Please cite this paper as:
Pal, P.K., Sarkar, S., Chattopadhyay, A., Tan, D.X. and Bandyopadhyay, D. 2019. Enterochromaffin cells as the source of melatonin: key findings and functional relevance in mammals. Melatonin Research. 2, 4 (Dec. 2019), 61-82. DOI:https://doi.org/https://doi.org/10.32794/mr11250041.
Review
Enterochromaffin cells as the source of melatonin: key findings and functional relevance in mammals
Palash Kumar Pala^, Swaimanti Sarkara^, Aindrila Chattopadhyayb, Dun-Xian Tanc, Debasish Bandyopadhyaya*
aOxidative Stress and Free Radical Biology Laboratory, Department of Physiology, University of Calcutta, 92, APC Road, Kolkata-700009, India
bDepartment of Physiology, Vidyasagar College,39, Sankar Ghosh Lane, Kolkata-700006, India
cDepartment of Cellular and Structural Biology, Health Science Center, University of Texas San Antonio, United States
^Authors have equal contribution
*Correspondence: debasish63@gmail.com; Tel: +91-9433072066
Running title: Melatonin and enterochromaffin cells
Received: September 20, 2019; Accepted: December 23, 2019
ABSTRACT
The enteroendocrine cells in gastrointestinal (GI) tract synthesize more than thirty hormones in mammals. Among these cells, the enterochromaffin (EC) cells are probably the most important one due to the fact that they produce melatonin. The rate-limiting enzymes for melatonin synthesis including arylalkylamine-N-acetyltransferase (AANAT, currently the SNAT) and hydroxyindole-O-methyltransferase (HIOMT, currently the ASMT) have been identified in EC cells and this has confirmed the local melatonin production in GI tract by these cells. EC cells play a critical role in regulation of gastrointestinal physiology, particularly, in protection of the GI tract from free radical attack and inflammatory reaction. GI tract is the major site exposed to the oxidative stress and inflammation because of the food residue metabolism and the presence of trillions of microbes including the pathological bacteria. Thus, it requires strong protection. Melatonin synthesized by the EC cells provides the onsite protection in GI tract since this molecule is the potent free radical scavenger and effective ant-inflammatory agent. In this review we summarize the available information regarding the structural and functional variability of the EC cells as well as their pathophysiological roles in the GI tract. The focus is given to the protective effects of melatonin produced by the EC cells on the oxidative stress, inflammation and microbiota balance in GI tract.
Keywords: Gastrointestinal (GI) tract, enterochromaffin (EC) cells, oxidative stress, melatonin, inflammation, enteric microbiota.
___________________________________________________________________________
1. INTRODUCTION
Enterochromaffin (EC) cells are the most abundant enteroendocrine cells in gastrointestinal (GI) tract and they synthesize more than thirty hormones, hence they are referred as ‘the largest endocrine organ in the body’ of mammals (1). Melatonin is one of these secretory products. Originally, serotonin was believed to be the prime hormone secreted by EC cells (2) However, further studies by use of immunohistochemical assay have identified that melatonin is the primary product of EC cells. Identification of melatonin synthetic system is the hallmark event in EC cell research (3-6). This observation raises a question as to what is the contribution of the GI melatonin on the circadian rhythm since the amounts of melatonin generated in the GI tract surpasses pineal melatonin by 10-100 folds (7-12). It was speculated that majority of circulatory melatonin during the day was derived from the GI tract since pinealectomy does not alter the daytime serum melatonin level (13-14). In addition, even though pinealectomy caused a decrease in night time circulatory melatonin level but it was unable to alter the melatonin concentration in the GI tract (10). Administration of L-tryptophan (Trp), the precursor of melatonin synthesis, in pinealectomized rats enhanced the circulatory melatonin level (15-16). The evidence mentioned above strongly supports melatonin synthesis in GI tract. A final proving of GI melatonin synthesis came from the identification of its rate-limiting enzyme, arylalkylamine-N-acetyltransferase (AANAT or SNAT), (12, 17-20) and hydroxyindole-O-methyltransferase (HIOMT or ASMT) (21) in GI tract, particularly in the EC cells of the digestive mucosa. Considering the large surface area of the GI tract and the relatively high concentrations of melatonin per gram of GI tissue it was calculated that the amounts of melatonin generated in GI tract of mammals would exceed the amounts of melatonin generated in pineal gland by roughly 400 times (22). This number may vary among the different species (23). Currently, the regulatory mechanisms on melatonin synthesis in GI tract have not been fully elucidated, but feeding regimen (meal frequency and timing of meals) seems to be the key environmental cue to synchronize the daily levels of GI melatonin in mammals (24-27).
Melatonin released from the EC cells seemed to act on a paracrine manner (12, 28-30) since the submucosa and muscularis tissue layer of the gastrointestinal wall possessed relatively low melatonin binding sites (28, 31-32). The GI melatonin can be transported into lamina propria and submucosa via blood vessels and then acts on the muscularis, where a substantial amount of melatonin was found (33). Physiologically, melatonin can either directly act on the intestinal muscles (6) or, produces its activities via myenteric nervous system (34). The presence of melatonin receptors and/or binding sites in the GI tissues supported the conjecture mentioned above (31, 34-36). All these clearly indicate the pleiotropic roles of melatonin played in the GI tract (20, 37-38). For example, as a signal molecule of photoperiodic clue melatonin has significant effect on the digestive physiology in mammals (12, 37). The constant light and constant darkness affected the activity of the digestive enzymes probably mediated by alterations in the levels of melatonin (39). Thus, the present review will summarize the functional relationships among EC cells, its melatonin production and the effects of melatonin on GI heath in mammals.
2. EC CELLS
The gastrointestinal mucosa is comprised of numerous types of endocrine cells with distinct appearances, localizations and functional characteristics. EC cells (or Type I cells) are one of the five enteroendocrine cell types (40). EC cell is designated its name by its occurrence in the intestinal epithelium and its ability to bind with chromium salts. It was first identified in the stomach of dog and rabbit (41) and then in many species. Erspamer classified EC cell as a collection of cells containing chromaffin and argentaffin granules that can bind with diazonium salts and exhibit fluorescence under Wood’s light. EC cells are found in the GI tract of different vertebrate and non-vertebrate species including Amphioxus, Ascidia, Octopoda, Muricidae and Amphibia (42).
2.1. Structural features of EC cells.
Up to 1969, there are different opinions as to the classification and categorization of endocrine cells in the gut. The opinion of only single type of endocrine cell in gut was rejected following the identification of different endocrine cells in the digestive tract of rats (40). Morphologically, epithelium of the GI mucosa has, at least, five different types of endocrine cells and the EC cell is one of them (40). However, EC cells are the major type of endocrine cells and they are structurally characterized by the presence of tapering end with numerous microvilli similar to intestinal columnar epithelial cells (40). The junctional proteins, including zonula adherens, zonula occludens and macula adherens, connect the apical region of the EC cells to the adjacent epithelial cells (40, 43). The rough endoplasmic reticulum is around the nuclei, whereas mitochondria are in the perinuclear as well as in the basal region of cytoplasm. Golgi apparatus occupy the cytoplasm of the apical zone, while the secretory granules are distributed in the wide basal end. Secretory granules are membrane bound molecules with slender, spherical, oval or bean shaped appearances (40, 43). These granules contain uniform opaque substance which can be stained with simple and fluorescent dyes or has ninhydrin vapour and alkallinethionidoxyl reactions. The presence of 5-hydroxytrypatamine (serotonin, 5-HT) in these granules was confirmed by auto-radiographic assay. In fact, about 95% of serotonin in the body are produced by EC cells (44-48). Motilin (49), substance P (50), and enkephalin (51) were also identified in the secretory granules. The lamina propria beneath the basement membrane has rich supply of fenestrated blood capillaries and lymphatic vessels. EC cells have the capacity to make direct anatomical connections with afferent and efferent nerve fibres involving both extrinsic neural pathways and the enteric nervous system of the GI tract (43).
2.2. Functional variability of the EC cells.
EC cells play pivotal roles in gastric secretion and motility and both are mainly mediated by serotonin and melatonin released from EC cells. Activations of specific EC cell receptors and signalling pathways orchestrate a variety of functions including propulsion, mixing and digestion of food, host-microbial signalling and modulating the gut immunity (52-54). A variety of membrane receptors of EC cells have been identified and these include 5HT receptors, cholinergic receptors, γ-amino butyric acid receptors, adrenoceptors (both α and β), corticotrophin releasing hormone receptors, irritant receptors (transient receptor potential A1) and pituitary adenylate cyclase-activating polypeptide receptors (55-57). Additionally, it has been observed that serotonin release is stimulated by addition of odorants and tastants to the EC cell cultures indicating the presence of olfactory and gustatory receptors too on the EC cells (58).
2.2.1. Role of EC cells in modulating gut motility in response to chemical and mechanical stimuli.
Mechanical and chemical stimulations of the gut luminal wall increase serotonin secretion and this reaction is, at least partially, mediated by neural reflexes (54, 57). The afferent vagal nerve does not make direct contact with the luminal contents of the gut. Instead, many bioactive substances in the intestinal lumen activate the EC cells to release serotonin as response to the chemical stimulation. These stimuli include glucose, oxygen, short-chain fatty acids, amino acids, peptides, purines, change in osmolarity and pH, certain drugs and even the products released by the enteric microbiota (57, 59-62). Besides, the mechanical forces generated during mixing and propulsion of food, defecation, increased stretch or, distension also lead to the release of serotonin from EC cells (54). Serotonin binds to 5HT3R receptors at the nerve endings of vagal sensory neurons, thereby activating the vagal afferents (52). Serotonin binding to 5HT4 receptors at the nerve terminals of the intrinsic afferents has also been observed. The stimulation of the enteric nervous system triggers excitation of the cholinergic neurons that makes efferent connections with the GI smooth muscles, finally leading to the smooth muscle contraction (63-64). Serotonin released from the EC cells also directly communicates with the serotonin receptors on smooth muscle cells of the GI tract to induce relaxation of the smooth muscles (65). Hence, serotonin released from EC cells is responsible for both contraction and relaxation of intestinal smooth muscle depending on the action positions. This makes the alternative intestinal segments to form peristaltic wave pattern (1).
EC cells are electrically excitable due to the presence of voltage-gated sodium and calcium channels in their membrane. This has been confirmed with the whole cell patch clamp study in transgenic mice. This feature seems to be partially responsible for signal transduction in EC cells (57). Mechanical stimulation also leads to intestinal mechano-sensory transduction. This is mediated by the activation of Piezo-2 mechano-gated channel in EC cells and the adjacent epithelial cells to cause purine release. The elevated purine level further triggers the release of serotonin from EC cells to perform its autocrine and paracrine functions, respectively. The purines, including ATP and UTP, activate IP3-DAG signalling pathway and consequently promote the release of calcium from endoplasmic reticulum. The calcium levels are responsible for peristalsis, mixing and propulsion of gastrointestinal contents (54, 66).
2.2.2. EC cell as the gut immunomodulator.
The role of EC cells in modulating immune functions has been well documented (67). A variety of toll-like receptors have been identified in mouse derived EC cell line (STC-1). The toll-like receptors can detect different microbial components and facilitate EC cell to participate in host-pathogen interaction (68). The severe combined immunodeficiency (SCID) mice without T-cell receptors are suffered from lack of EC cells and have low circulating level of serotonin (69-71). Similar situations have been observed in the inflammatory bowel disease and constipation-predominant irritable bowel syndrome (C-IBS) (69, 71-72). Conversely, diarrhoea-predominant irritable bowel syndrome (D-IBS) is characterized by rise in plasma serotonin levels. D-IBS can be attenuated by 5-HT3 receptor antagonist or, inhibitor of tryptophan hydroxylase-1 (the rate-limiting enzyme in the synthesis of serotonin) (69). All evidence points out to the fact that EC cells contribute to the patho-physiological mechanisms of these inflammatory disorders in the GI tract (69, 71-72). In addition to serotonin, melatonin is another important tryptophan derivative found in the EC cells. Discovery of melatonin being a potent antioxidant and an immunomodulatory molecule is a major breakthrough in understanding the functional diversity of the EC cells in GI tract. The importance is discussed in the following sections.
3. EC CELLS ARE THE PRIME SOURCE OF MELATONIN PRODUCTION IN THE GI TRACT
The presence of melatonin in GI tract is well documented without debate (4, 20, 73-75). By the use of different methodologies including immunohistology, radioimmunoassay and high performance liquid chromatography, it is confirmed that the abundance of melatonin primarily localizes in the EC cells (3, 22, 28, 76).The result from autoradiographic studies indicated that melatonin utmost bound to the mucosa and villi of the GI tract (32). At the sub-cellular distributions, the maximum binding of melatonin was detected in the nuclear fraction followed by microsomal, mitochondrial and cytosolic fractions, respectively (28, 77). The EC cells are the active melatonin synthetic cells among other cells in GI tract (4). The immunohistochemical assay has identified that the antibodies of melatonin and its immediate precursors (serotonin and N-acetylserotonin) all are present in the EC cells (78-79). The different methodologies including Coon’s indirect immunofluorescent and immunoperoxidase further detected the large quantity of melatonin and its precursors in the GI tract of mammals and humans (80). A question raised is whether the melatonin is synthesized by the EC cells, or it is taken up from the other sources by these cells? To answer this question, it requires to identify whether the melatonin synthetic machinery is also present in these cells. Not surprisingly, the melatonin synthetic rate-limiting enzymes, HIOMT (or SAMT) and AANAT (or SNAT), have been found in EC cells (12, 20, 38, 81-82). These observations have unambiguously proven that EC cells de novo synthesize melatonin. Since the total number of EC cells greatly surpasses the number of the pinealocytes and EC cells are responsible for 95% of serotonin synthesis (precursor of melatonin) in mammals (83), a mathematical calculation indicated that the amounts of melatonin produced by the EC cells might be several hundred-fold higher that that produced by pineal gland.
4. BIOSYNTHESIS OF MELATONIN IN EC CELLS
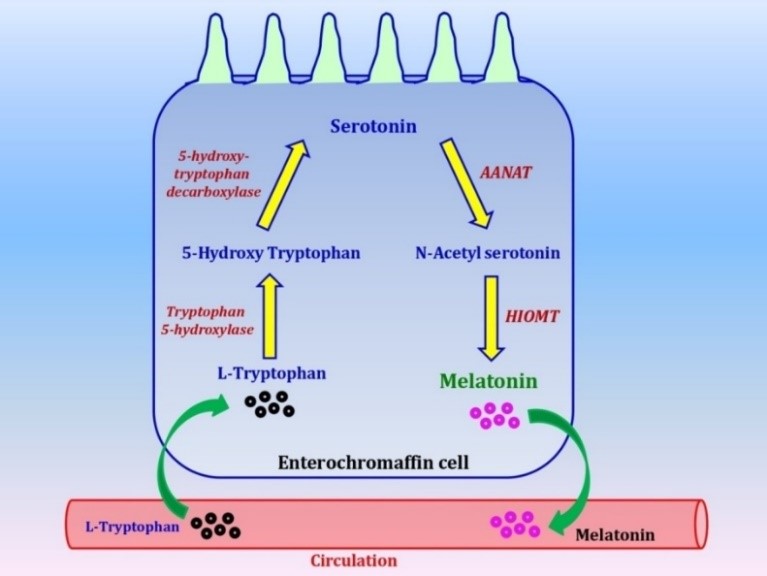
The melatonin synthetic pathway in the EC cells should be as same as in pinealocytes since they share the same enzymes. This pathway is illustrated in the Figure 1. Briefly, EC cells take up L-tryptophan from the circulation. Tryptophan-5-hydroxylase or, monooxygenase converts L-tryptophan to 5-hydroxy-tryptophan (5-HTP) (84). L-aromatic amino acid decarboxylase then decarboxlates 5-HTP to form serotonin or, 5-hydroxy tryptamine (5-HT) (85). AANAT (or SNAT) acetylates serotonin to form N-acetyl serotonin (NAS) (86) which is further o-methylated by HIOMT (or ASMT) to form melatonin (87). The melatonin synthetic sites in EC cells probably occur in mitochondria as it occurs in pinealocytes (88-90). The regulatory mechanism of melatonin synthesis in EC cells is not available currently, but it is not regulated by light as in the pineal gland. It seems that its synthesis is under central regulation triggered by intestinal contents (80).

Fig. 1. Biosynthetic pathway of melatonin in the EC cells in mammals.
AANAT:arylalkylamine-N-acetyl transferase, HIOMT:hydroxyindole-O-methyltransferase, arrows indicated the direction of the reactions.
5. RECEPTORS OF MELATONIN IN THE GI TRACT
Identification of melatonin receptors and/or their binding sites in the gastrointestinal cells suggests the paracrine actions of melatonin generated by EC cells locally (76, 91). Melatonin acts on its two primary membrane receptors MT1 and MT2 to produce different biological consequences in GI tissues. For example, activation of MT1 triggers G-protein mediated signalling, but inhibits cAMP signalling (92). On the other hand, activation of MT2 regulates phosphoinositol signalling pathway, but suppresses adenylyl cyclase and guanylyl cyclase mediated signalling pathways (92). Both MT1 and MT2 act synergistically to mediate melatonin’s signal. MT3 receptor, which is actually human quinone reductase-2, is also thought to be involved in melatonin mediated signalling (38, 93). Apart from its membrane receptors, melatonin also can bind to several nuclear receptors including RZR/RORγ (94) to mediate some of its biological activities (95-96). Currently, MT1 and MT2 receptors have been identified in the mitochondrial membrane of gastric endothelial cells (97). The authors speculated that some physiological effects of melatonin on GI tract, for example, angiogenesis, might be mediated by its mitochondrial melatonin receptors rather than the membrane receptors. This observation remains to be confirmed.
6. FUNCTIONAL DIVERSITY OF MELATONIN IN THE GI TRACT
Being an amphiphilic molecule melatonin can diffuse through any biological membrane to reach its targets inside and outside of the cells. Presence of its specific transporters in cellular and mitochondrial membranes (98-101) facilitates melatonin’s transportation against its concentration gradient. In this way, melatonin can accumulate in extremely high concentrations in some sub-cellular sites such as in mitochondria. Additionally, its wide presence in the inside/outside of the cells and its variety of receptors render this molecule to have pleiotropic physiological as well as pharmacological effects in GI tract (20, 37-38, 102-103).
6.1. Melatonin as an antioxidant against gastrointestinal injuries induced by oxidative stress.
The advantage of melatonin as an antioxidant over other antioxidants is its ubiquitously protective effects on GI injuries caused by a variety of etiologies (Figure 2) (12, 20, 38, 104). Melatonin not only directly scavenges a broad spectrum of reactive oxygen species (ROS), but it also upregulates different antioxidative enzymes and downregulates prooxiative enzymes. These are classified as its receptor-independent or dependent antioxidant activities (103, 105-112).
As to its receptor-independent activity, melatonin interacts with highly toxic ROS including hydroxyl radical, peroxynitrite anion, peroxinitrite, peroxyl radicals and singlet oxygen (106-110). This direct antioxidant property of melatonin has been confirmed not only through in vitro but also in many animal studies (109, 113). A protective role of melatonin has been frequently reported in ischemia-reperfusion induced GI injuries by caused by diverse stressors including bacteria (114-120). In addition, melatonin treatment upregulates matrix-metalloproteinase-2 (MMP-2) but downregulates MMP-9 levels which is mediated by MT2 receptor in GI tract, finally suppressing the tissue ROS level (121-122). The interactions of melatonin with ROS generate several products which also exhibit profound antioxidant capacity (107-108, 123-124). The continuously free radical scavenging activity of melatonin with its metabolites has been classified as the antioxidant cascade reaction. From this reaction one melatonin molecule can scavenge up to 10 ROS (125).
The indirect antioxidant activity of melatonin is mediated by its membrane and nuclear receptors (126-127). In this pathway, melatonin upregulates a serial of stress responsive genes including AMPK, HIFα, Sirt, etc. (128-129). Activations of these pathways lead to upregulation of variety of antioxidant enzymes including SODs, catalase, glutathione peroxidase and glutathione reductase (103, 127, 129-131), thus, further reduces the oxidative stress and protect the tissue injuries in GI tact.

Fig. 2. Effects of melatonin produced by the EC cells on multiple oxidative stresses in the GI tract.
NSAIDs: Non-steroidal anti-inflammatory drugs; ROS: reactive oxygen species; Arrows: indicated the direction of the reaction, Bars: blocking activity.
6.2. Effects of melatonin on GI tissue injury induced by heavy metal toxicity.
Heavy metal pollution is a serious global problem. It not only destroys the ecosystem but also is a major hazard for human health. Usually, the GI tract is the primary site to first contact with heavy metals. Heavy metals can be extracted in GI system by contaminated food and water. If not properly treated, it will damage the GI tissues via oxidative stress (132-133). For example, cadmium (Cd)-induced GI injury is mediated through two possible pathways by disturbing mitochondrial fusion and fission process (134-137) and by activating transcription factor EB mediated autophagy (135). The alterations induced by Cd in GI tract can be protected by melatonin (138-140). In addition to Cd, melatonin also protects GI from injuries associated with the toxicity of mercury (138), arsenic (139) and lead (140). One of the protective mechanisms is its antioxidant capacity. For example, in lead-induced GI toxicity, pre-treatment with melatonin efficiently reduced the tissue level of lipid peroxidation and protein carbonyl content, possibly by restoring the activities/levels of different enzymatic and non-enzymatic antioxidants in the GI tract of rats (140).
6.3. Effects of melatonin on non-steroidal anti-inflammatory drug (NSAID) induced GI damages.
NSAIDs cause severe GI injuries including bleeding, ulceration and apoptosis. The protective roles of melatonin on NSAID-induced GI tissue damage are frequently reported. These include the beneficial effects of melatonin on indomethacin, aspirin and piroxicam induced GI injuries (114, 141-147). Melatonin not only protects the GI tissues from the adverse effects of these drugs, but also accelerates healing process of the ulcer induced by NSAID (141). Orally application of both melatonin and its precursor (L-tryptophan) accelerated the healing process of the GI mucosal damages caused by unregulated aspirin intake in patients (142, 146). Importantly, the data showed that the endogenously produced melatonin also significantly elevated in these patients. This is probably the auto-response of the oxidatively stressed GI tissues to increase their melatonin against the oxidative stress. This stress-stimulated and stress-released melatonin phenomenon has been reported previously (127). Melatonin treatment effectively reduced diclofenac induced intestinal damage with the mechanism to restore the intestinal permeability and mucosal integrity (143-144). To increase the activities of antioxidant enzymes including peroxidase, superoxide dismutase and catalase in GI tract is another mechanism of melatonin protect against NSAIDS-induced GI damages (145). In addition, other mechanisms of melatonin also enhance its protective effects on the NSAIDS-induced GI damages. These include that melatonin inhibits gastric acid secretion, suppresses infiltration of neutrophils, increases mucosal blood flow in the inflamed tissues, enhances bicarbonate secretion in the duodenum and promotes synthesis of prostaglandins (142, 146-147).
6.4. Melatonin as an anti-inflammatory agent in the GI tract.
Among the diverse pathophysiological conditions, inflammation plays an important role in GI disorders. Actually, melatonin is a profound anti-inflammatory molecule in the GI tract (102, 148-153). A major signal transduction pathway of inflammation in GI is possibly mediated by necrosis factor kappa β (NFkβ) (154-157). Melatonin inhibits the translocation of NFkβ to the nucleus, hence reduces its binding to DNA (158). The NFkβ mediated inflammatory pathway is primarily triggered by TLR4 and TLR5. Melatonin downregulates the expression of TLR4 and its signal associated genes, such as MyD88 (159). Melatonin also enhances the levels of Ikβ, eventually suppressing the expression of NFkβ (159). The multiple blocking of NFkβ pathway by melatonin lead to the suppression of overproduction of leukocytes, the adhesion agents and the implementation of different inflammatory cells (158) and all these result in the reduced inflammatory reaction in the GI tissue. On the other hand, aflatoxin B1 mediated intestinal lesions in rat is known to increase circulating level of proinflammatory cytokine IL-1β and melatonin administration profoundly reduces its level (160). Presence of melatonin receptors in mast cells plays a key role in modulation of anti-inflammatory pathway and activation of these receptors by melatonin inhibits the release of TNF-α in the circulation or, tissue (161). In addition, metabolites of melatonin, AFMK and AMK, are also known to exert similar anti-inflammatory functions as melatonin (162). Other mechanisms also involve the anti-inflammatory activity of melatonin including suppression of synthesis of prostaglandins and adhesion molecules (109), inhibition of leukocyte transendothelial cell migration (102) and cyclooxygenase 2 expressions in the macrophages (163) as well as reduction of the recruitment of different pro-inflammatory cells to the sites of inflammation (109, 162). Similarly, melatonin administration reduced the circulating levels of different pro-inflammatory cytokines including IL-1β, IL-6, IL-17, interferon-γ and TNF-α and downregulated the expressions of protein kinase Cζ (PKCζ) and calmodulin 3 (CALM3) (164). In an animal study, melatonin treatment significantly reversed the colonic mucosal injury induced by acetic acid (AA) and further confirmed its anti-inflammatory function in GI tract (165).
6.5. Effect of melatonin on the balance of intestinal microbiota.
Roughly 1014 microbes belonging to nearly 500 diverse species exist in GI tract of animals (166). The normal microbial distribution defines the health and function of GI system. The disruptions of microbial signalling and their normal distribution pave the way for a number of gut related pathologies. The balance of gut microbial community with enteric microenvironment keeps GI healthy. A variety of factors contribute to the GI microbial balance. Melatonin generated by pineal gland and EC cells is one of the most important factors that maintain the GI healthy (167). An interesting correlation between melatonin and gut microbial profile was observed in high fat diet (HFD) fed mice, a model of lipid metabolism imbalance. Antibiotic treatment disturbed the gut microbiota and thus, promoted the metabolic impairment in HFD fed mice while melatonin supplementation significantly improved the metabolic disturbances. Further analysis indicated that melatonin had the capacity to re-establish the balance of GI microbiota by promoting the growth of Alistipes and Bacteroides which are beneficial bacteria in GI tract (168). It is speculated that disturbance of gut motility may lead to the development of irritable bowel syndrome (IBS) and gastroesophageal reflux disease (GERD) (169). Actually, these disorders may be associated with dysregulation of intestinal microflora. Melatonin treatment preserved the abundance and diversity of enteric microbiota in dextran sulfate sodium (DSS) induced murine collitis model (166). Besides restoring healthy gut microbial population, melatonin has the potency to regulate altered gut permeability and immune response initiated by Escherichia coli (170). On the other hand, Helicobacter pylori infection downregulated the expression of AANAT and HIOMT and reduced the production of melatonin in the GIT tissues. This may be one of the factors to promote the gastro ulceration associated with this bacterium. Once the infection subsides, melatonin production returns back to its normal level (171). Undoubtedly, the crosstalk between gut microbial population and the gastrointestinal melatonin is a fascinating area to be explored.
6.6. Effects of melatonin on GI physiology.
In addition to the protective effects of melatonin on the GI tissues, it also participates in diverse of GI physiologies. Melatonin exhibits inhibitory effect on the motor activity of GI tract. This inhibition is directly proportional to the tone and intensity of contractions in the different portions of the GI tract (172-173) possibly by blocking nicotinic acetylcholine receptors on the submucosal nervous plexus (33). Melatonin administration suppressed cell proliferation and gastrointestinal motor activity induced by gastrin (174). The structural similarity of melatonin with the gastrin receptor antagonist (benzotript) may render melatonin having this effect (80). It has been known that melatonin inhibits cAMP production which serves potent role in hydrochloric acid secretion by the parietal cells; therefore, melatonin is thought to suppress HCl production. In contrast, histamine usually enhances cAMP production and cAMP is crucial for melatonin secretion, thus melatonin secretion from the EC cells may be regulated by histamine (80). Interestingly, the effects of cholecystokinin on the motor activities in GI tract are also mediated by melatonin. Melatonin might be the utmost regulator of cell proliferation in the mucosa of the GI tract (80). There are many physiological functions of melatonin on the GI tract and those are not the focus of the current review.
7. CONCLUDING REMARK
EC cells are the important cell type in GI tract. The importance of these cells is not because of their specific morphology but they synthesize melatonin. Originally, melatonin was thought to be solely a pineal derived hormone, but the discovery of melatonin synthesis in EC cells has greatly expanded the spectrum of extra-pineal melatonin sources in mammals. Judging from the cell numbers, it was calculated that the amounts of melatonin generated by the EC cells are several hundred-fold greater than that produce by pineal gland. This melatonin may contribute to the day time circulatory melatonin level. The gastrointestinal melatonin mainly functions as autocrine and paracrine to participate in diverse pathophysiological activities in GI tract. It protects GI tissues against damage caused by oxidative stress and inflammation. Melatonin can bind to its membrane receptors and/or its intra- as well as extra-cellular signalling molecules in exerting its physiological activities in GI tract. These activities include regulation of the GI movement, HCl production, cell proliferation, microbiota balance and prostaglandin synthesis. Collectively, discovery of melatonin synthesis in the EC cells in GI tract of mammals can be considered as a hallmark event in the field of endocrine as well as melatonin researches.
ACKNOWLEDGEMENTS
Dr. PKP thankfully acknowledges the receipt of UGC Dr. D. S. Kothari Post Doctoral Fellowship (BL/16-17/0502), Govt. of India. A financial assistance as Junior Research Fellow (JRF) [709/(CSIR-UGC NET DEC. 2018] under UGC, Govt. of India to Swaimanti Sarkar is also thankfully and gratefully acknowledged. Dr. AC is supported by funds available to her from Department of Science and Technology, Govt. of West Bengal. DB also gratefully acknowledges the support he received from Departmental BI Grant and DST-PURSE Program awarded to the University of Calcutta.
AUTHORSHIP
Dr. DB and Dr. AC contributed to conception, revised the manuscript critically and approved it. Dr. PKP prepared figures, drafted and edited the manuscript. SS contributed in drafting the manuscript and edited it. Dr. DXT edited the manuscript critically.
CONFLICT OF INTEREST
Authors declare no conflict of interest.
REFERENCES
Gunawardene AR, Corfe BM, Staton CA (2011) Classification and functions of enteroendocrine cells of the lower gastrointestinal tract. Int. J. Exp. Pathol. 92: 219-231. DOI: 10.1111/j.1365-2613.2011.00767.x.
Ahlman H, Nilsson O (2003) The gut as the largest endocrine organ in the body. Ann. Oncol. 12 (Suppl. 2): S63–S68. DOI: 10.1093/annonc/12.suppl2.s63.
Raikhlin NT, Kvetnoy IM (1974) Lightening effect of the extract of human appendix mucosa on frog skin melanophores. Bull. Exp. Biol. Med. 8: 114–116.
Raikhlin NT, Kvetnoy IM, Tolkachev VN (1975) Melatonin may be synthesized in enterochro-maffin cells. Nature 255: 344–345. DOI: 10.1038/255344a0.
Bubenik GA, Brown G, Grota L (1977) Immuno-histological localization of melatonin in the rat digestive system. Experientia. 33: 662–663. https://doi.org/10.1007/BF01946561.
Bubenik GA (2001) Localization, physiological significance and possible clinical implication of gastrointestinal melatonin. Biol. Signals Recept. 10: 350–366. DOI: 10.1159/000046903.
Huether G, Poeggeler B, Reimer A, George A (1992) Effect of tryptophan administration on circulating melatonin levels in chicks and rats: evidence for stimulation of melatonin synthesis and release in the gastrointestinal tract. Life Sci. 51: 945–953. DOI: 10.1016/0024-3205(92)90402-b.
Huether G (1994) Melatonin synthesis in the gastrointestinal tract and the impact of nutritional factors on circulating melatonin. Ann. NY. Acad. Sci. 719: 146–158. DOI: 10.1111/j.1749-6632.1994.tb56826.x.
Bubenik GA, Pang SF, Hacker RR, Smith PS (1996) Melatonin concentrations in serum and tissues of porcine gastrointestinal tract and their relationship to the intake and passage of blood. J. Pineal Res. 21: 251–256. DOI:10.1111/j.1600-079x.1996.tb00294.x.
Bubenik GA, Brown GM (1997) Pinealectomy reduces melatonin levels in the serum but not in the gastrointestinal tract of the rat. Biol. Signals. 6: 40–44.DOI: 10.1159/000109107.
Bubenik GA (1999) Localization and physiological significance of gastrointestinal melatonin. In: R Watson (ed) Melatonin in Health Promotion. CRC press, Boca Raton, Florida, pp. 21–39.
Pal PK, Hasan KN, Maitra SK (2016) Gut melatonin response to microbial infection in carp Catla catla. Fish Physiol. Biochem. 42: 579–592. DOI: 10.1007/s10695-015-0161-7.
Kennaway DJ, Firth RG, Philipou G, Matthews CD, Seamark RF (1977) A specific radioimmunoassay for melatonin in biological tissue and fluids and its validation by gas chromatography-mass spectrometry. Endocrinology. 101: 119–127. DOI: 10.1210/endo-101-1-119.
Vaughan GM, Reiter RJ (1986) Pineal dependence of the Syrian hamster’s nocturnal serum melatonin surge. J. Pineal Res. 3: 9–14. https://doi.org/10.1111/j.1600-079X.1986.tb00721.x.
Huether G, Poegeller B, Reimer R, George A (1992) Effect of tryptophan administration on circulating melatonin levels in chicks and rats: evidence for stimulation of melatonin synthesis and release in the gastrointestinal tract. Life Sci. 51: 945–953. DOI: 10.1016/0024-3205(92)90402-b.
Kezuka H, Iigo I, Furukawa K, Aida K, Hanyu I (1992) Effects of photoperiod, pinealectomy and ophtalmectomy on circulating melatonin rhythms in the goldfish (Carassius auratus). Zool. Sci. 9: 1147–1153.
Klein DC (1985) Photoneural regulation of the mammalian pineal gland. In: Everet D, Clark E. (eds) Photoperiodism, melatonin and the pineal. Ciba Found Symp. 117: 38–56.
Klein DC, Roseboom PH, Coon SL (1996) New light is shining on the melatonin rhythm enzyme: the Wrst postcloning view. Trends Endocrinol. Metab. 7: 106–112. https://doi.org/10.1016/1043-2760(96)00033-1.
Mukherjee S, Moniruzzaman M, Maitra SK (2014) Daily and seasonal profiles of gut melatonin and their temporal relationship with pineal and serum melatonin in carp Catla catla under natural photo-thermal conditions. Biol. Rhythm Res. 45: 301–315. DOI: 10.1080/09291016.2013.817139.
Pal PK, Bhattacharjee B, Ghosh AK, Chattopadhyay A, Bandyopadhyay D (2019) Adrenaline induced disruption of endogenous melatonergic system, antioxidant and inflammatory responses in the gastrointestinal tissues of male Wistar rat: an in vitro study. Melatonin Res. 1: 109-131. DOI: 10.32794/mr11250007.
Zhao F, Ma C, Zhao G, Wang G, Li X, Yang K (2019) Rumen-protected 5-hydroxytryptophan improves sheep melatonin synthesis in the pineal gland and intestinal tract. Med. Sci. Monit. 25: 3605-3616. DOI: 10.12659/MSM.915909.
Huether G (1993) The contribution of extrapineal sites of melatonin synthesis to circulating melatonin levels in higher vertebrates. Experientia 49: 665–670. DOI: 10.1007/bf01923948.
Bubenik GA, Pang SF (1997) Melatonin level in the gastrointestinal tissue of fish, amphibians, and a reptile. Gen. Comp. Endocrinol. 106: 415–419. DOI: 10.1006/gcen.1997.6889.
Boujard T, Leatherland JF (1992) Circadian rhythms and feeding time in fishes. Environ. Biol. Fish 35: 109–131. https://doi.org/10.1007/BF00002186.
Escobar C, Díaz-Muñoz M, Encinas F, Aguilar-Roblero R (1998) Persistence of metabolic rhythmicity during fasting and its entrainment by restricted feeding schedule in rats. Am. J. Physiol. 274:1309–1316. DOI: 10.1152/ajpregu.1998.274.5.R1309.
Polakof S, Ceinos RM, Fernández-Durán B, Míguez JM, Soengas JL (2007) Daily changes in parameters of energy metabolism in brain of rainbow trout: dependence on feeding. Comp. Biochem. Physiol. 146: 265–273. DOI: 10.1016/j.cbpa.2006.10.026.
Polakof S, Míguez JM, Soengas JL (2007) Daily changes in parameters of energy metabolism in liver, white muscle, and gills of rainbow trout: dependence on feeding. Comp. Biochem. Physiol. A. 147: 363–374. DOI: 10.1016/j.cbpa.2007.01.009.
Chow PH, Lee PN, Poon AMS, Shiu SYW, Pang SF (1996) The gastrointestinal system: A site of melatonin paracrine action. In: Tang PL, Pang SF, Reiter RJ (eds.) Melatonin: A universal photoperiodic signal with diverse action. Front. Horm. Res. Basel, Karger. 21: 123–132. https://doi.org/10.1159/000425610.
Maitra SK, Pal PK (2017) Structural diversity and functional integrity of the fish pineal gland. In: Catalá A. (Ed.) Pineal gland: Research advances and clinical challenges. Nova Science Publishers, Inc. New York, USA. Pp. 51–92. https://www.novapublishers.com/ catalog/product_ info.php? products_id=62459.
Maitra SK, Pal PK (2017) Melatonin rhythms in the pineal and non-pineal tissues and their physiological implications in subtropical fish. Biol. Rhythm Res. 48: 757–776. https://doi.org/10.1080/09291016.2017.1345453.
Lee PPN, Pang SF (1993) Melatonin and its receptors in the gastrointestinal tract. Neurosignals 2: 181–193. DOI: 10.1159/000109491.
Lee PPN, Shiu SYU, Chow PH, Pang SF (1995) Regional and diurnal studies on melatonin binding sites in the duck gastrointestinal tract. Biol. Signals 4: 212–224. DOI:10.1159/000109445.
Bubenik GA, Niles LP, Pang SF, Pentney PJ (1993) Diurnal variation and binding characteristics of melatonin in the mouse brain and gastrointestinal tissues. Comp. Biochem. Physiol. C. 104: 221–224. DOI: 10.1016/0742-8413(93)90027-i.
Barajas-Lopez C, Pereso AL, Espinos-Luna R, Reyes-Vazquez C, Prieto-Gomez B (1996) Melatonin modulates cholinergic-transmission blocking nicotinic channels in the guinea-pig sub-mucous-plexus. Eur. J. Pharmacol. 312: 319–325. DOI: 10.1016/0014-2999(96)00481-5.
Poon AMS, Chow PH, Mak ASY, Pang SF (1997) Autoradiographic localization of [125I] iodomelatonin binding sites in the gastrointestinal tract of mammals including humans and birds. J. Pineal Res. 23: 5–14. https://doi.org/10.1111/j.1600-079X.1997.tb00328.x.
Kulczykowska E, Kalamarz H, Warne J, Balment R (2006) Day-night specific binding 2[I125]iodomelatonin and melatonin in chronically cannulated Xounder (Platichthys xesus). Comp. Biochem. Physiol.176: 277–285.
Pal PK, Maitra SK (2018) Response of gastrointestinal melatonin, antioxidants, and digestive enzymes to altered feeding conditions in carp (Catla catla). Fish Physiol. Biochem. 44: 1061–1073. https://doi.org/10.1007/s10695-018-0494-0.
Pal PK, Bhattacharjee B, Chattopadhyay A, Bandyopadhyay D (2019) Pleiotropic roles of melatonin against oxidative stress mediated tissue injury in the gastrointestinal tract: An overview. Melatonin Res.2: 158-184. DOI: 10.32794/mr11250027.
Khizhkin EA, Ilyukha VA, Vinogradova IA, Anisimov VN (2019) Absence of photoperiodism and digestive enzymes in rats: the role of the age and endogenous melatonin level. Adv. Gerontol. 32: 347-356.
Forssmann WG, Orci L, Pictet R, Renold AE, Rouiller C (1969) The endocrine cells in the epithelium of the gastrointestinal mucosa of the rat: An electron microscope study. J. Cell Biol. 40: 692-715. DOI: 10.1083/jcb.40.3.692.
Heidenhain R (1870) Untersuchungenüber den Bau der Labdrüsen. Archiv. Fürmikroskopische Anatomie. 6: 368-406. https://doi.org/10.1007/BF02933955.
Vialli M (1966) Histology of the enterochromaffin cell system. In 5-Hydroxytryptamine and related indolealkylamines (pp. 1-65). Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-85467-5_1.
Wade PR, Westfall JA (1985) Ultrastructure of enterochromaffin cells and associated neural and vascular elements in the mouse duodenum. Cell Tissue Res. 241: 557-563. https://doi.org/10.1007/BF00214576.
Erspamer V, Asero B (1952) Identification of enteramine, the specific hormone of the enterochromaffin cell system, as 5-hydroxytryptamine. Nature 169: 800-801. DOI: 10.1038/169800b0.
Barter R, Pearse AE (1953) Detection of 5-hydroxytryptamine in mammalian enterochromaffin cells. Nature.172: 810. DOI: 10.1038/172810a0.
Holcenberg J, Benditt E (1959) A new histochemical technique for demonstration of enterochromaffin cells- a reaction for indoleethylamines. J. Histochem. Cytochem. 7: 303-304.
Pearse AG (1968) Histochemistry: theoretical and applied. 3rd Edition. London, Churchill.
Sjölund K, Sanden G, Håkanson R, Sundler F (1983) Endocrine cells in human intestine: an immunocytochemical study. Gastroenterol. 85: 1120-1130. https://doi.org/10.1016/ S0016-5085(83)80080-8.
Pearse AG, Polak JM, Bloom SR, Adams C, Dryburgh JR, Brown JC (1974) Enterochromaffin cells of the mammalian small intestine as the source of motilin. Virchows Archiv. B Cell. Pathol. 16: 111-120. https://doi.org/10.1007/BF02894069.
Heitz P, Polak JM, Timson CM, Pearse AG (1976) Enterochromaffin cells as the endocrine source of gastrointestinal substance P. Histochem. 49: 343-347. DOI: 10.1007/bf00496138.
Alumets J, Håkanson R, Sundler F, Chang KJ (1978) Leu-enkephalin-like material in nerves and enterochromaffin cells in the gut. Histochem. 56: 187-196. https://doi.org/ 10.1007/BF00495979.
Rhee SH, Pothoulakis C, Mayer EA (2009) Principles and clinical implications of the brain–gut–enteric microbiota axis. Nature Rev. Gastroenterol. Hepatol. 6: 306-314. DOI: 10.1038/nrgastro.2009.35.
Shajib MS, Khan WI (2015) The role of serotonin and its receptors in activation of immune responses and inflammation. Acta Physiologica 213: 561-574. DOI: 10.1111/apha.12430.
Linan-Rico A, Ochoa-Cortes F, Beyder A, Soghomonyan S, Zuleta-Alarcon A, Coppola V, Christofi FL (2016) Mechanosensory signaling in enterochromaffin cells and 5-HT release: potential implications for gut inflammation. Frontiers Neurosci.10: 564. DOI: 10.3389/fnins.2016.00564.
Modlin IM, Kidd M, Pfragner R, Eick GN, Champaneria MC (2006) The functional characterization of normal and neoplastic human enterochromaffin cells. J. Clinic. Endocrinol. Metabol. 91: 2340-2348. https://doi.org/10.1210/jc.2006-0110.
Hansen MB, Witte AB (2008) The role of serotonin in intestinal luminal sensing and secretion. Acta Physiologica 193: 311-323. DOI: 10.1111/j.1748-1716.2008.01870.x.
Bellono NW, Bayrer JR, Leitch DB, Castro J, Zhang C, O’Donnell TA, Brierley SM, Ingraham HA, Julius D (2017) Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell 170: 185-198. DOI: 10.1016/j.cell.2017.05.034.
Braun T, Voland P, Kunz L, Prinz C, Gratzl M (2007) Enterochromaffin cells of the human gut: sensors for spices and odorants. Gastroenterol. 132: 1890-1901. DOI: 10.1053/j.gastro.2007.02.036.
Linan-Rico A, Ochoa-Cortes F, Zuleta-Alarcon A, Alhaj M, Tili E, Enneking J, Harzman A, Grants I, Bergese S, Christofi FL (2017) UTP–Gated signaling pathways of 5-HT release from BON cells as a model of human enterochromaffin cells. Front. Pharmacol. 8: 429. DOI: 10.3389/fphar.2017.00429.
Kim M, Cooke HJ, Javed NH, Carey HV, Christofi F, Raybould HE (2001) D-glucose releases 5-hydroxytryptamine from human BON cells as a model of enterochromaffin cells. Gastroenterol. 121:1400-1406. DOI: 10.1053/gast.2001.29567.
Cooke HJ, Wunderlich J, Christofi FL (2003) “The force be with you”: ATP in gut mechanosensory transduction. News Physiol. Sci. 18: 43-49. https://doi.org/10.1152/nips.01411.2002.
Haugen M, Dammen R, Svejda B, Gustafsson BI, Pfragner R, Modlin I, Kidd M (2012) Differential signal pathway activation and 5-HT function: the role of gut enterochromaffin cells as oxygen sensors. Am. J. Physiol-Gastr. L. 303: G1164-1173. DOI: 10.1152/ajpgi.00027.2012.
Costedio MM, Hyman N, Mawe GM (2007) Serotonin and its role in colonic function and in gastrointestinal disorders. Dis. Colon Rectum. 50: 376-388. DOI: 10.1007/s10350-006-0763-3.
Gershon MD, Tack J (2007) The serotonin signalling system: from basic understanding to drug development for functional GI disorders. Gastroenterol. 132: 397-414. DOI: 10.1053/j.gastro.2006.11.002.
Björnsson ES, Chey WD, Hooper F, Woods ML, Owyang C, Hasler WL (2002) Impaired gastrocolonic response and peristaltic reflex in slow-transit constipation: role of 5-HT3 pathways. Am. J. Physiol-Gastr. L. 283: G400-407. DOI: 10.1152/ajpgi.00082.2001.
Alcaino C, Knutson KR, Treichel AJ, Yildiz G, Strege PR, Linden DR, Li JH, Leiter AB, Szurszewski JH, Farrugia G, Beyder A (2018) A population of gut epithelial enterochromaffin cells is mechanosensitive and requires Piezo2 to convert force into serotonin release. Proc. Natl. Acad. Sci. 115: E7632-7641. DOI: 10.1073/pnas.1804938115.
Khan WI, Ghia JE (2010) Gut hormones: emerging role in immune activation and inflammation. Clin. Exp. Immunol. 161: 19-27. DOI: 10.1111/j.1365-2249.2010.04150.x.
Bogunovic M, Davé SH, Tilstra JS, Chang DT, Harpaz N, Xiong H, Mayer LF, Plevy SE (2007) Enteroendocrine cells express functional Toll-like receptors. Am. J. Physiol-Gastr. L. 292: G1770-1783. DOI: 10.1152/ajpgi.00249.2006.
Wang H, Steeds J, Motomura Y, Deng Y, Verma-Gandhu M, El-Sharkawy RT, McLaughlin JT, Grencis RK, Khan WI (2007) CD4+ T cell-mediated immunological control of enterochromaffin cell hyperplasia and 5-hydroxytryptamine production in enteric infection. Gut 56: 949-957. DOI: 10.1136/gut.2006.103226.
Spiller R (2008) Serotonin and GI clinical disorders. Neuropharmacol. 55: 1072-1080. DOI: 10.1016/j.neuropharm.2008.07.016.
Jun S, Kohen R, Cain KC, Jarrett ME, Heitkemper MM (2011) Associations of tryptophan hydroxylase gene polymorphisms with irritable bowel syndrome. Neurogastroent. Motil. 23: 233-e116. DOI: 10.1111/j.1365-2982.2010.01623.x.
Bischoff SC, Mailer R, Pabst O, Weier G, Sedlik W, Li Z, Chen JJ, Murphy DL, Gershon MD (2009) Role of serotonin in intestinal inflammation: knockout of serotonin reuptake transporter exacerbates 2, 4, 6-trinitrobenzene sulfonic acid colitis in mice. Am. J. Physiol-Gastr. L. 296: G685-695. DOI: 10.1152/ajpgi.90685.2008.
Pal PK, Maitra SK (2017) Neuronal control of gut melatoninergic system in carp. BAOJ Neuro. 2: 024. https://bioaccent.org/neurology/neurology24.pdf.
Bubenik GA, Brown G, Grota L (1977) Immuno-histological localization of melatonin in the rat digestive system. Experientia 33: 662–663. https://doi.org/10.1007/BF01946561.
Huether G (1996) Melatonin as an antiaging drug: Between facts and fantasy. Gerontolog. 42: 87–96. DOI: 10.1159/000213777.
Bubenik GA (1999) Localization and physiological significance of gastrointestinal melatonin. In: R Watson (ed.) Melatonin in Health Promotion. CRC press, Boca Raton, Florida, pp. 21–39.
Menendez-Pelaez A, Buzzell GR (1992) Harderian gland indoles. In: Webb SM, Hoffman RA, Puig-Domingo ML, Reiter RJ (eds.) Harderian glands: Porphyrin metabolism, behavioral, and endocrine effects. Springer, Berlin, pp. 219–234.
Raikhlin NT, Kvetnoy IM, Kadagidze ZG, Soko-lov AA (1976) Immunohistochemical evidence of lo-calization of melatonin and N-acetylserotonin in enterochromaffin cells. Bull Exp. Biol. Med. 82:1400–1401.
Raikhlin NT, Kvetnoy IM, Kadagidze ZG, Sokolov AA (1978) Immunomorphological studies on synthesis of melatonin in enterochromaffin cells. Acta Histochem. Cytochem. 11: 75–77.
Kvetnoy IM, Ingel IE, Kvetnaia TV Malinovskaya NK, Rapoport SI, Raikhlin NT, Trofimov AV, Yuzhakov VV (2002) Gastrointestinal melatonin: cellular identification and biological role. Neuro. Endocrinol. Lett. 23: 121–132.
Quay WB, Ma YH (1976) Demonstration of gastro-intestinal hydroxyindole-O-methyltransferase. IRCS Med. Sci. 4:563.
Stefulj J, Hörtner M, Ghosh M, Schauenstein K, Rinner I, Wölfler A, Semmler J, Liebmann PM (2001) Gene expression of the key enzymes of melatonin synthesis in extrapineal tissues of the rat. J. Pineal Res. 30: 243–247.
Kvetnoy IM, Yuzhakov VV (1993) Extrapinealmelato-nin: advances in microscopical identification of hormones in endocrine and non-endocrine cells. Microsc. Anal. 21: 27–29.
Lovenberg W, Jequier E, Sjoerdsma A (1967) Tryptophan hydroxylation: Measurement in pineal gland, brain stem and carcinoid tumor. Science 155: 217–219.
Balemans MGM, Bary FAM, Legerstee WC, van Benthem J (1978) Estimation of the methylating capacity in the pineal gland of the rat with special reference to the methylation of N-acetylserotonin and 5-hydroxytryptophol separately. Experientia 34: 1434–1435.
Voisin P, Namboodiri MAA, Klein DC (1984) Arylamine N-acetyltransferase and arylalkylamine N-acetyltransferase in the mammalian pineal gland. J. Biol. Chem. 259: 10913–10918.
Axelrod J, Weissbach H (1960) Enzymatic O-methylation of N-acetylserotonin to melatonin. Science 131: 1312–1312.
Tan DX, Manchester LC, Liu X, Rosales-Corral SA, Acuna-Castroviejo D, Reiter RJ (2013) Mitochondria and chloroplasts as the original sites of melatonin synthesis: a hypothesis related to melatonin's primary function and evolution in eukaryotes. J. Pineal Res. 54 (2): 127-38. DOI: 10.1111/jpi.12026).
He C, Wang J, Zhang Z, Yang M, Li Y, Tian X, Ma T, Tao J, Zhu K, Song Y, Ji P, Liu G (2016) Mitochondria synthesize melatonin to ameliorate its function and improve mice oocyte's quality under in vitro conditions. Int. J. Mol. Sci. 14: 17(6). pii: E939. DOI: 10.3390/ijms17060939.
Suofu Y, Li W, Jean-Alphonse FG, Jia J, Khattar NK, Li J, Baranov SV, Leronni D, Mihalik AC, He Y, Cecon E, Wehbi VL, Kim J, Heath BE, Baranova OV, Wang X, Gable MJ, Kretz ES, Di Benedetto G, Lezon TR, Ferrando LM, Larkin TM, Sullivan M, Yablonska S, Wang J, Minnigh MB, Guillaumet G, Suzenet F, Richardson RM, Poloyac SM, Stolz DB, Jockers R, Witt-Enderby PA, Carlisle DL, Vilardaga JP, Friedlander RM (2017) Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc. Natl. Acad. Sci. 114 (38): E7997-E8006. DOI: 10.1073/pnas.1705768114.
Dobocovich M, Mankowsha M (2005) Functional MP1 and MP2 melatonin receptors in mammals. Endocrine 27: 101–110. DOI: 10.1385/ENDO:27:2:101.
Boutin JA, Audinot V, Ferry G, Delagrange P (2005) Molecular tools to study melatonin pathways and actions. Trends Pharmacol. Sci. 26: 412–419. DOI:10.1016/j.tips. 2005.06.006.
Srinivasan V, Lauterbach EC, Ho KY, Acuña-Castroviejo D, Zakaria R, Brzezinski A (2012) Melatonin in antinociception: Its therapeutic applications. Curr. Neuropharmacol. 10: 167–178. DOI: 10.2174/157015912800604489.
Wang RX, Liu H, Xu L, Zhang H, Zhou RX (2015) Involvement of nuclear receptor RZR/RORγ in melatonin-induced HIF-1α inactivation in SGC-7901 human gastric cancer cells. Oncol. Rep. 34 (5): 2541-2546. DOI: 10.3892/or.2015.4238.
Hardeland R (2018) Review Melatonin and retinoid orphan receptors: Demand for new interpretations after their exclusion as nuclear melatonin receptors. Melatonin Res.1: 78-93. DOI: 10.32794/mr11250005.
Witt-Enderby PA, Radio NM, Doctor JS, Davis VL (2006) Therapeutic treatments potentially mediated by melatonin receptors: potential clinical uses in the prevention of osteoporosis, cancer and as an adjuvant therapy. J. Pineal Res. 41: 297–305. DOI: 10.1111/j.1600-079X.2006.00369.x.
Ahluwalia A, Brzozowska IM, Hoa N, Jones MK, Tarnawski AS (2018) Melatonin signaling in mitochondria extends beyond neurons and neuroprotection: Implications for angiogenesis and cardio/gastroprotection. Proc. Natl. Acad. Sci. 115 (9): E1942-E1943. DOI: 10.1073/pnas.1722131115.
Hevia D, González-Menéndez P, Quiros-González I, Miar A, Rodríguez-García A, Tan DX, Reiter RJ, Mayo JC, Sainz RM (2015) Melatonin uptake through glucose transporters: a new target for melatonin inhibition of cancer. J. Pineal Res. 58 (2): 234-250. DOI: 10.1111/jpi.12210.
Tan DX, Manchester LC, Qin L, Reiter RJ (2016) Melatonin: A mitochondrial targeting molecule involving mitochondrial protection and dynamics. Int. J. Mol. Sci. 17 (12): 2124. DOI: 10.3390/ijms17122124.
Huo X, Wang C, Yu Z, Peng Y, Wang S, Feng S, Zhang S, Tian X, Sun C, Liu K, Deng S, Ma X (2017) Human transporters, PEPT1/2, facilitate melatonin transportation into mitochondria of cancer cells: An implication of the therapeutic potential. J. Pineal Res. 62 (4). DOI: 10.1111/jpi.12390.
Mayo JC, Aguado A, Cernuda-Cernuda R, Álvarez-Artime A, Cepas V, Quirós-González I, Hevia D, Sáinz RM (2018) Melatonin Uptake by Cells: An Answer to Its Relationship with Glucose? Molecules 23 (8). pii: E1999. DOI: 10.3390/molecules23081999.
Reiter RJ (2000) Melatonin: Lowering the high price of free radicals. News Physiol. Sci. 15: 246–250.
Rodriguez C, Mayo JC, Sainz RM, Antolin I, Herrera F, Martin V, Reiter RJ (2004) Regulation of antioxidant enzymes: A significant role for melatonin. J. Pineal Res. 36: 1–9.
Pal PK, Hasan NK and Maitra SK (2016b) Temporal relationship between the daily profiles of gut melatonin, oxidative status and major digestive enzymes in carp Catla catla. Biol. Rhythm Res. 47: 755–771. DOI: 10.1080/09291016.2016.1191697.
Martín M, Macías M, León J, Escames G, Khaldy H, Acuña- Castroviejo D (2002) Melatonin increases the activity of the oxidative phosphorylation enzymes and the production of ATP in rat brain and liver mitochondria. Int. J. Biochem. Cell. Biol. 34: 348–357. https://doi.org/10.1016/S1357-2725(01)00138-8.
Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre-Jimenez M, Qin L (2016) Melatonin as an antioxidant: under promises but over delivers. J. Pineal Res. 61: 253–278. DOI: 10.1111/jpi.12360.
Reiter RJ, Tan DX, Manchester LC, Qi W (2001) Biochemical reactivity of melatonin with reactive oxygen and reactive nitrogen species: A review of the evidence. Cell. Biochem. Biophys. 34: 237–256. DOI: 10.1385/CBB:34:2:237.
Tan DX, Reiter RJ, Manchester LC, Yan MT, El-Sawi M, Sainz RM, Mayo JC, Kohen R, Allegra M, Hardeland R (2002) Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr. Top. Med. Chem. 2: 181-197. https://www.ncbi.nlm.nih.gov/pubmed/11899100.
Cuzzocrea S, Reiter JR (2002) Pharmacological action of melatonin in acute and chronic inflammation. Curr. Top. Med. Chem. 2: 153–165. DOI: 10.2174/ 1568026023394425.
Manchester LC, Coto-Montes A, Boga JA, Andersen LP, Zhou Z, Galano A, Vriend J, Tan DX, Reiter RJ (2015) Melatonin: an ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 59: 403–419. DOI: 10.1111/jpi.12267.
Tan DX, Reiter RJ (2019) Mitochondria: the birth place, battle ground and the site of melatonin metabolism in cells. Melatonin Res. 2: 44-66. DOI: https://doi.org/https:// doi.org/10.32794/mr11250011.
Torres-Farfan C, Richter HG, Rojas-García P, Vergara M, Forcelledo ML, Valladares LE, Torrealba F, Valenzuela GJ, Seron- Ferre M (2003) mt1 Melatonin receptor in the primate adrenal gland: inhibition of adrenocorticotropin-stimulated cortisol production by melatonin. J. Clin. Endocrinol. Metab. 88: 450–458. DOI: 10.1210/jc.2002-021048.
Saito S, Tachibana T, Choi YH, Denbow DM, Furuse M (2005) ICV melatonin reduces stress responses in neonatal chicks. Behav. Brain Res. 165: 197–203. DOI: 10.1016/j.bbr.2005.06.045.
Brozozowski T, Konturek PC, Konturek SJ, Bubenik GA (1997) The role of melatonin and L-tryptophan in prevention of acute gastric lesions induced by stress, ethanol, ischemia and aspirin. J. Pineal Res. 23: 79–89. https://pdfs.semanticscholar.org/ e851/bd31b7bf03f282a83ade3193e1f76aa6338d.pdf.
Konturek PC, Konturek SJ, Majka J, Zembala M, Hahn EG (1997) Melatonin affords protection against gastric lesions induced by ishemia-reperfu-sion possibly due to its antioxidant and muco-sal microcirculatory effects. Eur. J. Pharmacol. 322: 73–77.DOI: 10.1016/S0014-2999(97)00051-4.
Cabeza J, Alarcón-de-la-Lastra C, Jiménez D, Martín MJ, Motilva V (2003) Melatonin modulates the effects of gastric injury in rats: role of prostaglandins and nitric oxide. Neurosignals 12: 71–77. DOI: 10.1159/000071816.
Ates B, Yilmaz I, Geckil H, Iraz M, Birincioglu M, Fiskin K (2004) Protective role of melatonin given either before ischemia or prior to reperfusion on intestinal ischemia-reperfusion damage. J. Pineal Res. 37: 149–152. DOI:10.1111/j.1600079X.2004. 00148.x.
Sileri P, Sica GS, Gentileschi P, Venza M, Benavoli D, Jarzembowski T, Manzelli A, Gaspari AL (2004) Melatonin reduces bacterial translocation after intestinal ischemia-reperfusion injury. Transplant Proc. 36: 2944–2946. DOI:10.1016/j.transproceed. 2004.10.085.
Ustundag B, Kazez A, Demirbag M, Canatan H, Halifeoglu I, and Ozercan IH (2000) Protective effect of melatonin on antioxidative system in experimental ischemia-reperfusion of rat small intestine. Cell Physiol. Biochem. 10: 229–236. DOI: 10.1159/000016354.
Ozacmak VH, Sayan H, Arslan SO, Altaner S, Aktas RG (2005) Protective effect of melatonin on contractile activity and oxidative injury induced by ischemia and reperfusion of rat ileum. Life Sci. 76: 1575–1588. DOI: 10.1016/j.lfs.2004.08.031.
Ganguly K, Maity P, Reiter RJ, Swarnakar S (2005) Effect of melatonin on secreted and induced matrix metalloproteinase -9 and -2 activity during prevention of indomethacin-induced gastric ulcer. J. Pineal Res. 39: 307–315. DOI: 10.1111/j.1600-079x.2005.00250.x.
Ganguly K, Kundu P, Banerjee A, Reiter RJ, Swarnakar S (2006) Hydrogen peroxide-mediated down regulation of matrixmetalloprotease-2 in indomethacin-induced acute gastric ulceration is blocked by melatonin and other antioxidants. Free Radic. Biol. Med. 41: 911–925. https://doi.org/10.1016/j.freeradbiomed.2006.04.022.
Poeggeler B, Reiter RJ, Hardeland R, Sewerynek E, Melchiorri D, Barlow-Walden LR (1995) Melatonin, a mediator of electron transfer and repair reactions acts synergistically with the chain breaking antioxidants ascorbate trolox and glutathione. Neuroendocrinol. Lett. 17: 87-92. DOI: 10.1186/1743-7075-2-22.
Taslidere E, Vardi N, Parlakpinar H, Yıldız A, Taslidere B, Karaaslan MG (2018) Effects of melatonin on acetylsalicylic acid induced gastroduodenal and jejunal mucosal injury. Biotech. Histochem. 93: 485-495. DOI: 10.1080/10520295.2018.1442020.
Tan DX, Manchester LC, Terron MP, Flores LJ, Reiter RJ (2007) One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 42 (1): 28-42. DOI: 10.1111/j.1600-079X.2006.00407.x.
Tordjman S, Chokron S, Delorme R, Charrier A, Bellissant E, Jaafari N, Fougerou C (2017) Melatonin: Pharmacology, functions and therapeutic benefits. Curr. Neuropharmacol. 15 (3): 434–443. DOI: 10.2174/1570159X14666161228122115.
Reiter RJ, Tan DX, Rosales-Corral S, Galano A, Zhou XJ, Xu B (2018) Mitochondria: Central organelles for melatonin's antioxidant and anti-aging actions. Molecules 23 (2). pii: E509. DOI: 10.3390/molecules23020509.
Jenwitheesuk A, Nopparat C, Mukda S, Wongchitrat P, Govitrapong P (2014) Melatonin regulates aging and neurodegeneration through energy metabolism, epigenetics, autophagy and circadian rhythm pathways. Int. J. Mol. Sci. 15: 16848-16884. DOI:10.3390/ijms150916848.
Rehman SU, Ikram M, Ullah N, Alam SI, Park HY, Badshah H, Choe K, Kim MO (2019) Neurological enhancement effects of melatonin against brain injury-induced oxidative stress, neuroinflammation, and neurodegeneration via AMPK/CREB signaling. Cells 8: 760. DOI:10.3390/cells8070760.
Bonnefont-Rousselot D, Collin F (2010) Melatonin: action as antioxidant and potential applications in human disease and aging. Toxicology 278 (1): 55-67. DOI: 10.1016/j.tox.2010.04.008.
Hacışevki A, Baba B (2018) An overview of melatonin as an antioxidant molecule: a biochemical approach. In: Dragoi CM (Ed.) Melatonin molecular biology, clinical and pharmaceutical approaches Pp. 59-85. DOI: 10.5772/intechopen.79421.
Faixi S, Faixova Z, Boldizarova K, Javorsky P (2005) The effect of long-term high heavy metal intake on lipid peroxidation of gastrointestinal tissue in sheep. Vet. Med. Czech. 50 (9): 401–405.
Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE (2014) Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 94: 329–354. DOI:10.1152/physrev.00040.2012.
León J, Acuña-Castroviejo D, Escames G, Tan DX, Reiter RJ (2005) Melatonin mitigates mitochondrial malfunction. J. Pineal Res. 38: 1–9. DOI: 10.1111/j.1600-079x.2004.00181.x.
Xu S, Pi H, Zhang L, Zhang N1, Li Y, Zhang H, Tang J, Li H, Feng M, Deng P, Guo P, Tian L, Xie J, He M, Lu Y, Zhong M, Zhang Y, Wang W, Reiter RJ, Yu Z, Zhou Z (2016) Melatonin prevents abnormal mitochondrial dynamics resulting from the neurotoxicity of cadmium by blocking calcium-dependent translocation of Drp1 to the mitochondria. J. Pineal Res. 60: 291-302. DOI: 10.1111/jpi.12310.
Mitra E, Bhattacharjee B, Pal PK, Ghosh AK, Mishra S, Chattopadhyay A, Bandyopadhyay D (2019) Melatonin protects against cadmium-induced oxidative damage in different tissues of rat: a mechanistic insight. Melatonin Res. 2: 1-21. DOI: 10.32794/mr11250018.
Li M, Pi H, Yang Z, Reiter RJ, Xu S, Chen X, Chen C1, Zhang L, Yang M, Li Y, Guo P1, Li G, Tu M, Tian L, Xie J, He M, Lu Y, Zhong M, Zhang Y, Yu Z, Zhou Z (2016) Melatonin antagonizes cadmium-induced neurotoxicity by activating the transcription factor EB-dependent autophagy-lysosome machinery in mouse neuroblastoma cells. J. Pineal Res. 61: 353-369. DOI: 10.1111/jpi.12353.
Sener G, Sehirli AO, Ayanoglu-Dülger G (2003) Melatonin protects against mercury (II)-induced oxidative tissue damage in rats. Pharmacol. Toxicol. 93: 290-296. DOI: 10.1111/j.1600-0773.2003.pto930607.x.
Zhang Y, Wei Z, Liu W, Wang J, He X, Huang H, Zhang J, Yang Z (2017) Melatonin protects against arsenic trioxide-induced liver injury by the upregulation of Nrf2 expression through the activation of PI3K/AKT pathway. Oncotarget 8: 3773–3780. DOI: 10.18632/oncotarget.13931.
Mishra S, Ghosh D, Dutta M, Chattopadhyay A, Bandyopadhyay D (2013) Melatonin protects against lead-induced oxidative stress in stomach, duodenum and spleen of male Wistar rats. J. Pharm. Res.1: 997-1004.
Pandi-Perumal SR, Srinivasan V, Maestroni GJ, Cardinali DP, Poeggeler B, Hardeland R (2006) Melatonin: Nature's most versatile biological signal? FEBS J. 273: 2813–2838. DOI: 10.1111/j.1742-4658.2006.05322.x.
Konturek PC, Konturek SJ, Burnat G, Brzozowski T, Brzozowska I, Reiter RJ (2008) Dynamic physiological and molecular changes in gastric ulcer healing achieved by melatonin and its precursor L-tryptophan in rats. J. Pineal Res. 45: 180–190. DOI: 10.1111/j.1600-079x.2008.00574.x.
Kusuhura H, Komatsu H, Sumichika H, Sugahara K (1999) Reactive oxygen species are involved in the apoptosis induced by nonsteroidal anti-inflammatory drugs in cultured gastric cells. Eur. J. Pharmacol. 383: 331–337. https://doi.org/10.1016/S0014-2999(99)00599-3.
Mei Q, Diao L, Xu J, Liu X, Jin J (2011) A protective effect of melatonin on intestinal permeability is induced by diclofenac via regulation of mitochondrial function in mice. Acta Pharmacol. Sin. 32: 495–502. DOI: 10.1038/aps.2010.225.
Bandyopadhyay D, Ghosh G, Bandyopadhyay A, Reiter RJ (2004) Melatonin protects against piroxicam-induced gastric ulceration. J. Pineal Res. 36: 195–203. https://doi.org/ 10.1111/j.1600-079X.2004.00118.x.
Brzozowska I, Konturek PC, Brzozowski T, Konturek SJ, Kwiecien S, Pajdo R, Drozdowicz D, Pawlik M, Ptak A, Hahn EG (2002) Role of prostaglandins, nitric oxide, sensory nerves and gastrin in acceleration of ulcer healing by melatonin and its precursor, L-tryptophan. J. Pineal Res. 32: 149–162. https://doi.org/10.1034/j.1600-079x.2002.1o811.x.
Brzozowski T, Konturek PC, ZwirskaKorczala K, Konturek SJ, Brzozowska I, Drozdowicz D, Sliwowski Z, Pawlik M, Pawlik WW, Hahn EG (2005) Importance of the pineal gland, endogenous prostaglandins and sensory nerves in the gastroprotective actions of central and peripheral melatonin against stress-induced damage. J. Pineal Res. 39: 375–385. https://doi.org/10.1111/j.1600-079X.2005.00264.x.
Lissoni P, Rovelli F, MeregalliS, Fumagalli L, Musco F, Brivio F, Brivio O, Esposti G (1997) Melatonin as a new possible anti-inflammatory agent. J. Biol. Regul. Homeost. Agents. 11: 157–159.
Cuzzocrea S, Reiter JR (2002) Pharmacological action of melatonin in acute and chronic inflammation. Curr. Top. Med. Chem. 2: 153–165. DOI: 10.2174/1568026023394425.
Galano A, Tan DX, Reiter RJ (2011) Melatonin as a natural ally against oxidative stress: a physicochemical examination. J. Pineal Res. 51: 1–16. DOI: 10.1111/j.1600-079X.2011.00916.x.
Carrillo-Vico A, Lardone PJ, Alvarez-Sánchez N, Rodríguez-Rodríguez A, Guerrero JM (2013) Melatonin: buffering the immune system. Int. J. Mol. Sci. 14: 8638–8683. DOI: 10.3390/ijms14048638.
Agil A, Reiter RJ, Jiménez-Aranda A, Ibán-Arias R, Navarro-Alarcón M, Marchal JA, Adem A, Fernández-Vázquez G (2013) Melatonin ameliorates low-grade inflammation and oxidative stress in young Zucker diabetic fatty rats. J. Pineal Res. 54: 381–388. DOI: 10.1111/jpi.12012.
Dong Y, Fan C, Hu W, Jiang S, Ma Z, Yan X, Deng C, Di S, Xin Z, Wu G, Yang Y, Reiter RJ, Liang G (2016) Melatonin attenuated early brain injury induced by subarachnoid hemorrhage via regulating NLRP3 inflammasome and apoptosis signalling. J. Pineal Res. 60: 253–262. DOI: 10.1111/jpi.12300.
Cuzzocrea S, Reiter RJ (2001) Pharmacological action of melatonin in shock, inflammation and ischemia/reperfusion injury. Eur. J. Pharmacol. 426: 1–10. https://doi.org/10.1016/S0014-2999(01)01175-X.
Li JH, Yu JP, Yu HG Xi-Ming Xu, Liang-Liang Yu, Jin Liu, He-Sheng Luo (2005) Melatonin reduces inflammatory injury through inhibiting NF-kappaB activation in rats with colitis. Mediators Inflamm. 2005: 185–193. DOI: 10.1155/MI.2005.185.
Mauriz JL, Collado PS, Veneroso C, Reiter RJ, González-Gallego J (2013) A review of the molecular aspects of melatonin’s anti-inflammatory actions: recent insights and new perspectives. J. Pineal Res. 54: 1–14. DOI: 10.1111/j.1600-079X.2012.01014.x.
Vriend J, Reiter RJ (2014) Melatonin as a proteasome inhibitor. Is there any clinical evidence? Life Sci. 115: 8–14. DOI: 10.1016/j.lfs.2014.08.024.
Najafi M, Shirazi A, Motevaseli E, Rezaeyan AH, Salajegheh A, Rezapoor S (2017) Melatonin as an anti-inflammatory agent in radiotherapy. Inflammopharmacol. 25: 403-413. DOI 10.1007/s10787-017-0332-5.
Hu ZP, Fang XL, Fang N, Wang XB, Qian HY, Cao Z, Cheng Y, Wang BN, Wang Y (2013) Melatonin ameliorates vascular endothelial dysfunction, inflammation, and atherosclerosis by suppressing the TLR4/NF-kB system in high-fat-fed rabbits. J. Pineal Res. 55: 388–398. DOI: 10.1111/jpi.12085.
Akinrinmade FJ, Akinrinde AS, Amid A (2016) Changes in serum cytokine levels, hepatic and intestinal morphology in aflatoxin B1-induced injury: modulatory roles of melatonin and flavonoid-rich fractions from Chromolenaodorata. Mycotoxin Res. 32:53-60.DOI 10.1007/s12550-016-0239-9.
Carrillo-Vico A, García-Mauriño S, Calvo JR, Guerrero JM (2003) Melatonin counteracts the inhibitory effect of PGE2 on IL-2 production in human lymphocytes via its mt1 membrane receptor. FASEB J. 17: 755–757.
Favero G, Franceschetti L, Bonomini F, Rodella LF, Rezzani R (2017) Melatonin as an anti-inflammatory agent modulating inflammasome activation. Int. J. Endocrinol. 2017: 1835195. https://doi.org/10.1155/2017/1835195.
Hardeland R, Cardinali DP, Srinivasan V, SpenceDW, Brown GM, Pandi-Perumal SR, (2011) Melatonin—a pleiotropic, orchestrating regulator molecule. Progress Neurobiol. 93: 350–384.
Park YS, Chung SH, Lee SK, Kim JH, Kim JB, Kim TK, Kim DS, Baik HW (2015) Melatonin improves experimental colitis with sleep deprivation. Int. J. Mol. Med. 35: 979-986. DOI: 10.3892/ijmm.2015.2080.
Tahan G, Gramignoli R, Marongiu F, Aktolga S, Cetinkaya A, Tahan V, Dorko K (2011) Melatonin expresses powerful anti-inflammatory and antioxidant activities resulting in complete improvement of acetic-acid-induced colitis in rats. Dig. Dis. Sci. 56: 715-720. DOI: 10.1007/s10620-010-1364-5.
Zhu D, Ma Y, Ding S, Jiang H, Fang J (2018) Effects of melatonin on intestinal microbiota and oxidative stress in colitis mice. Biomed. Res. 2018: 2607679. DOI: 10.1155/2018/2607679.
Paulose JK, Cassone VM (2016) The melatonin-sensitive circadian clock of the enteric bacterium Enterobacter aerogenes. Gut Microbes. 7: 424-427. DOI: 10.1080/19490976.2016.1208892.
Yin J, Li Y, Han H, Chen S, Gao J, Liu G, Wu X, Deng J, Yu Q, Huang X, Fang R (2018) Melatonin reprogramming of gut microbiota improves lipid dysmetabolism in highfat dietfed mice. J. Pineal Res. 65: e12524. DOI: 10.1111/jpi.12524.
Bang CS, Yang YJ, Baik GH (2019) Melatonin for the treatment of gastroesophageal reflux disease; protocol for a systematic review and meta-analysis. Medicine (Baltimore). 98: e14241. DOI: 10.1097/MD.0000000000014241.
Gao J, Xu K, Liu H, Liu G, Bai M, Peng C, Li T and Yin Y (2018) Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front. Cell Infect. Microbiol. 8: 13. DOI: 10.3389/fcimb.2018.00013.
Chojnacki C, Wiśniewska-Jarosińska M, Kulig G, Majsterek I, Reiter RJ, Chojnacki J (2013) Evaluation of enterochromaffin cells and melatonin secretion exponents in ulcerative colitis. World J. Gastroenterol. 19: 3602-3607. DOI:10.3748/wjg.v19.i23.3602.
Brzezinski A (1997) Melatonin in humans. N. Engl. J. Med. 336: 186–195. DOI: 10.1056/NEJM199701163360306.
Bubenik GA, Dhanvantari S (1989) Influence of serotonin and melatonin on some parameters of gastrointestinal activity. J. Pineal Res. 7: 333–344. DOI: 10.1111/j.1600-079x.1989.tb00909.x
Lewinski A, Rybicka I, Wajs E, Szkudlinski M, Pawlikowski M (1991) Influence of pineal indol-amines on the mitotic activity of gastric and colonic mucosa epithelial cells in the rat: Interaction with omeprazole. J. Pineal Res. 10: 104–108.DOI: 10.1111/j.1600-079x.1991.tb00018.x.
This work is licensed under a Creative Commons Attribution 4.0 International License