These actions can be carried out by various mechanisms, among which the following stand out:
4.1. Its role as an antioxidant.
An important effect of melatonin in the treatment of patients with COVID-19 is based on its action to reduce tissue and cell damage caused by the virus and its pathophysiological consequences. One of these functions is its potent antioxidant activity, in this sense we know that antioxidants, such as GSH, and the antioxidant enzymatic activity of CAT, SOD, GPx and GR, are the main contributors of total antioxidant capacity (TAC) in human plasma (93). The beneficial effects of melatonin on GSH levels have been confirmed in many studies (94, 96) and are explained by its direct antioxidant and scavenging effect, even more effectively than any other endogenous antioxidants such as GSH (97).
It has been reported that melatonin reduces MDA levels (98-100), an action related to its ability to scavenge free radicals (101), such as the peroxynitrite anion (102), the hydroxyl radical (103), and to detoxify the hydrogen peroxide (104), remove peroxynitrous acid (105), nitric oxide (106) and singlet oxygen (107), which normally contribute to increased MDA levels by elevating lipid peroxidation (108). Melatonin significantly decreases MDA levels (109).
Melatonin improves the activity of the main antioxidant defense enzymes, such as GPx (110, 111) and the activity of the enzyme glucose 6-phosphate dehydrogenase (G6PD) (112-114). Thus, melatonin supplementation may lead to a decrease of DNA damage by increasing the activity of the aforementioned enzymes and increasing ROS scavenging through high GSH levels (115).
Similarly, melatonin administration increases SOD activity as indicated by the elevated mRNA gene expression for both Mn-SOD and Zn-Cu-SOD (116, 117). SOD preserves and protects the mitochondrial membrane from oxidative damage (118, 119) and melatonin increases mitochondrial membrane protection and decreases calcium overload (120). It also has a critical role in mitochondrial physiology through its impact on SOD gene expression and high GSH recycling (121). In conclusion, melatonin administration can increase TAC, GSH levels, and GPx, GR, and SOD activity, and significantly decrease MDA levels (109).
4.2. Melatonin as an anti-inflammatory molecule
Among the different effects exerted by melatonin, its role as an anti-inflammatory molecule through several pathways (122-125), including down-regulation of NF-κB activation in T cells and lung tissue (126, 127) and upregulation of Nrf2 (128). Since inflammation has a direct relationship with cytokine and chemokine produc-tion, it appears that melatonin not only reduces the proinflammatory cytokines TNF-α, IL-1β, IL-6 and IL-8, but also increases level of the anti-inflammatory cytokine IL-10 (129,130). Certainly, it is possible that under certain conditions, such as in immunosuppression or in its excessively high doses melatonin may promotes pro-inflammatory actions to induce the proinflammatory cytokines, IL-1β, IL-2, IL -6, IL-12, TNF-α and IFN-γ (130). However, generally,melatonin exhibits an anti-inflammatory and protective function (131) in models of infection presenting acute lung injury.
As previously mentioned, the use of melatonin attenuates circulating cytokine levels caused by lung tissue damage, this was confirmed by a recent a randomized controlled trial (132). In this work they treated patients with diabetes mellitus and periodontitis, who were orally administered 6 mg/day of melatonin for a period of 8 continuous weeks. This caused a significant decrease in serum levels of IL-6, TNF-α and hs-C reactive protein (hs-CRP) as described by the authors. In patients suffering from severe multiple sclerosis (133), melatonin 25 mg/day was administered orally, for a period of 6 months a significant reduction of serum levels of TNF-α, IL-6, IL-1β and lipoper-oxides was observed (133). In this line,there are different works implemented during the acute phase of inflammation or even during surgical stress (134), cerebral reperfusion (135), and coronary artery reperfusion (136), the melatonin intake at different doses achieved reduction in the levels of proinflammatory cytokines. For a corroboration of all these studies, a meta-analysis consisting of a total of 22 randomized controlled trials was performed and the results showed that the supplementation of melatonin was associated with a significant reduction in the level of TNF-α and IL-6 as clinical evidence (137). It can be derived from this evidence that the use of melatonin as a supplement can effectively reduce the levels of circulating cytokines and, potentially, can also reduce the levels of proinflammatory cytokines in patients with COVID-19.
With these considerations and the data provided in this study seem rather conclusive: we found no deaths or seriously ill patients, and without the need to admit any of the surveyed and infected patients who take melatonin to the Intensive Care Unit. We consider that these are new arguments to recommend, as already indicated, to use melatonin as a drug according to the protocol that has been proposed by these authors (138). Although we have the feeling that higher doses could be even more effective, but these statements we believe are still pending the results of clinical trials that are already underway.
4.3. Immunoregulatory role of melatonin.
The COVID-19 pandemic has made it possible to study melatonin as a pharmacological agent to control the immune response triggered by SARS-CoV-2. Both virus and cell apoptosis trigger and amplify the immune response. Exacerbation of cytokine production, excessive recruitment of immune cells, and uncontrollable epithelial damage create a vicious cycle related to this vitus infection (139). In this context, melatonin can modulate the immune response, on the one hand, by reducing the excessive response of innate immunity and, on the other, by facilitating the response of adaptive immunity (140).
The innate immune system uses receptors for pathogen-associated molecular patterns (PAMPs) that recognize the RNA, DNA, proteins, and lipids of pathogens. These receptors help cells of the innate immune system to eliminate pathogens. A response that has capacity to produce colleteral tissue damage. For example in the case of TLR4 in sepsis, TLR4 recognizes bacterial LPS and participates in the defense of pathogens, but its overactivation can exacerbate the septic condition (141), Same as the TLR-9 and cGAS, which recognize the double-stranded pathogen DNA and their overactivations induce the formation of the NLRP3 inflammasome. In this sense, melatonin can inhibit the overactivation of TLR4, TLR9 and cGAS and balance the innate immune response and consequently reduce tissue damage caused by their overaction (142-145).
The NLRP3 inflammasome is part of the innate immune response during pulmonary infection. The pathogen would trigger the activation of NLRP3 to amplify inflammation. There is probably a balance between the protective and damaging actions of NLRP3 in the lung. In an experiment with mice, inhibition of NLRP3 early in infection increased mortality, while suppression of NLRP3 at peak infection had a protective effect (146). This supports the use of melatonin when inflammation is most intense. The efficacy of melatonin in regulating NLRP3 has been tested in models of radiation-induced lung injury, allergic airway inflammation, and oxidative stress and LPS-induced lung damage, in which melatonin reduced macrophage and neutrophil infiltration into the lung due to inhibition of the NLRP3 inflammasome, (147; 148; 149).
Melatonin also participates directly in the cellular mechanisms of the innate immune response, especially by decreasing its intensity, as occurs with the migration of neutrophils to inflammatory areas. In this sense, the ability of melatonin to block ERK phosphorylation enables the inhibition of neutrophil migration and associated tissue damage (150). Macrophages and mast cells participate in antigen processing, and their exaggerated involvement leads to cytokine storm and subsequent tissue damage. Melatonin receptors have been identified on these cells, indicating that melatonin involves in the physiological functions of these immune cells (124,151), indeed, melatonin treatment down-regulates mast cell activation and it reduces the production of TNF-α and IL-6, and inhibits the IKK/NF-κB signal transduction pathway in activated mast cells (152).
Regarding macrophages, in the evolution of COVID-19 in severely ill patients, there is a depletion of the anti-inflammatory macrophage M2 and an increase in the proinflammatory phenotype M1 (153). Mechanisms that have been shown to be reversed by administration of melatonin (154), such that increased M2 macrophages help cleaning SARS-CoV-2 and suppress the hyperinflammatory response mediated by M1 macrophages (155). Under physiological conditions, melatonin favors the mechanisms of innate immunity (156), without influencing the protective effects of this system during the arrival of pathogens.
Melatonin exerts regulatory actions on the immune system and directly enhances the immune response by enhancing the proliferation and maturation of natural killing cells, T and B lymphocytes, granulocytes, and monocytes in both bone marrow and other tissues (157). Consequently, during viral infection, melatonin reduces the exaggerated response of the innate immune system (158), and secondarily improves the adaptive immune response, including the balance of the ratio of populations of T and B lymphocytes (75, 76).
d. Melatonin and fourth level prevention.
Initially we must consider that melatonin offers an acceptable safety profile, especially when it has been used in specific problems and with defined doses, such as in insomnia, cancer, neurological problems, etc. (44, 46, 47, 91, 159-163). But, as shown in Figure 2, there are several aspects to consider when discussing the possible role that melatonin may play at this level of prevention. Probably the lack of randomized, double-blind clinical trials that more precisely define aspects such as safety, dosage, routes of administration, adaptation to circadian rhythmicity, usefulness in specific diseases, pharmacological interactions, adverse effects, etc., is the fundamental reason why we still do not have specific guidelines for its use in the clinic, except for some contributions such as the protocol recently proposed by Reiter et al. (138).
In any case, the available scientific literature allows us to comment on some of these aspects. In a meta-analysis, the safety of melatonin has been studied when high doses ≥10 mg are used, analyzing the number of adverse events (AEs), serious adverse events (SAEs) and withdrawals due to AE, concluding that melatonin seems to have a good security profile. Across all studies included in the review, there were approximately 913 AEs in 2114 participants receiving melatonin, effects referred to as tiredness, fever, headache, and diarrhea. However, these side effects were also present in the control groups, with 708 AEs reported from a total of 2,258 participants (33). We have found no adverse effects in survey results with much higher doses and in the long term, results that are consistent with a study in trained young athletes taking 100 mg of melatonin daily for 4 weeks (164). In the data that we provide, we have 3 respondents who take 500 mg, 300 mg and 245 mg respectively during the entire period of time that has elapsed without noticing any adverse effect. With respect to the rest of the respondents, only one person has collected a "weight gain", and in no case were they recorded: headaches, fever, tiredness, etc. Results in line with two other well-designed, double-blind, randomized studies with controls in which the toxicity and adverse effects of melatonin have been analyzed (80, 165). In short, and as has already been mentioned in another study, melatonin is safe in the short term, even when administered in high doses (79).
AS drug interactions, melatonin metabolism is primarily mediated by CYP1A enzymes. Therefore, interactions between melatonin and other active ingredients may occur because of their effect on CYP1A. In practice, melatonin is often given as a substitute for drugs with known AD profiles, such as glucocorticoids, opioids, benzodiazepines, and nonsteroidal anti-inflammatory drugs (166).
Pharmacokinetic studies in humans have documented that drugs are metabolized by hepatic CYP (1A2) enzymes, such as fluvoxamine (167), caffeine (168), and oral contraceptives (169), increase plasma melatonin levels after exogenous administration, as does smoking cigarettes (170). In contrast, plasma levels are reduced when combined with carbamazepine, rifampin, and zinc. Considerations that do not seem to have a special repercussion in the clinic, at least they have not been clearly communicated. Melatonin in combination with a hypnotic (Zolpidem) for a few hours affects psychomotor and driving performance, with no pharmacokinetic interactions detected (171).
In clinical trials, melatonin is usually administered as a hypnotic, anxiolytic, analgesic, or anti-inflammatory drug (172, 173). Therefore, the adverse effects and safety profile should be compared with drugs such as benzodiazepines, opiates, nonsteroidal anti-inflammatory drugs (NSAIDs), and corticoids. These drugs induce both short-and long-term adverse effects and complications (174). Benzodiazepines induce amnesia, confusion, dizziness, respiratory dysfunction, and sedation (175). Opioids cause constipation, dizziness, itching, postoperative nausea and vomiting, respiratory dysfunction, and sedation (176). Benzodiazepines and opiates also induce tolerance and may pose a risk of addiction. An aspect that does not happen with melatonin.
Nonsteroidal anti-inflammatory drugs increase the risk of gastrointestinal diseases, bleeding, renal failure, cardiac and cerebral morbidity (177-180), and increase the risk of serious postoperative complications (181). Finally, corticosteroids cause a series of serious adverse effects, including osteoporosis, edema, hyperglycemia, glaucoma, and increased risk of infections (182). In this context, the adverse effects of melatonin described above should be interpreted as mild. However, the unestablished clinical efficacy of melatonin within most indications does not allow it to replace drug regimens at this time, possibly with the exception of pharmacological treatments in sleep disorders and as a preoperative anxiolytic (177, 183, 184).
e. Future implications.
A thorough knowledge of the characteristics of the pharmacokinetics of melatonin (185) and its circadian variation is important for its clinical use. We believe that some of these aspects should already be resolved based on the data listed above and the candidate patients are deserved to test the benificial effects of melatonin. In this sense, it is a fundamental aspect to know the dose to be administered in each pathological process, the route of administration and its fractionation according to circadian variation, so that the dose adjustment can be precise.
It has already been shown that the doses that have been administered are low and certain studies suggest that in certain processes considerably higher doses are required to achieve therapeutic efficacy (186, 187). Current trials are investigating doses of 500 mg IV/d to reduce mortality in COVID-19 patients in the ICU (NCT04568863), as well as 30 mg orally in outpatients with COVID-19, with encouraging preliminary results and no AEs flashy (NCT04474483).
Regarding the results of this study after two years of intake, with no side effects being found, we believe that 20 mg may be useful for primary prevention, at least in exposed contacts and with a higher probability of contracting the infection by the SARS-CoV-2, as well as in people considered at risk due to their characteristics (obesity, hypertension, immunosuppression, cancer, etc.). Once the individual has been infected and the local defense mechanisms cannot stop viral replication and the progress of the disease, the recommended dose should be ≥40 mg, it would be a dosage that allows viral replication to stop, and reach the mitochondria (63, 188). When the disease has progressed and the patient is hospitalized even with intensive care, the proposal by Reiter et al (138) (Figure 4), is undoubtedly a good starting point to progress in the knowledge of the clinical use of melatonin.
Furthermore, it has been argued that treatment of COVID-19 with melatonin, due to the urgency of the pandemic, can be used before clinical trials are conducted, as current knowledge demonstrates its acceptable tolerability (30, 83, 137) in fact there are two double-blind randomized clinical trial protocols using melatonin doses of 5 mg twice daily orally in capsule for 7 days and 5 mg per kg body weight intravenously every 6 hours for 7 days (22. 35). The considerations, which are certainly not definitive, but are along the lines of clarifying the problems described, will undoubtedly become clearer when the results of ongoing clinical trials are provided.

Fig. 4. Proposal for melatonin treatment in patients with COVID-19 based on its severity and clinical course.
Modified from Reiter et al. [137].
f. Conclusions.
Knowing the characteristics of the pharmacokinetics of melatonin (185) and its circadian variation, we believe that there are aspects that should already be resolved and candidate patients should be given melatonin for its benificial effects. In this sense, it is important to know the dose that must be given in each pathological process, the route of administration and its fractionation based on the circadian variation.
ACKNOWLEDGMENTS
We thank the participants in the survey, for their contributions. Funding: N.A.
AUTHORSHIP
The concept of the article was developed by AMC, AMH. Moreover, AJC; MLFL and MCAM contributed to colect data and in drafting the manuscript, prepared the figures, and edited it. AMC, AMH and AA revised the manuscript critically and finally approved it.
CONFLICTS OF INTEREST:
Authors declare no condlicts of interests.
REFERENCES
Levels of Prevention: Health Education, Advocacy and Community Mobilization Chapter: 4. Human Behaviour and Health: 1.
Porta M (2014) A dictionary of epidemiology: Oxford University Press.
Leavel HR, Clark EG (1965) Preventive Medicine for the Doctor in His Community. New York, NY: McGraw-Hill.
Chen Y, Zhou R, Chen B et al. (2020) Knowledge, perceived beliefs, and preventive behaviors related to COVID-19 among chinese older adults: Cross-sectional web-based survey. J. Med. Internet. Res. 22: e23729.
Li Y, Li L, Niu YC et al. (2020) Several considerations on the establishment of a new public health and preventive medicine system in national level. Zhonghua Yu Fang Yi Xue Za Zhi 54: 469-474.
Cross KM, Landis DM, Sehgal L et al. (2021) Melatonin for the early treatment of COVID-19: A narrative review of current evidence and possible efficacy. Endocr. Pract. 27: 850-855.
Regodón S, Martín-Palomino P, Fernández-Montesinos R et al. (2005) The use of melatonin as a vaccine agent. Vaccine 23: 5321-5327.
Regodón S, Ramos A, Míguez MP et al. (2012) Vaccination prepartum enhances the beneficial effects of melatonin on the immune response and reduces platelet responsiveness in sheep. BMC Vet. Res. 8: 84.
Regodón S, Ramos A, Morgado S et al. (2009) Melatonin enhances the immune response to vaccination against A1 and C strains of Dichelobacter nodosus. Vaccine 27: 1566-1570.
Wang YX, Yang GH, Zhang LL et al. (2021) Melatonin as immune potentiator for enhancing subunit vaccine efficacy against bovine viral diarrhea virus. Vaccines (Basel) 9: 1030.
Cardinali DP, Brown GM, Pandi-Perumal SR (2020) Can Melatonin Be a Potential "Silver Bullet" in Treating COVID-19 Patients? Diseases 8: 44.
Kow CS, Ramachandram DS, Hasan SS (2021) Melatonin: Revisited role as vaccine adjuvant during outbreaks of COVID-19 caused by the delta variant. J. Neuroimmune Pharmacol. 17: 425-426.
Guerrero JM, Reiter RJ (2002) Melatonin-immune system relationships. Curr. Top. Med. Chem. 2: 167-179.
Csaba G (2013) The pineal regulation of the immune system: 40 years since the discovery. Acta Microbiol. Immunol. Hung. 60: 77-91.
Carrillo-Vico A, Reiter RJ, Lardone PJ et al. (2006) The modulatory role of melatonin on immune responsiveness. Curr. Opin. Investig. Drugs 7: 423-431.
Markus RP, Sousa KS, da Silveira Cruz-Machado S et al. (2021) Possible role of pineal and extra-pineal melatonin in surveillance, immunity, and first-line defense. Int. J. Mol. Sci. 22: 12143.
Huggard D, Kelly L, Worrall A et al. (2021) Melatonin as an immunomodulator in children with Down syndrome. Pediatr. Res. 91: 1812-1820.
Ramos González EJ, Ramirez Jirano LJ, García Martínez DZ et al. (2021) A comparative study of melatonin and immunomodulatory therapy with interferon beta and glatiramer acetate in a mouse model of multiple sclerosis. Neurologia (Engl Ed) 36: 262-270.
Cernysiov V, Gerasimcik N, Mauricas M et al. (2010) Regulation of T-cell-independent and T-cell-dependent antibody production by circadian rhythm and melatonin. Int. Immunol. 22: 25-34.
NaveenKumar SK, Hemshekhar M, Jagadish S et al. (2020) Melatonin restores neutrophil functions and prevents apoptosis amid dysfunctional glutathione redox system. J. Pineal Res. 69: e12676.
Sánchez-González M, Mahíllo-Fernández I, Villar-Álvarez F et al. (2022) What if melatonin could help patients with severe COVID-19? J. Clin. Sleep Med. 18: 335-336.
Ameri A, Asadi MF, Kamali M et al. (2021) Evaluation of the effect of melatonin in patients with COVID-19-induced pneumonia admitted to the Intensive Care Unit: A structured summary of a study protocol for a randomized controlled trial. Trials 22: 194.
Tan DX, Reiter RJ (2022) Mechanisms and clinical evidence to support melatonin's use in severe COVID-19 patients to lower mortality. Life Sci, 294: 120368.
Ramlall V, Zucker J, Tatonetti N (2020) Melatonin is significantly associated with survival of intubated COVID-19 patients. medRxiv. PMID: 33083812.
Chitimus DM, Popescu MR, Voiculescu SE et al. (2020) Melatonin's impact on antioxidative and anti-inflammatory reprogramming in homeostasis and disease. Biomolecules 10: 1211.
Nair AS (2022) Perioperative melatonin in COVID-19 patients: benefits beyond sedation and analgesia. Med. Gas Res. 12: 41-43.
Mousavi SA, Heydari K, Mehravaran H et al. (2022) Melatonin effects on sleep quality and outcomes of COVID-19 patients: An open-label, randomized, controlled trial. J. Med. Virol. 94: 263-271.
Shchetinin E, Baturin V, Arushanyan E et al. (2022) Potential and possible therapeutic effects of melatonin on SARS-CoV-2 infection. Antioxidants (Basel) 11: 140.
Hasan ZT, Atrakji D, Mehuaiden DAK (2022) The Effect of Melatonin on thrombosis, sepsis and mortality rate in COVID-19 patients. Int. J. Infect. Dis. 114: 79-84.
El-Missiry MA, El-Missiry ZMA, Othman AI (2020) Melatonin is a potential adjuvant to improve clinical outcomes in individuals with obesity and diabetes with coexistence of Covid-19. Eur. J. Pharmacol. 882: 173329.
Sileri P, Sica GS, Gentileschi P et al. (2004) Melatonin reduces bacterial translocation after intestinal ischemia-reperfusion injury. Transplant. Proc. 36: 2944-2946.
Parlakpinar H, Polat S, Acet HA (2021) Pharmacological agents under investigation in the treatment of coronavirus disease 2019 and the importance of melatonin. Fundam. Clin. Pharmacol. 35: 62-75.
Menczel Schrire Z, Phillips CL, Chapman JL et al. (2022) Safety of higher doses of melatonin in adults: A systematic review and meta-analysis. J. Pineal Res. 72: e12782.
Acuña-Castroviejo D, Escames G, Figueira JC et al. (2020) Clinical trial to test the efficacy of melatonin in COVID-19. J. Pineal Res. 69: e12683.
Rodríguez-Rubio M, Figueira JC, Acuña-Castroviejo D et al. (2020) A phase II, single-center, double-blind, randomized placebo-controlled trial to explore the efficacy and safety of intravenous melatonin in patients with COVID-19 admitted to the intensive care unit (MelCOVID study): a structured summary of a study protocol for a randomized controlled trial. Trials 21: 699.
Ziaei A, Davoodian P, Dadvand H et al. (2020) Evaluation of the efficacy and safety of Melatonin in moderately ill patients with COVID-19: A structured summary of a study protocol for a randomized controlled trial. Trials 21: 882.
Cardinali DP (2021) Melatonin and healthy aging. Vitam. Horm. 115: 67-88.
Zhang R, Wang X, Ni L et al. (2020) COVID-19: Melatonin as a potential adjuvant treatment. Life Sci. 250: 117583.
Galano A, Tan DX, Reiter RJ (2011) Melatonin as a natural ally against oxidative stress: a physicochemical examination. J. Pineal Res. 51: 1-16.
Reiter RJ (1997) Aging and oxygen toxicity: Relation to changes in melatonin. Age (Omaha) 20: 201-213.
Reiter R, Tang L, Garcia JJ et al. (1997) Pharmacological actions of melatonin in oxygen radical pathophysiology. Life Sci. 60: 2255-2271.
Liu Z, Gan L, Xu Y et al. (2017) Melatonin alleviates inflammasome-induced pyroptosis through inhibiting NF-κB/GSDMD signal in mice adipose tissue. J. Pineal Res. 63: e12414.
Touitou Y, Reinberg A, Touitou D (2017) Association between light at night, melatonin secretion, sleep deprivation, and the internal clock: Health impacts and mechanisms of circadian disruption. Life Sci. 173: 94-106.
Chahbouni M, López MDS, Molina-Carballo A et al. (2017) Melatonin treatment reduces oxidative damage and normalizes plasma pro-inflammatory cytokines in patients suffering from charcot-marie-tooth neuropathy: A pilot study in three children. Molecules 22: 1728.
Uberos J, Augustin-Morales MC, Molina Carballo A et al. (2011) Normalization of the sleep-wake pattern and melatonin and 6-sulphatoxy-melatonin levels after a therapeutic trial with melatonin in children with severe epilepsy. J. Pineal Res. 50: 192-196.
Muñoz-Hoyos A, Sánchez-Forte M, Molina-Carballo A et al. (1998) Melatonin's role as an anticonvulsant and neuronal protector: experimental and clinical evidence. J. Child. Neurol. 13: 501-509.
Acuña-Castroviejo D, Escames G, Macías M et al. (1995) Cell protective role of melatonin in the brain. J. Pineal Res. 19: 57-63.
Liu H, Wang F, Zhao J et al. (2022) The effect and mechanisms of melatonin on the proliferation and apoptosis of lung cancer cells. Bioengineered 13: 3462-3469.
Muñoz A, Palomo EJ, Jerez-Calero A (2019) Use of an ANN to value MTF and melatonin effect on ADHD affected children. IEEE Access 7: 127254-127264.
Checa-Ros A, Muñoz-Hoyos A, Molina-Carballo A et al. (2017) Analysis of different melatonin secretion patterns in children with sleep disorders: melatonin secretion patterns in children. J. Child. Neurol. 32: 1000-1008.
Checa-Ros A, Muñoz-Gallego A, Muñoz-Gallego M et al. (2018) Clinical considerations derived from the administration of melatonin to children with sleep disorders. Pediatr. Neurol. 78: 61-69.
Zhao D, Yu Y, Shen Y et al. (2019) Melatonin synthesis and function: evolutionary history in animals and plants. Front. Endocrinol. (Lausanne) 10: 249.
53. Reiter RJ (1991) Pineal melatonin: cell biology of its synthesis and of its physiological interactions. Endocr. Rev. 12: 151-180.
Tan DX, Manchester LC, Reiter RJ et al. (1999) Identification of highly elevated levels of melatonin in bone marrow: its origin and significance. Biochim. Biophys. Acta 1472: 206-214.
Acuña-Castroviejo D, Pablos MI, Menendez-Pelaez A et al. (1993) Melatonin receptors in purified cell nuclei of liver. Res. Commun. Chem. Pathol. Pharmacol. 82: 253-256.
Acuña Castroviejo D, Escames G, Carazo A et al. (2002) Melatonin, mitochondrial homeostasis and mitochondrial-related diseases. Curr. Top. Med. Chem. 2: 133-151.
Morgan PJ, Barrett P, Howell HE et al. (1994) Melatonin receptors: localization, molecular pharmacology and physiological significance. Neurochem. Int. 24: 101-146.
Dubocovich ML (1995) Melatonin receptors: are there multiple subtypes? Trends Pharmacol. Sci. 16: 50-56.
Benítez-King G, Antón-Tay F (1993) Calmodulin mediates melatonin cytoskeletal effects. Experientia 49: 635-641.
Becker-André M, Wiesenberg I, Schaeren-Wiemers N et al. (1994) Pineal gland hormone melatonin binds and activates an orphan of the nuclear receptor superfamily. J. Biol. Chem. 269: 28531-28534.
Ekmekcioglu C (2006) Melatonin receptors in humans: biological role and clinical relevance. Biomed. Pharmacother. 60: 97-108.
He F, Wu X, Zhang Q et al. (2021) Bacteriostatic potential of melatonin: therapeutic standing and mechanistic insights. Front. Immunol. 12: 683879.
Reiter RJ, Rosales-Corral S, Tan DX et al. (2017) Melatonin as a mitochondria-targeted antioxidant: one of evolution's best ideas. Cell Mol. Life Sci. 74: 3863-3881.
Bubenik GA (2001) Localization, physiological significance and possible clinical implication of gastrointestinal melatonin. Biol. Signals Recept. 10: 350-366.
Carvalho-Sousa CE, Pereira EP, Kinker GS et al. (2020) Immune-pineal axis protects rat lungs exposed to polluted air. J. Pineal Res. 68: e12636.
Woo YD, Jeong D, Chung DH (2021) Development and functions of alveolar macrophages. Mol. Cells 44: 292-300.
Rubins JB (2003) Alveolar macrophages: wielding the double-edged sword of inflammation. Am. J. Respir. Crit. Care Med. 167: 103-104.
Schagat TL, Wofford JA, Wright JR (2001) Surfactant protein A enhances alveolar macrophage phagocytosis of apoptotic neutrophils. J. Immunol. 166: 2727-2733.
Knapp S, Leemans JC, Florquin S et al. (2003) Alveolar macrophages have a protective antiinflammatory role during murine pneumococcal pneumonia. Am. J. Respir. Crit. Care Med. 167: 171-179.
Gibbs JL, Dallon BW, Lewis JB et al. (2019) Diesel exhaust particle exposure compromises alveolar macrophage mitochondrial bioenergetics. Int. J. Mol. Sci. 20: 5598.
Slominski A, Tobin DJ, Zmijewski MA et al. (2008) Melatonin in the skin: synthesis, metabolism and functions. Trends Endocrinol. Metab. 19: 17-24.
Fernandes PA, Kinker GS, Navarro BV et al. (2021) Melatonin-Index as a biomarker for predicting the distribution of presymptomatic and asymptomatic SARS-CoV-2 carriers. Melatonin Res, 4: 189-205.
Xia D, Yang L, Li Y et al. (2021) Melatonin alleviates Ochratoxin A-induced liver inflammation involved intestinal microbiota homeostasis and microbiota-independent manner. J. Hazard. Mater. 413: 125239.
Bonmati-Carrion MA, Tomas-Loba A (2021) Melatonin and cancer: A polyhedral network where the source matters. Antioxidants (Basel) 10: 210.
Castrillón PO, Esquifino AI, Varas A et al. (2000) Effect of melatonin treatment on 24-h variations in responses to mitogens and lymphocyte subset populations in rat submaxillary lymph nodes. J. Neuroendocrinol. 12: 758-765.
Ramos A, Míguez MP, Morgado S et al. (2018) Melatonin enhances responsiveness to Dichelobacter nodosus vaccine in sheep and increases peripheral blood CD4 T lymphocytes and IgG-expressing B lymphocytes. Vet. Immunol. Immunopathol. 206: 1-8.
Gurunathan S, Kang M-H, Choi Y et al. (2021) Melatonin: A potential therapeutic agent against COVID-19. Melatonin Res, 4: 30-69.
Shiu SY, Reiter RJ, Tan DX et al. (2003) Urgent search for safe and effective treatments of severe acute respiratory syndrome: is melatonin a promising candidate drug? J. Pineal Res. 35: 69-70.
Andersen LP, Gögenur I, Rosenberg J et al. (2016) The safety of melatonin in humans. Clin. Drug Investig. 36: 169-175.
Seabra ML, Bignotto M, Pinto LR, Jr. et al. (2000) Randomized, double-blind clinical trial, controlled with placebo, of the toxicology of chronic melatonin treatment. J. Pineal Res. 29: 193-200.
Citera G, Arias MA, Maldonado-Cocco JA et al. (2000) The effect of melatonin in patients with fibromyalgia: a pilot study. Clin. Rheumatol. 19: 9-13.
Sánchez-Barceló EJ, Mediavilla MD, Tan DX et al. (2010) Clinical uses of melatonin: evaluation of human trials. Curr. Med. Chem. 17: 2070-2095.
Sehirli AO, Sayiner S, Serakinci N (2020) Role of melatonin in the treatment of COVID-19; as an adjuvant through cluster differentiation 147 (CD147). Mol. Biol. Rep. 47: 8229-8233.
Maestroni GJ (1993) The immunoneuroendocrine role of melatonin. J. Pineal Res. 14: 1-10.
Huang SH, Cao XJ, Liu W et al. (2010) Inhibitory effect of melatonin on lung oxidative stress induced by respiratory syncytial virus infection in mice. J. Pineal Res. 48: 109-116.
Cardinali DP, Brown GM, Reiter RJ et al. (2020) Elderly as a high-risk group during COVID-19 pandemic: Effect of circadian misalignment, sleep dysregulation and melatonin administration. sleep vigil. 4: 81-87.
Nunes Oda S, Pereira Rde S (2008) Regression of herpes viral infection symptoms using melatonin and SB-73: comparison with Acyclovir. J. Pineal Res. 44: 373-378.
Boga JA, Coto-Montes A, Rosales-Corral SA et al. (2012) Beneficial actions of melatonin in the management of viral infections: a new use for this "molecular handyman"? Rev. Med. Virol. 22: 323-338.
Reiter RJ, Tan DX, Sainz RM et al. (2002) Melatonin: reducing the toxicity and increasing the efficacy of drugs. J. Pharm. Pharmacol. 54: 1299-1321.
Balduini W, Weiss MD, Carloni S et al. (2019) Melatonin pharmacokinetics and dose extrapolation after enteral infusion in neonates subjected to hypothermia. J. Pineal Res. 66: e12565.
Besag FMC, Vasey MJ, Lao KSJ et al. (2019) Adverse events associated with melatonin for the treatment of primary or secondary sleep disorders: A systematic review. CNS Drugs 33: 1167-1186.
Artigas L, Coma M, Matos-Filipe P et al. (2020) In-silico drug repurposing study predicts the combination of pirfenidone and melatonin as a promising candidate therapy to reduce SARS-CoV-2 infection progression and respiratory distress caused by cytokine storm. PLoS One 15: e0240149.
Yeum KJ, Russell RM, Krinsky NI et al. (2004) Biomarkers of antioxidant capacity in the hydrophilic and lipophilic compartments of human plasma. Arch. Biochem. Biophys. 430: 97-103.
Jamilian M, Foroozanfard F, Mirhosseini N et al. (2019) Effects of melatonin supplementation on hormonal, inflammatory, genetic, and oxidative stress parameters in women with polycystic ovary syndrome. Front. Endocrinol. (Lausanne) 10: 273.
Daneshvar Kakhaki R, Ostadmohammadi V, Kouchaki E et al. (2020) Melatonin supplementation and the effects on clinical and metabolic status in Parkinson's disease: A randomized, double-blind, placebo-controlled trial. Clin. Neurol. Neurosurg. 195: 105878.
Estaras M, Moreno N, Santofimia-Castaño P et al. (2019) Melatonin induces reactive oxygen species generation and changes in glutathione levels and reduces viability in human pancreatic stellate cells. J. Physiol. Biochem. 75: 185-197.
Fischer TW, Scholz G, Knöll B et al. (2004) Melatonin suppresses reactive oxygen species induced by UV irradiation in leukocytes. J. Pineal Res. 37: 107-112.
Fulia F, Gitto E, Cuzzocrea S et al. (2001) Increased levels of malondialdehyde and nitrite/nitrate in the blood of asphyxiated newborns: reduction by melatonin. J. Pineal Res. 31: 343-349.
Allegra M, Gentile C, Tesoriere L et al. (2002) Protective effect of melatonin against cytotoxic actions of malondialdehyde: an in vitro study on human erythrocytes. J. Pineal Res. 32: 187-193.
Ziaadini F, Aminae M, Rastegar MM et al. (2017) Melatonin supplementation decreases aerobic exercise training induced-lipid peroxidation and malondialdehyde in sedentary young women. Polish J. Food Nutr. Sci. 67: 225-232.
Reina M, Martínez A (2018) A new free radical scavenging cascade involving melatonin and three of its metabolites (3OHM, AFMK and AMK). Computational Theoretical Chem. 1123: 111-118.
Goc Z, Szaroma W, Kapusta E et al. (2017) Protective effects of melatonin on the activity of SOD, CAT, GSH-Px and GSH content in organs of mice after administration of SNP. Chin. J. Physiol. 60: 1-10.
Purushothaman A, Sheeja AA, Janardanan D (2020) Hydroxyl radical scavenging activity of melatonin and its related indolamines. Free Radic. Res. 54: 373-383.
Mehrzadi S, Safa M, Kamrava SK et al. (2017) Protective mechanisms of melatonin against hydrogen-peroxide-induced toxicity in human bone-marrow-derived mesenchymal stem cells. Can. J. Physiol. Pharmacol. 95: 773-786.
Loren P, Sánchez R, Arias ME et al. (2017) Melatonin scavenger properties against oxidative and nitrosative stress: impact on gamete handling and in vitro embryo production in humans and other mammals. Int. J. Mol. Sci. 18: 1119.
Fan W, He Y, Guan X et al. (2018) Involvement of the nitric oxide in melatonin-mediated protection against injury. Life Sci. 200: 142-147.
Hardeland R (2017) Taxon- and Site-specific melatonin catabolism. Molecules 22: 2015.
Ayala A, Muñoz MF, Argüelles S (2014) Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell Longev. 2014: 360438.
Morvaridzadeh M, Sadeghi E, Agah S et al. (2020) Effect of melatonin supplementation on oxidative stress parameters: A systematic review and meta-analysis. Pharmacol. Res. 161: 105210.
Erat M, Ciftci M (2006) Effect of melatonin on enzyme activities of glutathione reductase from human erythrocytes in vitro and from rat erythrocytes in vivo. Eur. J. Pharmacol. 537: 59-63.
Fischer TW, Kleszczyński K, Hardkop LH et al. (2013) Melatonin enhances antioxidative enzyme gene expression (CAT, GPx, SOD), prevents their UVR-induced depletion, and protects against the formation of DNA damage (8-hydroxy-2'-deoxyguanosine) in ex vivo human skin. J. Pineal Res. 54: 303-312.
Pierrefiche G, Laborit H (1995) Oxygen free radicals, melatonin, and aging. Exp. Gerontol. 30: 213-227.
Ciftçi M, Bilici D, Küfrevioğlu OI (2001) Effects of melatonin on enzyme activities of glucose-6-phosphate dehydrogenase from human erythrocytes in vitro and from rat erythrocytes in vivo. Pharmacol. Res. 44: 7-11.
Gupta M, Gupta YK, Agarwal S et al. (2004) A randomized, double-blind, placebo controlled trial of melatonin add-on therapy in epileptic children on valproate monotherapy: effect on glutathione peroxidase and glutathione reductase enzymes. Br. J. Clin. Pharmacol. 58: 542-547.
Okatani Y, Wakatsuki A, Shinohara K et al. (2001) Melatonin stimulates glutathione peroxidase activity in human chorion. J. Pineal Res. 30: 199-205.
Sharafati-Chaleshtori R, Shirzad H, Rafieian-Kopaei M et al. (2017) Melatonin and human mitochondrial diseases. J. Res. Med. Sci. 22: 2.
Ozturk G, Coşkun S, Erbaş D et al. (2000) The effect of melatonin on liver superoxide dismutase activity, serum nitrate and thyroid hormone levels. Jpn. J. Physiol. 50: 149-153.
Madsen-Bouterse SA, Zhong Q, Mohammad G et al. (2010) Oxidative damage of mitochondrial DNA in diabetes and its protection by manganese superoxide dismutase. Free Radic. Res. 44: 313-321.
Majima HJ, Oberley TD, Furukawa K et al. (1998) Prevention of mitochondrial injury by manganese superoxide dismutase reveals a primary mechanism for alkaline-induced cell death. J. Biol. Chem. 273: 8217-8224.
Suwanjang W, Abramov AY, Charngkaew K et al. (2016) Melatonin prevents cytosolic calcium overload, mitochondrial damage and cell death due to toxically high doses of dexamethasone-induced oxidative stress in human neuroblastoma SH-SY5Y cells. Neurochem. Int .97: 34-41.
Genario R, Morello E, Bueno AA et al. (2019) The usefulness of melatonin in the field of obstetrics and gynecology. Pharmacol. Res. 147: 104337.
Hardeland R (2018) Melatonin and inflammation-story of a double-edged blade. J. Pineal Res. 65: e12525.
Wang QL, Yang L, Peng Y et al. (2019) Ginsenoside Rg1 regulates SIRT1 to ameliorate sepsis-induced lung inflammation and injury via inhibiting endoplasmic reticulum stress and inflammation. Mediators Inflamm. 2019: 6453296.
Sun CK, Lee FY, Kao YH et al. (2015) Systemic combined melatonin-mitochondria treatment improves acute respiratory distress syndrome in the rat. J. Pineal Res. 58: 137-150.
Ling Y, Li ZZ, Zhang JF et al. (2018) MicroRNA-494 inhibition alleviates acute lung injury through Nrf2 signaling pathway via NQO1 in sepsis-associated acute respiratory distress syndrome. Life Sci. 210: 1-8.
Pedrosa AM, Weinlich R, Mognol GP et al. (2010) Melatonin protects CD4+ T cells from activation-induced cell death by blocking NFAT-mediated CD95 ligand upregulation. J. Immunol. 184: 3487-3494.
Shang Y, Xu SP, Wu Y et al. (2009) Melatonin reduces acute lung injury in endotoxemic rats. Chin. Med. J. (Engl) 122: 1388-1393.
Ahmadi Z, Ashrafizadeh M (2020) Melatonin as a potential modulator of Nrf2. Fundam. Clin. Pharmacol. 34: 11-19.
Habtemariam S, Daglia M, Sureda A et al. (2017) Melatonin and respiratory diseases: A review. Curr. Top. Med. Chem. 17: 467-488.
Hardeland R (2019) Aging, melatonin, and the pro- and anti-inflammatory networks. Int. J. Mol. Sci. 20: 1223.
Carrascal L, Nunez-Abades P, Ayala A et al. (2018) Role of melatonin in the inflammatory process and its therapeutic potential. Curr. Pharm. Des. 24: 1563-1588.
Bazyar H, Gholinezhad H, Moradi L et al. (2019) The effects of melatonin supplementation in adjunct with non-surgical periodontal therapy on periodontal status, serum melatonin and inflammatory markers in type 2 diabetes mellitus patients with chronic periodontitis: a double-blind, placebo-controlled trial. Inflammopharmacology 27: 67-76.
Sánchez-López AL, Ortiz GG, Pacheco-Moises FP et al. (2018) Efficacy of melatonin on serum pro-inflammatory cytokines and oxidative stress markers in relapsing remitting multiple sclerosis. Arch. Med. Res. 49: 391-398.
Kücükakin B, Lykkesfeldt J, Nielsen HJ et al. (2008) Utility of melatonin to treat surgical stress after major vascular surgery--a safety study. J. Pineal Res. 44: 426-431.
Zhao Z, Lu C, Li T et al. (2018) The protective effect of melatonin on brain ischemia and reperfusion in rats and humans: In vivo assessment and a randomized controlled trial. J. Pineal Res. 65: e12521.
Shafiei E, Bahtoei M, Raj P et al. (2018) Effects of N-acetyl cysteine and melatonin on early reperfusion injury in patients undergoing coronary artery bypass grafting: A randomized, open-labeled, placebo-controlled trial. Medicine (Baltimore) 97: e11383.
Zarezadeh M, Khorshidi M, Emami M et al. (2020) Melatonin supplementation and pro-inflammatory mediators: a systematic review and meta-analysis of clinical trials. Eur. J. Nutr. 59: 1803-1813.
Reiter RJ, Abreu-Gonzalez P, Marik PE et al. (2020) Therapeutic algorithm for use of melatonin in patients with COVID-19. Front. Med. (Lausanne) 7: 226.
Yang CY, Chen CS, Yiang GT et al. (2018) New insights into the immune molecular regulation of the pathogenesis of acute respiratory distress syndrome. Int. J. Mol. Sci. 19: 588.
Tan DX, Hardeland R (2020) Targeting host defense system and rescuing compromised mitochondria to increase tolerance against pathogens by melatonin may impact outcome of deadly virus infection pertinent to COVID-19. Molecules 25: 4410.
Sygitowicz G, Sitkiewicz D (2020) Molecular mechanisms of organ damage in sepsis: an overview. Braz J. Infect. Dis. 24: 552-560.
Luo J, Song J, Zhang H et al. (2018) Melatonin mediated Foxp3-downregulation decreases cytokines production via the TLR2 and TLR4 pathways in H. pylori infected mice. Int. Immunopharmacol. 64: 116-122.
Jauhari A, Baranov SV, Suofu Y et al. (2020) Melatonin inhibits cytosolic mitochondrial DNA-induced neuroinflammatory signaling in accelerated aging and neurodegeneration. J. Clin. Invest. 130: 3124-3136.
Xu X, Wang G, Ai L et al. (2018) Melatonin suppresses TLR9-triggered proinflammatory cytokine production in macrophages by inhibiting ERK1/2 and AKT activation. Sci. Rep. 8: 15579.
Feng R, Adeniran SO, Huang F et al. (2022) The ameliorative effect of melatonin on LPS-induced Sertoli cells inflammatory and tight junctions damage via suppression of the TLR4/MyD88/NF-κB signaling pathway in newborn calf. Theriogenology 179: 103-116.
Tate MD, Ong JDH, Dowling JK et al. (2016) Reassessing the role of the NLRP3 inflammasome during pathogenic influenza A virus infection via temporal inhibition. Sci. Rep. 6: 27912.
Wu X, Ji H, Wang Y et al. (2019) Melatonin alleviates radiation-induced lung injury via regulation of miR-30e/NLRP3 axis. Oxid. Med. Cell Longev. 2019: 4087298.
Wu HM, Xie QM, Zhao CC et al. (2019) Melatonin biosynthesis restored by CpG oligodeoxynucleotides attenuates allergic airway inflammation via regulating NLRP3 inflammasome. Life Sci. 239: 117067.
Zhang Y, Li X, Grailer JJ et al. (2016) Melatonin alleviates acute lung injury through inhibiting the NLRP3 inflammasome. J. Pineal Res. 60: 405-414.
Ren DL, Sun AA, Li YJ et al. (2015) Exogenous melatonin inhibits neutrophil migration through suppression of ERK activation. J. Endocrinol. 227: 49-60.
Maldonado MD, Mora-Santos M, Naji L et al. (2010) Evidence of melatonin synthesis and release by mast cells. Possible modulatory role on inflammation. Pharmacol. Res. 62: 282-287.
Muxel SM, Pires-Lapa MA, Monteiro AW et al. (2012) NF-κB drives the synthesis of melatonin in RAW 264.7 macrophages by inducing the transcription of the arylalkylamine-N-acetyltransferase (AA-NAT) gene. PLoS One 7: e52010.
Maldonado MD, García-Moreno H, González-Yanes C et al. (2016) Possible involvement of the inhibition of NF-κB factor in anti-inflammatory actions that melatonin exerts on mast cells. J. Cell Biochem. 117: 1926-1933.
Hasan MZ, Islam S, Matsumoto K et al. (2021) Meta-analysis of single-cell RNA-seq data reveals phenotypic switching of immune cells in severe COVID-19 patients. Comput. Biol. Med. 137: 104792.
Reiter RJ, Sharma R, Ma Q et al. (2020) Plasticity of glucose metabolism in activated immune cells: advantages for melatonin inhibition of COVID-19 disease. Melatonin Res. 3,:362–379.
Duan F, Guo L, Yang L et al. (2020) Modeling COVID-19 with human pluripotent stem cell-derived cells reveals synergistic effects of anti-inflammatory macrophages with ACE2 inhibition Against SARS-CoV-2. Res. Sq. 3: rs-62758.
Cuesta A, Cerezuela R, Esteban MA et al. (2008) In vivo actions of melatonin on the innate immune parameters in the teleost fish gilthead seabream. J. Pineal Res. 45: 70-78.
Crespo I, Fernández-Palanca P, San-Miguel B et al. (2020) Melatonin modulates mitophagy, innate immunity and circadian clocks in a model of viral-induced fulminant hepatic failure. J. Cell Mol. Med. 24: 7625-7636.
Foley HM, Steel AE (2019) Adverse events associated with oral administration of melatonin: A critical systematic review of clinical evidence. Complement. Ther. Med. 42: 65-81.
Mills E, Wu P, Seely D et al. (2005) Melatonin in the treatment of cancer: a systematic review of randomized controlled trials and meta-analysis. J. Pineal Res. 39: 360-366.
Herxheimer A, Petrie KJ (2002) Melatonin for the prevention and treatment of jet lag. Cochrane Database Syst. Rev: Cd001520.
Alghamdi BS (2018) The neuroprotective role of melatonin in neurological disorders. J. Neurosci. Res. 96: 1136-1149.
Jerez-Calero A, Salvatierra-Cuenca MT, Benitez-Feliponi Á et al. (2020) Hypothermia plus melatonin in asphyctic newborns: A randomized-controlled pilot study. Pediatr. Crit. Care Med. 21: 647-655.
Leonardo-Mendonça RC, Martinez-Nicolas A, de Teresa Galván C et al. (2015) The benefits of four weeks of melatonin treatment on circadian patterns in resistance-trained athletes. Chronobiol. Int. 32: 1125-1134.
Jahnke G, Marr M, Myers C et al. (1999) Maternal and developmental toxicity evaluation of melatonin administered orally to pregnant Sprague-Dawley rats. Toxicol. Sci. 50: 271-279.
Cardinali DP, Srinivasan V, Brzezinski A et al. (2012) Melatonin and its analogs in insomnia and depression. J. Pineal Res. 52: 365-375.
Härtter S, Nordmark A, Rose DM et al. (2003) Effects of caffeine intake on the pharmacokinetics of melatonin, a probe drug for CYP1A2 activity. Br. J. Clin. Pharmacol. 56: 679-682.
Härtter S, Grözinger M, Weigmann H et al. (2000) Increased bioavailability of oral melatonin after fluvoxamine coadministration. Clin. Pharmacol. Ther. 67: 1-6.
Hilli J, Korhonen T, Turpeinen M et al. (2008) The effect of oral contraceptives on the pharmacokinetics of melatonin in healthy subjects with CYP1A2 g.-163C>A polymorphism. J. Clin. Pharmacol. 48: 986-994.
Ursing C, von Bahr C, Brismar K et al. (2005) Influence of cigarette smoking on melatonin levels in man. Eur. J. Clin. Pharmacol. 61: 197-201.
Otmani S, Demazières A, Staner C et al. (2008) Effects of prolonged-release melatonin, zolpidem, and their combination on psychomotor functions, memory recall, and driving skills in healthy middle aged and elderly volunteers. Hum. Psychopharmacol. 23: 693-705.
Andersen LP, Werner MU, Rosenberg J et al. (2014) A systematic review of peri-operative melatonin. Anaesthesia 69: 1163-1171.
Brzezinski A, Vangel MG, Wurtman RJ et al. (2005) Effects of exogenous melatonin on sleep: a meta-analysis. Sleep Med. Rev. 9: 41-50.
Kehlet H, Wilmore DW (2008) Evidence-based surgical care and the evolution of fast-track surgery. Ann. Surg. 248: 189-198.
Lader M (2014) Benzodiazepine harm: how can it be reduced? Br. J. Clin. Pharmacol. 77: 295-301.
Kehlet H (2005) Postoperative opioid sparing to hasten recovery: what are the issues? Anesthesiology 102: 1083-1085.
Ingrasciotta Y, Sultana J, Giorgianni F et al. (2015) Association of individual non-steroidal anti-inflammatory drugs and chronic kidney disease: a population-based case control study. PLoS One 10: e0122899.
Lanas Á, Carrera-Lasfuentes P, Arguedas Y et al. (2015) Risk of upper and lower gastrointestinal bleeding in patients taking nonsteroidal anti-inflammatory drugs, antiplatelet agents, or anticoagulants. Clin. Gastroenterol. Hepatol. 13: 906-912.e902.
Schjerning Olsen AM, Fosbøl EL, Lindhardsen J et al. (2011) Duration of treatment with nonsteroidal anti-inflammatory drugs and impact on risk of death and recurrent myocardial infarction in patients with prior myocardial infarction: a nationwide cohort study. Circulation 123: 2226-2235.
Barthélémy O, Limbourg T, Collet JP et al. (2013) Impact of non-steroidal anti-inflammatory drugs (NSAIDs) on cardiovascular outcomes in patients with stable atherothrombosis or multiple risk factors. Int. J. Cardiol. 163: 266-271.
Klein M, Gögenur I, Rosenberg J (2012) Postoperative use of non-steroidal anti-inflammatory drugs in patients with anastomotic leakage requiring reoperation after colorectal resection: cohort study based on prospective data. BMJ 345: e6166.
Davis GF (1986) Adverse effects of corticosteroids: II. Systemic. Clin. Dermatol. 4: 161-169.
Ferracioli-Oda E, Qawasmi A, Bloch MH (2018) Meta-analysis: Melatonin for the treatment of primary sleep disorders. Focus (Am Psychiatr Publ) 16: 113-118.
Genario R, Giacomini A, de Abreu MS et al. (2020) Sex differences in adult zebrafish anxiolytic-like responses to diazepam and melatonin. Neurosci. Lett. 714: 134548.
Harpsøe NG, Andersen LP, Gögenur I et al. (2015) Clinical pharmacokinetics of melatonin: a systematic review. Eur. J. Clin. Pharmacol. 71: 901-909.
Ramos E, Farré-Alins V, Egea J et al. (2020) Melatonin's efficacy in stroke patients; a matter of dose? A systematic review. Toxicol. Appl. Pharmacol. 392: 114933.
Sugden D (1983) Psychopharmacological effects of melatonin in mouse and rat. J. Pharmacol. Exp. Ther. 227: 587-591.
Mehrzadi S, Karimi MY, Fatemi A et al. (2021) SARS-CoV-2 and other coronaviruses negatively influence mitochondrial quality control: beneficial effects of melatonin. Pharmacol. Ther. 224: 107825.

This work is licensed under a Creative Commons Attribution 4.0 International License