
Please cite this paper as:
Sarkar, S., Chattopadhyay, A. and Bandyopadhyay, D. 2020. Melatonin, the advance-guard in oxidative myocardial assault instigated by exercise stress: a physiological and biochemical insight. Melatonin Research. 3, 4 (Oct. 2020), 451-475. DOI:https://doi.org/https://doi.org/10.32794/mr11250072.
Review
Melatonin, the advance-guard in oxidative myocardial assault instigated by exercise stress: a physiological and biochemical insight
Swaimanti Sarkara, Aindrila Chattopadhyayb, Debasish Bandyopadhyaya*
aOxidative Stress and Free Radical Biology Laboratory, Department of Physiology, University of Calcutta, 92, APC Road, Kolkata-700009 bDepartment of Physiology, Vidyasagar College,39, Sankar Ghosh Lane, Kolkata-700006
*Correspondence: debasish63@gmail.com, Tel: +91-9433072066
Running title: Melatonin in exercise-induced cardiac stress
Received: May 11, 2020; Accepted: August 12, 2020
ABSTRACT
Exercise conducted at an optimum training load is usually beneficial for the overall health of an individual. However, an unaccustomed intense exercise carried out by untrained individuals or elite athletes during over-training and/or competition-related stress often bear inevitable cardiovascular risks. Although many alterations occurring in the cardiovascular system during exercise are the results of training adaptations, sudden cardiovascular deaths reported in competitive athletes is a matter of grave concern. Several oxidative biomarkers that depict the underlying structural and functional impairment of the myocardial tissue have been identified in the individuals subjected to extensive exercise. The exercise-mediated cardiomyopathy is free radical related and also associated with pro-inflammatory response. In this review we will highlight the possible role of melatonin in obviating irrevocable oxidative cardiovascular injury triggered by extensive exercise stress. Melatonin effectively reduces exercise-induced lipid peroxidation, restores natural cellular antioxidant pool and supresses the innate immune cascade reaction that, otherwise, jeopardize cardiovascular integrity. Melatonin blocks the IKK/IκB/NFκB signaling as well as suppress iNOS and COX-2 mediated inflammation in cardiac tissue. In addition, melatonin reduces blood lactate accumulation and accelerates glucose utilization, thereby, promoting energy metabolism in athletes during their training and competition. Physical exertion associated overheating and the resultant sympathetic outflow impede cardiovascular homeostasis. Melatonin not only attenuates the sympathomedullary stimulation but also protects the cardiac cells from the cytotoxic effect of catecholamines. The available information regarding the efficacy of melatonin in amelioration of exercise-driven oxidative insult in cardiac tissue has been discussed and summarized.
Key words: exercise, oxidative stress, cardiovascular system, inflammatory response, cardiac injury, antioxidants, melatonin.
__________________________________________________________________________________________
1. INTRODUCTION
The evolution of aerobic organism and the beginning of oxygen-dependent metabolism came up with a high cost to life. Although, aerobic metabolism is a rich source of ATP, its oxygen by-products, i.e., reactive oxygen species (ROS)/reactive nitrogen species (RNS), bear a huge physiological threat due to oxidative stress (1). Fortunately, organisms have developed an antioxidant defence system equipped with small molecular antioxidants and antioxidant enzymes to scavenge or neutralize these ROS/RNS. However, “disturbance of the oxidation-reduction balance in favour of oxidants” can lead to oxidative stress and severe tissue damage in many vital organs of organisms (2).
Exercise, when conducted regularly at optimum training intensity and duration elevates the antioxidant levels in blood, muscles, liver and heart and thus, prevents free radical induced damages (3-5). In contrast, a sedentary lifestyle leads to the development of chronic diseases. It has been reported that proper exercises prevent the onset of metabolic syndrome, obesity, non-alcoholic fatty liver disease, coronary heart disease, stroke, etc. (6). The benefits of exercise on the overall health and fitness of individuals are obvious. However, exercise seems as a double-edged sword since several studies have reported that both acute aerobic and anaerobic exercise cause oxidative damage to lipids (7-8), proteins (9) and DNA (10). Exercise-induced oxidative stress has been extensively studied over the past few decades. Dillard et al. were the first to come up with the idea that muscular exercise could induce lipid peroxidation in animals and humans in 1978 (11). Since then, many studies have reported that endurance exercise or short term high-intensity workout elevate oxidative stress biomarkers in blood and other tissues of the body (12).
Habitual physical activity lowers the risk of sudden death caused by exercise-related cardiac arrest. However, vigorous exertion can increase the risk of such cardiovascular events (13). Sports classified as static exercise including weight lifting, power-lifting, body-building poses a greater risk of cardiac muscle damage, due to their high potential for ROS generation (14). Dynamic exercises including running, cycling, swimming, when performed at an intense level with anaerobic metabolism also lead to free radical production (15). Therefore, research in the protective effects of antioxidants on exercise-stress induced cardiac injury has become a field of interest for the sportsman, coaches, exercise physiologists and free radical biologists. Several studies have indicated the potential benefits of antioxidant consumption either through diet or supplementation in preventing exercise associated oxidative damage (11, 16,17). Melatonin is a molecule synthesized by the pineal gland and other tissues (18). It plays important roles in circadian rhythm, immune system and reproduction. Melatonin is also a potent antioxidant (19). Its activities have been investigated in the field of sports science and medicine. Although there are conflicting data regarding the ergogenic effect of melatonin, the antioxidant property of this compound has certainly contributed to its protective effects against exercise-induced oxidative stress (20-22). Numerous studies have clearly shown that melatonin has an ameliorative role in myocardial oxidative damage (23). Melatonin was found to reduce acute-intense exercise stress related cardiac injury (24) and acute exercise induced elevation of pro-inflammatory markers (25). However, the underlying protective mechanisms of melatonin on exercise induced cardiopathy is yet to unfold.
2. PHYSIOLOGICAL ALTERATION IN CARDIOVASCULAR HOMEOSTASIS DURING EXERCISE
Changes in cardiovascular parameters to meet the metabolic demand of muscles during exercise is a natural response (26). These changes include increased heart rate and stroke volume with the consequent increase in cardiac output (27). Exercise, is also associated with decreased maximal heart rate in trained athletes. Therefore, the augmentation of stroke volume is a major contributor to enhanced cardiac output during exercise (28, 29). An increased stroke volume, on the other hand, is an outcome of multiple adaptive changes in the heart including alterations in morphological and functional aspects. Early in nineteenth century, Henschen et al., reported training-induced cardiac hypertrophy in cross country skiers (30). Although they used the simple method of chest percussion, the advent of radiographical techniques allowed researchers to confirm this study (30,31). This ultimately led to the development of a new concept called “athlete’s heart”. Echocardiography, magnetic resonance imaging and necropsy studies have demonstrated the exercise related increases in left ventricular (LV) mass, its end-diastolic volume, end diastolic diameter, right ventricular (RV) mass, its end-diastolic volume and left atrial volume (29,32). These structural modifications are, in turn, responsible for functional adjustments characterized by electrocardiographic abnormalities (33). Though such changes associated with “athlete’s heart” are not considered as pathological signs, several studies reporting mortality caused by sudden cardiac arrest in athletes have raised concern (34-36).
Activation of renin-angiotensin-aldosterone system in response to exercise-induced hyperosmolality due to enhanced metabolism, sweating and hyperalbuminemia leads to water retention and increased plasma volume (37-38). Exercise also instigates androgen-mediated erythropoietin stimulation and erythrocyte generation (39). All these along with sympathetic stimulation increases cardiac output and cause elevated systolic blood pressure. When the rise in blood pressure exceeds a certain limit [(i.e., a 60mmHg (in male) or 50mmHg (in female) difference between peak and resting blood pressure in response to exercise], it is referred to as “hypertensive response to exercise” (HRE). A study has highlighted the fatal consequences of HRE caused due to hypertensive load on the left ventricle (40).
3. SPORTS, COMPETITION AND STRESS IN GENERAL
Highly competitive sporting events demand the athletes to constantly perform at a peak level which often pushes the limits of their individual capacity. Apart from the professional athletes, many novice individuals, in order to cope with the growing aesthetic standards, often emulate themselves and practice heavy exercise recklessly, which cause tissue damage and jeopardize their health (41). During sporting events or competition, athletes experience a number of physical, emotional and psychosocial demands that evoke a complex set of physiological stress reactions (42). Cardiovascular performance is modulated by the physiological responses of the body to various stressful conditions that an athlete encounters during the competition period. At the initial phase of successful control over the challenge, there is an increased release of testosterone, with optimum levels of gonadotrophins and oxytocin hormones which are known to maintain a state of stability in species (43). With constant challenge, the testosterone, oxytocin and gonadotrophin levels eventually fall (43-44). In this situation, the fight hormone, norepinephrine, comes into play and supports the individual who struggles to cope with the constantly increased stress (43). As anxiety, uncertainty and perception of probable defeat grow, epinephrine secretion increases as a manifestation of flight response (45). Such activation of the adreno-medullary system initiates the adreno-cortical response as the athlete suffers pronounced distress, helplessness, and over-exhaustion (46). On the other hand, daytime extensive endurance exercise or even a moderate exertion under hypoxic conditions will enhance melatonin secretion to serve as a defence mechanism against oxygen deficiency related free radical attack (47-48). A prolonged over-exhaustive exercise bout or competition can overload body’s hormonal defence including activation of adrenal gland, testicles and pineal gland. Over activities of these organs finally will fall off drastically, leading to loss of endocrine harmony (47).
Physiological stress is often manifested at molecular level as oxidative stress as unbalance between the generation and detoxification of ROS (2). ROS are by-products of mitochondrial respiration or xanthine oxidase, arachidonate cascade, nitric oxide synthase, and NADH/NADPH oxidase (49). For example, superoxide anion is dismutated to hydrogen peroxide (H2O2) under the action of superoxide dismutase. When the transition metals are available H2O2 is catalysed to most active hydroxyl radical (∙OH) by Fenton reaction, or by Haber-Weiss reaction, in which, superoxide anion is the catalyser. ∙OH has the ability to alter the structural integrity of membrane lipids and proteins, and even damages the ultrastructure of DNA (1). A consensus about the involvement of ROS/RNS in the pathogenesis of a myriad of disorders has been well documented in the scientific literature (50).
4. BIOMARKERS OF EXERCISE INDUCED OXIDATIVE CARDIAC INJURY
During exercise, increased aerobic demand promotes a huge flow of oxygen to the active tissues, which subsequently favours generation of ROS (51). After the first evidence on exercise mediated elevation of oxidative stress was published in the late 1970s (11), more and more studies have confirmed this early observation. Brady et al., in 1979 reported that swimming exercise was associated with significant increase in hepatic and muscular lipid peroxidation (LPO) and declined erythrocyte glutathione reductase as well as glucose-6-phosphate dehydrogenase activities (16). In a clinical study, patients with chronic heart failure (CHF) were asked to participate in a 30 minutes exercise regime of moderate-intensity and this resulted in their post-workout increase in plasma malondialdehyde which continued for an hour even after the exercise session. In addition, their plasma pro-inflammatory markers were also elevated immediately after exercise (52). Although the authors concluded that such alterations in blood parameters could be a result of adaptation in CHF patients (52), the irreversible damage caused by lipid peroxidation in these subjects must be taken into consideration. One of the serum indicators for cardiac stress is heart-specific troponin protein. Troponin complex comprises of three loosely attached subunits: troponin I (TnI), troponin C (TnC), and troponin T (TnT), each having discrete functions. TnI associates with actin, TnT with tropomyosin, and TnC has a binding affinity for calcium. This protein complex plays a critical role in calcium driven muscle contraction via actin-myosin interaction (53). Cardiac specific troponin I (cTnI) and troponin T (cTnT) are released into the blood from cardiomyocytes when cardiac tissue is subjected to extensive stress, including exercise stress (54-55). An increase in cTnI and cTnT above 99th percentile of the blood concentrations of control individuals is a marker of myocardial injury (56-57). Cardiac troponins can remain elevated in blood for 24 hours to 5 days or more depending on the severity of myocardial damage (58). Two possible mechanisms for exercise-induced cardiac troponin release have been proposed. One is that when the exercise-induced damage is less severe, the membrane leakage of cardiac myocyte leads to the release of cTnI (3%) and cTnT (6%) from cytosolic troponin pool. The other is that an intense exercise can cause disruption of contractile machinery in cardiomyocytes, thus resulting in erosion of structurally bound cardiac troponin into the circulation (53). In addition, functional impairment is also a result of exercise-induced myocardial stress. the biomarkers including brain natriuretic peptide (BNP) (or B-type natriuretic peptide) and N-terminal prohormone of brain natriuretic peptide (NT-proBNP) are reflected in myocardial dysfunction since both are released from atrium and ventricle of heart caused by increased intravascular pressure (59-61). The endurance exercise can cause the release of NT-proBNP in an age-dependent manner (62-63). Animal studies and clinical investigations have revealed the role of free radicals in exercise associated elevation of plasma NT-proBNP (53). There are still disputes regarding whether the increases in circulatory cardiac troponin and BNP attribute to cardiac damage or they are the protective mechanisms. This issue deserves further investigation. Circulatory creatine kinase and its cardiac specific isozyme, CK-MB, are the additional markers of cardiac tissue damage (64). A rise in CK-MB level and/or activity during acute exercises such as intermittent training and marathon running indicate a significant cardiac stress (65-66). Ischaemia-modified albumin (IMA), a biomarker for myocardial ischaemia, has been implemented for testing exercise-driven cardiac ischaemia and systemic acidosis (67). This novel marker detects the binding affinity of albumin with cobalt, which is particularly diminished in ischaemic condition, leading to an increasing IMA concentration (68). In athletes, prolonged exercise causes an elevation of mean IMA concentration during 24-48 hours post-exercise (69-70). However, albumin concentration also increases during an acute bout of exercise and ischaemia in tissues other than the cardiac muscles. This will influence the determination whether this increase of IMA is dedicated to cardiac damage (67), thus, this biomarker has its limitation in its use in detecting the impact of exercise on cardiac injury.
5. INFLAMMATORY BIOMARKERS OF CARDIAC TISSUE INDUCED BY EXTENSIVE EXERCISE
There is a strong association between oxidative stress and circulatory inflammatory cytokines and adhesion molecules. This is also the case following an intense exercise session (25). For the readers’ convenience, we illustrate these associations in Figure 1.

Fig. 1. Summarization of the potential outcomes of strenuous exercise associated oxidative and inflammatory responses in cardiac cells.
PG: Prostaglandin; NO: Nitric oxide; BNP: B-type natriuretic peptide; CK-MB: Cardiac specific isozyme of creatine kinase; COX-2: Cyclooxygenase-2; cTNI: Cardiac specific troponin I; cTNT: Cardiac specific troponin T; IKK: I kappa B kinases; IL: Interleukin; IMA: Ischaemia-modified albumin; iNOS: Inducible nitric oxide synthase; IκB: I kappa B; LPO: Lipid peroxidation; MPO: Myeloperoxidase; NFκB: Nuclear factor kappa-light-chain-enhancer of activated B cells; NT-proBNP: N-terminal prohormone of brain natriuretic peptide; proBNP: Prohormone of brain natriuretic peptide; ROS: Reactive oxygen species; TNF-α: Tumour necrosis factor alpha.
Increased serum malondialdehyde level along with a rise in plasma pro-inflammatory cytokines are observed in patients with cardiac morbidity performing moderate exercise (52). Prolonged exercises cause impairment in cardiac function with left systolic and diastolic dysfunction (71). Exercise-induced pro-inflammatory response is directly associated with right ventricular dysfunction (72). High-intensity exercise for long duration leads to the release of pro-inflammatory cytokines including tumour necrosis factor alpha (TNF-α), interleukin 12p70 (IL-12p70) and interleukin 1β (IL-1β) in athletes performing ultra-endurance triathion (72). A significant number of trained ultra-endurance athletes have myocardial dysfunction with elevated BNP and cTnI levels following completion of ultra-endurance activities (72). The results definitely raised a vital question as to whether these changes are indications for underlying inflammatory and necrotic damage in cardiac tissue. A correlation between raised circulatory troponin and BNP levels with an escalation of plasma myeloperoxidase (MPO) concentration has been observed in marathon runners (73). A high plasma MPO level is often considered as a prodrome of possible cardiac arrest and death following a myocardial infarction (74). Oxidative stress orchestrates a series of signalling events leading to generation of Nuclear Factor kappa-light-chain-enhancer of activated B cells (NFκB) which up-regulates inflammatory cytokines, adhesion molecules as well as enzymes participating in inflammatory cascades such as inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) (75). Exercise also activates I kappa B kinases (IKK) which promotes degradation of IκB, an inhibitor of NFκB (76). In mice, acute exercise activates NFκB in skeletal and cardiac muscles (25, 77). A significant increase in both COX-2 and iNOS transcription and translation was observed in myocardial tissue of rat after acute bouts of exercise (25).
6. APPLICATION OF ANTIOXIDANTS IN EXERCISE AND SPORTS
The recommendation of antioxidants to athletes should be individualized, based on exercise intensity, duration, the type of sports and training regime. Micronutrients, viz., vitamins and minerals play a vital role in maintaining the natural antioxidant defence function to avoid oxidative stress (78).
Vitamin C is very effective in mitigating tissue oxidative damage impelled by exhaustive exercise (79). Muscular damage, fatigue, and immune dysfunction (80) are common in athletes who play intense sports, perform in harsh weather conditions and have to travel across time zones. Vitamin C consumed in an adequate amount through diet has beneficial effects in protecting against these disturbances (79). However, some studies found that vitamin C consumed in doses higher than 1 gram a day has impaired training adaptations and athletic performance by possible disruption of mitochondrial biogenesis (81,82).
Vitamin E, another well-known antioxidant, enhances sport performance upon acute parenteral administration (83). Nevertheless, long term use of vitamin E has been found to have a negative impact on performance as documented in a number of surveys related to elite athletes (84-86). Studies conducted on mountain climbers; however, have indicated that vitamin E might have a promisingly ameliorative effect against oxidative damage of erythrocytes that otherwise would have led to a deterioration in performance (79, 87-88).
Quercetin is a natural flavonoid found in many fruits and vegetables and it exhibits a plethora of biological functions including the antioxidant, anti-inflammatory and cardioprotective activities among others (89). Quercetin not only promotes mitochondrial biogenesis and exercise tolerance marked by enhanced endurance capacity (90), but it also regulates the immune response in individuals under loaded training regime (91). These results indicate that quercetin can be a protective molecule against exercise-induced oxidative stress in animals, but the data in human subjects are scanty.
Resveratrol, a phytochemical present in the Mediterranean diet, is also known to have antioxidant activities (92). This compound has been found to protect against oxidative stress generated by strenuous treadmill running in rats. Elevated levels of malondialdehyde, 4-hydroxy-2-nonenal, 8-hydroxy-2'-deoxyguanosine, creatine kinase, and lactate dehydrogenase were reduced after resveratrol administration (93). Other studies have revealed that resveratrol can induce mitochondrial growth and increase endurance capacity in actively exercising animals (94). However, resveratrol has shown potential deleterious effect in inactive rodents and sedentary elderly humans (79, 95). Thus, its use as a sport supplement seems to depend on the activity status of the individuals and one should take precautions before using it as a sport supplement agent.
Curcumin is a popular spice, a natural food colouring and flavouring agent (96). At moderate concentrations, it displays significant antioxidant and anti-inflammatory property (97, 98). Studies have demonstrated that curcumin potentially reduces serum lipid peroxidation level and restore normal serum reduced glutathione (GSH) content in subjects participating in intense running (99). Curcumin reduces inflammatory reaction in mice performing full-body exercise (100), improves the antioxidant status in humans participating in exercise (101) and decreases muscle soreness and damage in participants performing moderate to high-intensity aerobic exercises (102).
Other substances may also have beneficial effects on the sport related oxidative injuries. For example, N-acetyl cysteine (NAC) that acts as a cysteine donor is known to replenish the GSH pool in the body. Hence the supplementation of NAC delays free radical-mediated muscular fatigue and enhances performance in endurance athletes (102-104). Besides, the use of coenzyme Q10, β carotene, and several polyphenols as sport supplements for reducing exercise-induced oxidative stress has been reported in the literature (105).
7. MELATONIN
Melatonin was first isolated from the bovine pineal gland in 1958, and its chemical identity was revealed as N-acetyl-5methoxytryptamine (106, 107). Melatonin is consistently reported to be a pineal hormone associated with the regulation of circadian rhythm (108). However, extensive research over the past few decades have explored the diverse physiological roles of this tryptamine derivative. These include the metabolic (109), reproductive (110), gastro-protective (111-112), cardioprotective (113), neuropsychiatric (114), immuno-stimulatory (115), oncostatic (116), antioxidant (19,117-118) and anti-inflammatory functions. In addition to its pleiotropic effects, melatonin is known to be synthesized in a number of extra-pineal sites, including the cardiac tissue (23), therefore, it can produce its autacoid and paracoid actions.
7.1. Melatonin as a protector against oxidative damage.
The excessively long-lasting biochemical reactions or the exposure of exogenous stressors lead to the obligatory production of ROS in biological system. In the absence of adequate antioxidant defense, the ROS attack the bio-molecules that mark the onset of several pathological mechanisms. Melatonin is a potent antioxidant and free radical scavenger that can effectively protect against these detrimental outcomes (1). Melatonin exhibits its antioxidant ability by either its direct free radical scavenging property or indirectly by acting on its receptors (117). Owing to its amphiphilic nature, melatonin can efficiently cross the cellular membrane and present itself in the site of ROS generation, to detoxify ROS (119). This on-site detoxifying action can annihilate up to ten ROS with a single melatonin molecule (120). Melatonin also interacts with transition metals to abolish the formation of hydroxyl radicals (121). In addition, its receptor mediated action can boost up the body’s inherent antioxidant capacity to alleviate the oxidative stress. Melatonin receptor1 (MT1) and melatonin receptor2 (MT2) are G-protein coupled receptors abundantly distributed in different regions of the central nervous system as well as the peripheral organs including the cardiac and vascular tissues, where these receptors synergistically operate to regulate the phosphoinositol, cGMP and cAMP signalings (119). Melatonin receptor3 (MT3) is an enzyme (quinone reductase-2) having antioxidant activity too and is also involved in promoting the efficacy of chemotherapy (122). Additionally, melatonin has been reported to act as a ligand for retinoic acid-related orphan receptors (RORα/RZR), although such interaction of melatonin is still a matter of debate (119). Melatonin can promote both gene expression and activity of a number of cellular antioxidant enzymes including superoxide dismutase, catalase, and glutathione peroxidase (117).
7.2. Melatonin and its effect on cardiovascular functions.
The potent beneficial effect of endogenous and exogenous melatonin for optimum cardiovascular functions has been well established by both animal and clinical studies (23). Melatonin exerts its physiological effect in the cardiovascular system either by its receptor-independent or receptor mediated actions (123). Both MT1 and MT2 are present in the cardiac tissue and blood vessels, particularly in the cardiomyocytes and coronary arteries (124,125). Activation of MT-1 is associated with arterial vasoconstriction, while vasodilation is mediated by MT-2 type receptor (126). Plasma melatonin concentration is elevated at the night, which is responsible for the alleviation of nocturnal hypertension and rapid heart rate (127). Melatonin reduces blood pressure either by central action or by antagonizing the effect of catecholamines and by relaxation of vascular smooth muscle via inhibition of α1adrenoceptor, endothelial nitric oxide production and/or antioxidant and anti-inflammatory mechanism (23, 128, 129). All of these activities of melatonin contribute to the prevention of hypertensive effect of nitric oxide synthase inhibitor (viz., NLG-nitro-L-arginine methylester) in a renin-angiotensin-aldosterone system (RAAS) independent manner (130). Mitochondrial stability takes a crucial part in preserving vascular health and restoring a normotensive state. Melatonin potentially inhibits mitochondrial ROS generation and intrinsic apoptotic pathway thereby, preventing mitochondrial permeability transition pore opening (mPTP). Further, melatonin promotes mitofusin-2 driven protection of mitochondria Such protective functions of melatonin in mitochondria are not only involved in reducing hypertension but are also associated with metabolic stability (129). RAAS activation usually causes mitochondrial dysfunction and pro-inflammatory response via NFκB signalling and inflammasome activation. Thus, RAAS activation has been associated with impaired glucose homeostasis, insulin resistance, and obesity, which in turn disrupts cardiovascular harmony. Melatonin effectively protects against such metabolic disturbances and the concomitant cardiovascular abnormalities (129).
Numerous animal and human studies have demonstrated the potential therapeutic effects of melatonin in a wide range of cardiovascular conditions (126). Melatonin treatment alleviates conduction disorders and lethal arrhythmic changes in the heart under different circumstances (131).This activity is mediated by antioxidant and anti-adrenergic action of melatonin, its ability to stimulate the expression of connexin-43 that facilitates cell-cell transmission of electrical impulse and up-regulation of the cardioprotective protein kinase C expression in cardiac tissue (132-134). Patients with coronary heart disease and myocardial ischaemia has lower night-time plasma melatonin than that in control subjects (127). Melatonin administration exhibits cardioprotective effect and reduces infarct size in patients with ST-segment elevation and myocardial infarction, depending upon the time of intervention, age-group, and disease severity, or whether the individuals receive percutaneous coronary intervention (135, 136). Biotransformation of melatonin into 6-sulfatoxymelatonin promotes its urinary excretion (137). Patients with coronary artery disease, stable and unstable angina pectoris, as well as those with acute and chronic congestive heart failure, have markedly lower levels of urinary 6-sulfatoxymelatonin (126, 138, 139). Furthermore, with the dramatic fall in melatonin concentration in the early morning from its night time peak, the highest occurrence of myocardial infarction and cardiac stroke has been noted (127). Day-time serum melatonin concentration has been implicated in cardiac re-synchronization therapy for patients with congestive heart failure and associated ventricular de-synchrony. This therapy improves morphological integrity and functional capacity of the left ventricle (140). Melatonin inhibits platelet aggregation and oxidative modification of low-density lipoprotein (LDL), thus probably having an anti-atherogenic property. In addition to that, the melatonin level was found to be significantly low in patients with high blood cholesterol or LDL levels (113). Due to the potent antioxidant, anti-inflammatory, and anti-apoptotic property, melatonin has been frequently suggested as a possible therapeutic agent in cardiac ischaemia-reperfusion injury and the other cardiovascular disorders.
7.3. Melatonin as a molecule of interest in the field of exercise physiology.
Evolutionary biologists speculated that melatonin had originated in nature when the early forms of life came into existence. The original and primary function of melatonin is to detoxify harmful free radicals generated in the aerobic metabolism of organisms (141). Such a detoxification function is crucial for the survival of species under the selective pressure of nature (142). In higher organisms, exercise mimics this natural stress condition that challenges the organism at the level of cell, tissue, organ, and system, particularly the cardiopulmonary system (143). Intriguingly, melatonin has been found to mitigate exercise-induced oxidative stress-mediated cellular damages (20-22).
Unlike the classical antioxidants such as glutathione, vitamin C and vitamin E, melatonin does not undergo redox reaction, but rather participates in additive reaction with ROS or RNS to generate stable water-soluble compounds that can be easily excreted (1). Considering the fact that melatonin lacks obvious toxic effect even at a considerably high dose and is available as a food supplement (144-145), these features have fascinated many melatonin researchers and exercise physiologists to explore the possible beneficial effect of melatonin against exercise stress. Melatonin supplementation decreases the lactate levels in rats with swimming exercise and also promotes their energy retention for a longer time, probably by regulating the circulatory zinc levels (146). Moreover, a study showed that the pre-exercise melatonin administration in the active individuals effectively induced early shift towards utilization of carbohydrates as an energy source during exercise (147). This might be beneficial for type-II diabetic patients, since it promotes glucose mobilization at an early stage during aerobic exercise. Melatonin ingestion caused a notable reduction in muscle pain which is attributable to the decline in blood lactate dehydrogenase and creatine kinase levels in soccer players participating in “intensive training camp” (148), suggesting a vital role of melatonin in muscle healing process. This is consistent with the studies on rodents where exogenous melatonin promoted post-injury skeletal muscle repair and regeneration (149, 150). Melatonin administration effectively prevented oxidative stress and the associated iNOS and NFκB up-regulation in rat skeletal muscles caused by intense exercise. The mode of action of melatonin follows the trajectory of IKK/IκB/NFκB signal abolition pathway to avert muscle damage caused by the master pro-inflammatory mediator, NFκB (75). Melatonin pre-treatment was able to alleviate swimming-induced lipid peroxidation and the decreased GSH/GSSG ratio in liver, muscle and brain in the swimming animals (151). In a recent work, melatonin was not only able to reduce circulatory malondialdehyde levels, but also stimulated the activity of superoxide dismutase, the enzyme serving as a frontline fighter against oxidative assault of superoxide anion (148). Melatonin was also reported to prevent oxidative stress-mediated bone damage in diabetic rats performing swimming exercise (152).
7.4. Melatonin as a prophylactic agent against oxidative cardiac injury actuated by strenuous exercise.
Heart being a highly metabolic organ, demands massive oxygenation leading to the excessive generation of ROS and RNS, which overwhelms the natural antioxidant defence machinery of the myocardium during strenuous exercise (153). This jeopardizes the heart to a diverse array of disease conditions greatly increasing the possibility of cardiovascular mortality (154). Vigorous exercise, both static and dynamic, exposes one to a huge risk of sudden cardiac event attributed by the generation of large amount of toxic free radicals and the consequent oxidative cardiovascular damage (14,15). In this scenario, it is important to find an efficient cardioprotective antioxidant that would not only scavenge the highly reactive free radicals, but would also restore the cardiovascular homeostasis. Although much research has been conducted on the beneficial roles of classical antioxidants in the enhancement of sports performance (79, 105), the pro-oxidant activity exhibited by these antioxidants questions their acceptability. Melatonin is considered to be a safe antioxidant molecule with strong therapeutic efficacy in cardiovascular disorders (23) without pro-oxidant activity (117). Interestingly, melatonin exhibits cardiovascular protection against exercise stress by regulating multiple physiological and biochemical pathways.
7.4.1. Melatonin protects against exercise induced cardiovascular damage by targeting the sympathomedullary pathway.
Physical exertion characterized by overheating and panting increases the chance of myocardial infarction by up to six-fold (155). Further, exercise and exercise-induced heat stress can enhance the sympathetic outflow which, in turn, increases heart rate and cardiac output that can overstress the cardiovascular system beyond its ability of regulation (156). Emotional stress and exertion during physical training and sport competition are also associated with the activation of the sympathetic nervous system and adrenal-medullary response. This triggers the accumulation of catecholamines in the blood (42). Indeed, plasma levels of both epinephrine and norepinephrine are elevated during aerobic and anaerobic exercises of varied intensities (157-159). Exercise-induced sympathetic activation possesses severe cardiovascular challenge due to the β-adrenergic receptor mediated positive dromotropic, ionotropic and chronotropic effects (160). Melatonin helps to attenuate sympathetic activity and considerably curtails norepinephrine turnover in cardiac tissue (161), thereby ameliorating β-adrenergic stimulated cardiac dysfunction. Besides, administration of a single dose of melatonin has been demonstrated to cause decreased rectal temperature in athletes performing intermittent exercise under hot climatic condition.
Catecholamines metabolism produces enormous amounts of reactive intermediates due to their auto-oxidant and pro-oxidant property (162). Administration of norepinephrine in ratspromotes lipid peroxidation, alters the activities of antioxidant enzymes, reduces GSH/GSSG ratio, as well as evokes a pro-inflammatory and apoptotic response in the cardiac tissue (162). Several studies substantiated the cardio-toxic role of adrenaline and pointed out to the fact that oxidative metabolites of epinephrine such as adrenochrome, adrenolutin and quinoproteins are the initiators of the biochemical pathway leading to cardiac damage (162, 164, 165). Melatonin protects against adrenaline induced cardio-toxicity (165). This indicates a plausible role of melatonin in amelioration of cardiac injury caused by catecholamine release and oxidative stress in heart tissue.
7.4.2. Melatonin, a powerful anti-inflammatory molecule, protects against acute exercise evoked inflammation in cardiac tissue.
Moderate to high-intensity exercise can induce myocardial stress and inflammatory reaction (52, 53). For example, rats undergoing acute swimming exercise had elevated levels of MDA and 3-nitrotyrosine in the cardiac tissue which was prevented by the administration of melatonin prior to exercise and melatonin also reduced impairment of antioxidant activities in the heart (24). Oxidative modification of tissue lipids and proteins are often associated with increased inflammatory response. In fact, pro-inflammatory cytokine levels were positively related to oxidative stress biomarkers in CHF patients under moderate workout (52). These inflammatory mediators of IL-1, IL-6, and TNF-α are responsible for aggravation of the existing cardiovascular condition (166, 167). Melatonin was able to significantly decrease elevated levels of TNF-α, IL-1 and IL-6 in rats under treadmill running (25). The cardiac damage markers, CK, and CK-MB increased upon acute exercise but was suppressed with melatonin treatment (25). Further cardiac damage is marked by neutrophil invasion in heart tissue with consequent myeloperoxidase (MPO) release (25,168). A rise in cardiac MPO activity was observed in rats upon prolonged running (167), which was prevented by pre-exercise melatonin treatment (25). Prostaglandins and nitric oxide are common mediators of tissue inflammatory response and are often reported to disrupt the integrity of cardiomyocytes. Therefore, the magnitude of tissue damage is often depicted by the levels of COX-2 and iNOS, which are the enzymes for prostaglandin and nitric oxide synthesis, respectively (169-170). Acute exercise mediated stimulation of both iNOS and COX-2 was proficiently suppressed with melatonin treatment by a mechanism of inhibiting NFκB activation in the myocardium of rats (25).
7.4.3. Melatonin as a possible therapeutic solution for exercise mediated cardiomyopathy.
A total or partial occlusion of the coronary artery may compromise the blood supply to heart leading to hypoxia/anoxia mediated injury to cardiomyocytes. Ironically, restoration of blood flow and re-oxygenation to cardiac tissue (reperfusion) cause a massive amount of ROS generation which is responsible for further detrimental effect on the heart (113). Besides inducing oxidative stress, the ischaemia reperfusion (I/R) injury also initiates inflammatory and apoptotic responses. All these events result in myocardial infarction and the associated cardiac dysfunction such as fibrillation and bradycardia (171). Acute hypoxic exercise is often associated with over-generation of ROS (172, 173). The heart is considered as the first organ to be the victim of hypoxia or anoxia caused by maximal exercise. Continuation of exercise beyond that point might put the subject at risk of developing myocardial ischaemia and angina pectoris (173). Although numerous studies have reported that exercise preconditioning is associated with increasing tolerance to I/R injury (174-178), the maximal hypoxic exercise often disrupts the prooxidant-antioxidant balance in myocardial tissue and potentially jeopardizes cardiac functions. Additionally, heavy exercise causes ischaemia associated ventricular arrhythmias (179). Tan and his team first reported the protective effects of melatonin on cardiac arrhythmia caused by I/R injury in isolated rat heart (171), which was confirmed by others in many studies (180-182). The protective role of melatonin against chronic intermittent hypoxic stress-induced cardiac insult has also been evidenced in rats (183). Athletes in competitive sports often experience anticipatory stress and anxiety which lead to an increase in circulatory epinephrine and other catecholamines (45). These molecules exert cardio-toxic effects (162,164). Melatonin administration provides protection against myocardial injury and cardiac arrhythmia caused by epinephrine (165, 184) or other β-adrenergic receptor agonists viz., isoproterenol (182, 185). Exercise in moderate-intensity also exacerbates the pathological state of patients suffering from CHF (52). Melatonin, being a strong antioxidant and anti-inflammatory molecule has been argued as a promising treatment for CHF (186, 187). All evidence suggests a possible therapeutic potency of melatonin in exercise-induced cardiovascular damage either caused by heavily loaded strenuous exercise or in the intermittent hypoxic exercise in CHF patients.
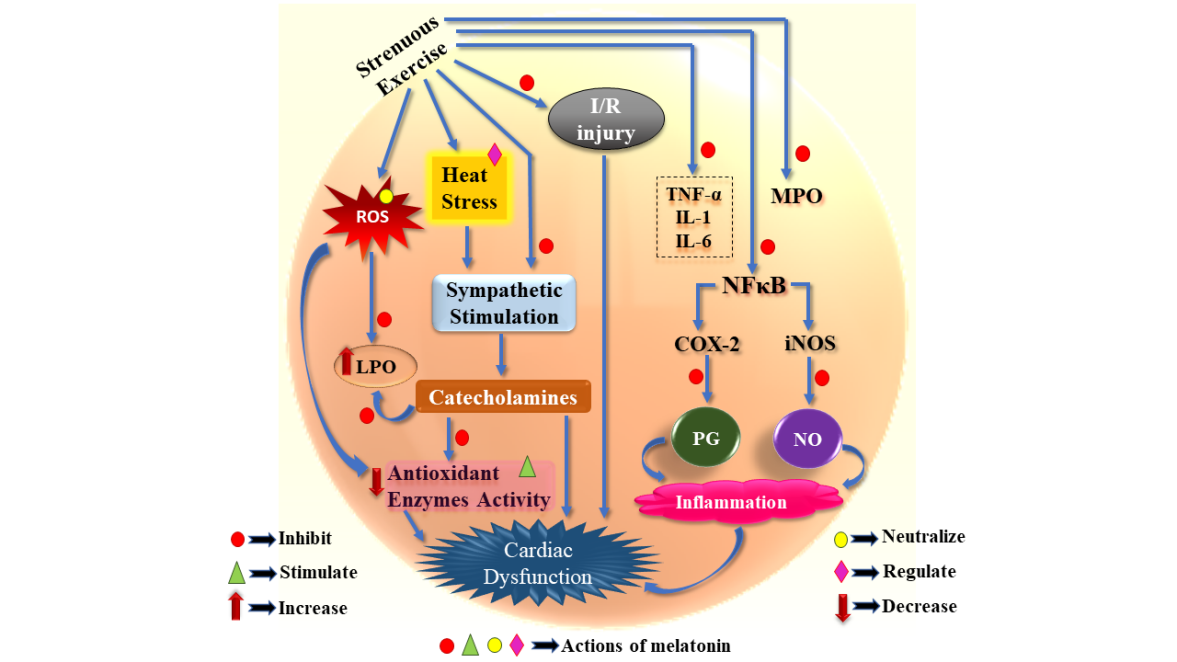
Fig. 2. Role of melatonin (i.e., inhibitory, stimulatory, neutralizing and regulatory actions) in combating strenuous exercise mediated cardiac dysfunction.
COX-2: Cyclooxygenase-2; I/R: Ischaemia reperfusion; IL: Interleukin; iNOS: Inducible nitric oxide synthase; LPO: Lipid peroxidation; MPO: Myeloperoxidase; NFκB: Nuclear factor kappa-light-chain-enhancer of activated B cells; NO: Nitric oxide; PG: Prostaglandin; ROS: Reactive oxygen species; TNF-α: Tumour necrosis factor alpha.
8. CONCLUSION AND PERSPECTIVES
Sports and exercise are a vital facet of life and are essential for one’s character development and overall fitness. However, competitive sports and unaccustomed exercises often evoke severe oxidative and inflammatory responses that may cause fatal cardiovascular events. Researchers therefore seek the effective prophylactic and therapeutic remedies to prevent the cardiac events occurring in athletes during vigorous exercise and over-training activity. Several antioxidants have been tested for these purposes in animals and in human subjects. Unexpectedly, most of them have either failed in providing effective protection against such damage or have been abandoned for their high pro-oxidant capacity. Melatonin, a potent antioxidant and a free radical scavenger, has profound cardio-protective effect. In this review, we have thoroughly discussed the possible pathways by which melatonin prevents cardiovascular injury triggered by acute physical exertion. Although several animal and human studies have been conducted in the concerned area, mechanistic details of the signaling processes are still lacking, and demands further investigations. Considering the great safety margin of melatonin, we strongly encourage melatonin to be tested as soon as possible regarding its protective effects on cardiopathy occurring in elite athletes during their sport competition or their strenuous training process.
ACKNOWLEDGMENTS
Swaimanti Sarkar is extremely grateful for the financial assistance that she has received as a Junior Research fellow (JRF) [709/(CSIR-UGC NET DEC. 2018] under Joint CSIR-UGC scheme, Govt. of India. Dr. Aindrila Chattopadhyay is supported by funds available to her from Department of Science and Technology, Govt. of West Bengal. Prof. Debasish Bandyopadhyay thankfully acknowledges the support he received from Departmental BI Grant and DST-PURSE Program awarded to the University of Calcutta. Prof. Debasish Bandyopadhyay expresses his grateful thanks to Dr. DunXian Tan, Editor-In-Chief, who have critically read the manuscript and made significant editorial corrections which surely have increased its clarity to readers.
AUTHORSHIP
Dr. DB and Dr. AC developed the concept and revised the manuscript thoroughly and approved it. SS contributed in the conception, drafted the manuscript, prepared the figures, and edited it.
CONFLICT OF INTEREST
Authors declare no conflict of interest.
REFERENCES
Tan DX, Manchester LC, Reiter RJ, Qi WB, Karbownik M, Calvo JR (2000) Significance of melatonin in antioxidative defense system: reactions and products. Neurosignals 9: 137-159. DOI: 10.1159/000014635.
Sies H (2015) Oxidative stress: a concept in redox biology and medicine. Redox Biol. 4: 180-183. DOI: 10.1016/j.redox.2015.01.002.
Higuchi M, Cartier LJ, Chen M, Holloszy JO (1985) Superoxide dismutase and catalase in skeletal muscle: adaptive response to exercise. J. Gerontol. 40: 281-286. DOI: 10.1093/geronj/40.3.281.
Kanter MM, Hamlin RL, Unverferth DV, Davis HW, Merola AJ (1985) Effect of exercise training on antioxidant enzymes and cardiotoxicity of doxorubicin. J. Appl. Physiol. 59: 1298-1303. DOI: 10.1152/jappl.1985.59.4.1298.
Sen CK, Marin EI, Kretzschmar MI, Hanninen OS (1992) Skeletal muscle and liver glutathione homeostasis in response to training, exercise, and immobilization. J. Appl. Physiol. 73: 1265-1272. DOI: 10.1152/jappl.1992.73.4.1265.
Booth FW, Roberts CK, Laye MJ (2011) Lack of exercise is a major cause of chronic diseases. Compr. Physiol. 2: 1143-1211. DOI: 10.1002/cphy.c110025.
Leaf DA, Kleinman MT, Hamilton M, Barstow TJ (1997) The effect of exercise intensity on lipid peroxidation. Med. Sci. Sports Exerc. 29: 1036-1039. DOI: 10.1097/00005768-199708000-00008.
Marzatico F, Pansarasa O, Bertorelli L, Somenzini L, Della GV (1997) Blood free radical antioxidant enzymes and lipid peroxides following long-distance and lactacidemic performances in highly trained aerobic and sprint athletes. J. Sport Med. Phys. Fit. 37: 235-239.
Alessio HM, Goldfarb AH, Cao G (1997) Exercise-induced oxidative stress before and after vitamin C supplementation. Int. J. Sport. Nutr. Exerc. Metab. 7: 1-9. DOI: 10.1123/ijsn.7.1.1.
Niess AM, Hartmann A, Grünert-Fuchs M, Poch B, Speit G (1996) DNA damage after exhaustive treadmill running in trained and untrained men. Int. J. Sports Med. 17: 397-403. DOI: 10.1055/s-2007-972868.
Dillard CJ, Litov RE, Savin WM, Dumelin EE, Tappel AL (1978) Effects of exercise, vitamin E, and ozone on pulmonary function and lipid peroxidation. J. Appl. Physiol. 45: 927-932. DOI: 10.1152/jappl.1978.45.6.927.
Powers SK, Radak Z, Ji LL (2016) Exercise‐induced oxidative stress: past, present and future. J. Physiol. 594: 5081-5092. DOI: 10.1113/JP270646.
Albert CM, Mittleman MA, Chae CU, Lee IM, Hennekens CH, Manson JE (2000) Triggering of sudden death from cardiac causes by vigorous exertion. N. Engl. J. Med. 343: 1355-1361. DOI: 10.1056/NEJM200011093431902.
Williams MA, Haskell WL, Ades PA, Amsterdam EA, Bittner V, Franklin BA, Gulanick M, Laing ST, Stewart KJ (2007) Resistance exercise in individuals with and without cardiovascular disease: 2007 update: a scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, Physical Activity, and Metabolism. Circulation 116: 572-584. DOI: 10.1161/CIRCULATIONAHA.107.185214.
Michailidis Y, Jamurtas AZ, Nikolaidis MG, Fatouros IG, Koutedakis Y, Papassotiriou I, Kouretas D (2007) Sampling time is crucial for measurement of aerobic exercise-induced oxidative stress. Med. Sci. Sport. Exer. 39: 1107-1113. DOI: 10.1249/01.mss.0b013e318053e7ba.
Brady PS, Brady LJ, Ullrey DE (1979) Selenium, vitamin E and the response to swimming stress in the rat. Nutr. J. 109: 1103-1109. DOI: 10.1093/jn/109.6.1103.
Coombes JS, Rowell B, Dodd SL, Demirel HA, Naito H, Shanely AR, Powers SK (2002) Effects of vitamin E deficiency on fatigue and muscle contractile properties. Eur. J. Appl. Physiol. 87: 272-277. DOI: 10.1007/s00421-002-0631-3.
Huether G (1993) The contribution of extrapineal sites of melatonin synthesis to circulating melatonin levels in higher vertebrates. Experientia 49: 665-670. DOI: 10.1007/BF01923948.
Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre‐Jimenez M, Qin L (2016) Melatonin as an antioxidant: under promises but over delivers. J. Pineal Res. 61: 253-278. DOI: 10.1111/jpi.12360.
Ochoa JJ, Díaz‐Castro J, Kajarabille N, García C, Guisado IM, De Teresa C, Guisado R (2011) Melatonin supplementation ameliorates oxidative stress and inflammatory signaling induced by strenuous exercise in adult human males. J. Pineal Res. 51: 373-380. DOI: 10.1111/j.1600-079X.2011.00899.x.
Borges LD, Dermargos A, Junior EP, Weimann E, Lambertucci RH, Hatanaka E (2015) Melatonin decreases muscular oxidative stress and inflammation induced by strenuous exercise and stimulates growth factor synthesis. J. Pineal Res. 58: 166-172. DOI: 10.1111/jpi.12202.
Leonardo-Mendonça RC, Ocaña-Wilhelmi J, de Haro T, de Teresa-Galván C, Guerra-Hernández E, Rusanova I, Fernández-Ortiz M, Sayed RK, Escames G, Acuña-Castroviejo D (2017) The benefit of a supplement with the antioxidant melatonin on redox status and muscle damage in resistance-trained athletes. Appl. Physiol. Nutr. Metab. 42: 700-707. DOI: 10.1139/apnm-2016-0677.
Jiki Z, Lecour S, Nduhirabandi F (2018) Cardiovascular benefits of dietary melatonin: a myth or a reality? Front. Physiol. 9: 528. DOI: 10.3389/fphys.2018.00528.
Cimen B, Uz A, Cetin I, Cimen L, Cetin A (2017) Melatonin supplementation ameliorates energy charge and oxidative stress induced by acute exercise in rat heart tissue. Acta Cardiol. Sin. 33: 530-538. DOI: 10.6515/acs20170331a.
Veneroso C, Tuñón MJ, González‐Gallego J, Collado PS (2009) Melatonin reduces cardiac inflammatory injury induced by acute exercise. J. Pineal Res. 47: 184-191. DOI: 10.1111/j.1600-079X.2009.00699.x.
Higginbotham MB, Morris KG, Williams RS, McHale PA, Coleman RE, Cobb FR (1986) Regulation of stroke volume during submaximal and maximal upright exercise in normal man. Circ. Res. 58: 281-291. DOI: 10.1161/01.res.58.2.281.
Ekblom B, Hermansen L (1968) Cardiac output in athletes. J. Appl. Physiol. 25: 619-625. DOI: 10.1152/jappl.1968.25.5.619.
Zavorsky GS (2000) Evidence and possible mechanisms of altered maximum heart rate with endurance training and tapering. Sports Med. 29: 13-26. DOI: 10.2165/00007256-200029010-00002.
Hellsten Y, Nyberg M (2011) Cardiovascular adaptations to exercise training. Compr. Physiol. 6: 1-32. DOI: 10.1002/cphy.c140080.
Thompson PD (2004) Historical concepts of the athlete's heart. Med. Sci. Sports Exerc. 36: 363-370. DOI: 10.1249/01.MSS.0000117117.67849.F6.
Roskamm H, Reindell H, Musshoff K, Koenig K (1961) Relations between heart size and physical efficiency in male and female athletes in comparison with normal male and female subjects. III. Archiv. Fur. Kreislaufforschung 35: 67-102.
Fagard R (2003) Athlete’s heart. Heart 89: 1455-1461. DOI: 10.1136/heart.89.12.1455.
Estes NA, Link MS, Homoud M, Wang PJ (2001) Electrocardiographic variants and cardiac rhythm and conduction disturbances in the athlete. Exercise and sports cardiology (New York: McGraw-Hill), pp 211-232.
Simons SM, Berry J, Bartsokas TW (1993) Preventing sudden death: the role of automated defibrillators. Phys. Sports Med. 21: 53-59. DOI: https://doi.org/10.1080/00913847.1993.11710429.
Maron BJ, Thompson PD, Puffer JC, McGrew CA, Strong WB, Douglas PS, Clark LT, Mitten MJ, Crawford MH, Atkins DL, Driscoll DJ (1996) Cardiovascular preparticipation screening of competitive athletes: a statement for health professionals from the Sudden Death Committee (clinical cardiology) and Congenital Cardiac Defects Committee (cardiovascular disease in the young), American Heart Association. Circulation 94: 850-856. DOI: 10.1161/01.cir.94.4.850.
Maron BJ (1998) Hypertrophic cardiomyopathy as a cause of sudden death in the young competitive athlete. Sudden Cardiac Death in the Athlete (Futura Publishing, Armonk), pp 301-317. DOI: https://doi.org/10.1016/S0146-2806(98)80008-X.
Convertino VA, Brock PJ, Keil LC, Bernauer EM, Greenleaf JE (1980) Exercise training-induced hypervolemia: role of plasma albumin, renin, and vasopressin. J. Appl. Physiol. 48: 665-669. DOI: 10.1152/jappl.1980.48.4.665.
Convertino VA, Keil LC, Bernauer EM, Greenleaf JE (1981) Plasma volume, osmolality, vasopressin, and renin activity during graded exercise in man. J. Appl. Physiol. 50: 123-128. DOI: 10.1152/jappl.1981.50.1.123.
Mairbäurl H (2013) Red blood cells in sports: effects of exercise and training on oxygen supply by red blood cells. Front. Physiol. 4: 332. DOI: 10.3389/fphys.2013.00332.
Kim D, Ha JW (2016) Hypertensive response to exercise: mechanisms and clinical implication. J. Clin. Hypertens. 22: 17. DOI: 10.1186/s40885-016-0052-y.
de Miranda Rohlfs ICP, de Mara LS, de Lima WC, de Carvalho T (2005) Relationship of the overtraining syndrome with stress, fatigue, and serotonin. Rev. Bras. Med. Esporte. 11: 333-337. DOI: https://doi.org/10.1590/S1517-86922005000600012.
van Paridon KN, Timmis MA, Nevison CM, Bristow M (2017) The anticipatory stress response to sport competition; a systematic review with meta-analysis of cortisol reactivity. BMJ Open Sport Exerc. Med. 3: e000261. DOI: 10.1136/bmjsem-2017-000261.
Henry JP (1992) Biological basis of the stress response. Integr. Psychol. Behav. Sci. 27: 66-83. DOI: 10.1007/BF02691093.
Smelik PG (1985) Stress and hormones. Organorama 22: 16-18.
de Boer SF, De Beun R, Slangen JL, Van der Gugten J (1990) Dynamics of plasma catecholamine and corticosterone concentrations during reinforced and extinguished operant behavior in rats. Physiol. Behav. 47: 691-698. DOI: https://doi.org/10.1016/0031-9384(90)90079-J.
Selye H. (1936) A syndrome produced by diverse noxious agents. Nature 138: 32-34. DOI:10.1038/138032a0.
Lucía A, Diaz B, Hoyos J, Fernandez C, Villa G, Bandres F, Chicharro JL (2001) Hormone levels of world class cyclists during the Tour of Spain stage race. Brit. J. Sport Med. 35: 424-430. DOI: 10.1136/bjsm.35.6.424.
Stacchiotti A, Favero G, Rodella LF (2020) Impact of melatonin on skeletal muscle and exercise. Cells 9: 288. DOI: 10.3390/cells9020288.
Pereira EJ, Smolko CM, Janes KA (2016) Computational models of reactive oxygen species as metabolic byproducts and signal-transduction modulators. Front. Pharmacol. 7: 457. DOI: 10.3389/fphar.2016.00457.
Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, Squadrito F, Altavilla D, Bitto A (2017) Oxidative stress: harms and benefits for human health. Oxid. Med. Cell. Longev. 2017: 8416763. DOI: 10.1155/2017/8416763.
Kelkar G, Subhadra K, Chengappa RK (2008) Effect of antioxidant supplementation on hematological parameters, oxidative stress and performance of Indian athletes. J. Hum. Ecol. 24: 209-213. DOI: 10.1080/09709274.2008.11906116.
Ribeiro-Samora GA, Rabelo LA, Ferreira AC, Favero M, Guedes GS, Pereira LS, Parreira VF, Britto RR (2017) Inflammation and oxidative stress in heart failure: effects of exercise intensity and duration. Braz. J. Med. Biol. Res. 50: e6393. DOI: 10.1590/1414-431X20176393.
König D, Neubauer O, Nics L, Kern N, Berg A, Bisse E, Wagner KH (2007) Biomarkers of exercise-induced myocardial stress in relation to inflammatory and oxidative stress. Exerc. Immunol. Rev. 13: 15-36.
Mair J, Thome-Kromer B, Wagner I, Lechleitner P, Dienstl F, Puschendorf B, Michel G (1994) Concentration time courses of troponin and myosin subunits after acute myocardial infarction. Coron. Artery Dis. 5: 865-872.
Neumayr G, Gaenzer H, Pfister R, Sturm W, Schwarzacher SP, Eibl G, Mitterbauer G, Hoertnagl H (2001) Plasma levels of cardiac troponin I after prolonged strenuous endurance exercise. Am. J. Cardiol. 87: 369-371. DOI: 10.1016/s0002-9149(00)01382-5.
Wu AH, Feng YJ, Moore R, Apple FS, McPherson PH, Buechler KF, Bodor G, Standardization CC (1998) Characterization of cardiac troponin subunit release into serum after acute myocardial infarction and comparison of assays for troponin T and I. Clin. Chem. 44:1198-1208.
Ooi DS, Isotalo PA, Veinot JP (2000) Correlation of antemortem serum creatine kinase, creatine kinase-MB, troponin I, and troponin T with cardiac pathology. Clin. Chem. 46:338-344.
Wu AH, Apple FS, Gibler WB, Jesse RL, Warshaw MM, Valdes R (1999) National academy of clinical biochemistry standards of laboratory practice: recommendations for the use of cardiac markers in coronary artery diseases. Clin. Chem. 45:1104-1121.
Kato M, Kinugawa T, Ogino K, Endo A, Osaki S, Igawa O, Hisatome I, Shigemasa C (2000) Augmented response in plasma brain natriuretic peptide to dynamic exercise in patients with left ventricular dysfunction and congestive heart failure. J. Intern. Med. 248: 309-315. DOI: 10.1046/j.1365-2796.2000.00736.x.
Sagnella GA (2001) Measurement and importance of plasma brain natriuretic peptide and related peptides. Ann. Clin. Biochem. 38: 83-93. DOI: 10.1258/0004563011900317.
McNairy M, Gardetto N, Clopton P, Garcia A, Krishnaswamy P, Kazanegra R, Ziegler M, Maisel AS (2002) Stability of B-type natriuretic peptide levels during exercise in patients with congestive heart failure: implications for outpatient monitoring with B-type natriuretic peptide. Am. Heart J. 143:406-411. DOI: 10.1067/mhj.2002.120148.
Friedl W, Mair J, Thomas S, Pichler M, Puschendorf B (1999) Relationship between natriuretic peptides and hemodynamics in patients with heart failure at rest and after ergometric exercise. Clinica chimica acta 281: 121-126. DOI: 10.1016/s0009-8981(98)00217-4.
Ohba H, Takada H, Musha H, Nagashima J, Mori N, Awaya T, Omiya K, Murayama M (2001) Effects of prolonged strenuous exercise on plasma levels of atrial natriuretic peptide and brain natriuretic peptide in healthy men. Am. Heart J. 141:751-758. DOI: 10.1067/mhj.2001.114371.
Jacob R, Khan M (2018) Cardiac Biomarkers: What Is and What Can Be. Ind. J. Cardiovascul. Dis. in Women 3: 240-244. DOI: 10.1055/s-0039-1679104.
Smith JE, Garbutt G, Lopes P, Pedoe DT (2004) Effects of prolonged strenuous exercise (marathon running) on biochemical and haematological markers used in the investigation of patients in the emergency department. Brit. J. Sport Med. 38:292-294. DOI: 10.1136/bjsm.2002.002873.
Rahnama N, Faramarzi M, Gaeini AA (2011) Effects of intermittent exercise on cardiac troponin I and creatine kinase-MB. Int. J. Prev. Med. 2:20.
Scharhag J, George K, Shave R, Urhausen A, Kindermann W (2008) Exercise-associated increases in cardiac biomarkers. Med. Sci. Sports Exerc .40: 1408-1415. DOI: 10.1249/MSS.0b013e318172cf22.
Shave R, George K, Gaze D (2007) The influence of exercise upon cardiac biomarkers: a practical guide for clinicians and scientists. Curr. Med. Chem. 14: 1427-1436. DOI: 10.2174/092986707780831177.
Apple FS, Quist HE, Otto AP, Mathews WE, Murakami MM (2002) Release characteristics of cardiac biomarkers and ischemia-modified albumin as measured by the albumin cobalt-binding test after a marathon race. Clin. Chem. 48: 1097-1100. DOI: 10.1093/clinchem/48.7.1097.
Middleton N, Shave R, George K, Whyte G, Forster J, Oxborough D, Gaze D, Collinson P (2006) Novel application of flow propagation velocity and ischaemia‐modified albumin in analysis of postexercise cardiac function in man. Exp. Physiol. 91: 511-519. DOI: 10.1113/expphysiol.2005.032631.
Dawson E, George K, Shave R, Whyte G, Ball D (2003) Does the human heart fatigue subsequent to prolonged exercise? Sports Med. 33: 365-380. DOI: 10.2165/00007256-200333050-00003.
La Gerche A, Inder WJ, Roberts TJ, Brosnan MJ, Heidbuchel H, Prior DL (2015) Relationship between inflammatory cytokines and indices of cardiac dysfunction following intense endurance exercise. PLoS One. 10: e0130031. DOI: 10.1371/journal.pone.0130031.
Melanson SE, Green SM, Wood MJ, Neilan TG, Lewandrowski EL (2006) Elevation of myeloperoxidase in conjunction with cardiac-specific markers after marathon running. Am. J. Clin. Pathol. 126: 888-893. DOI: 10.1309/1D62H6KRFTVQRJ0A.
Mocatta TJ, Pilbrow AP, Cameron VA, Senthilmohan R, Frampton CM, Richards AM, Winterbourn CC (2007) Plasma concentrations of myeloperoxidase predict mortality after myocardial infarction. J. Am. Coll. Cardiol. 49: 1993-2000. DOI: 10.1016/j.jacc.2007.02.040.
García-Mediavilla V, Crespo I, Collado PS, Esteller A, Sánchez-Campos S, Tuñón MJ, González-Gallego J (2007) The anti-inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and reactive C-protein, and down-regulation of the nuclear factor kappaB pathway in Chang Liver cells. Eur. J. Pharmacol. 557: 221-229. DOI: 10.1016/j.ejphar.2006.11.014.
Moreira AJ, Fraga C, Alonso M, Collado PS, Zetller C, Marroni C, Marroni N, González-Gallego J (2004) Quercetin prevents oxidative stress and NF-κB activation in gastric mucosa of portal hypertensive rats. Biochem. Pharmacol. 68: 1939-1946. DOI: 10.1016/j.bcp.2004.07.016.
Alonso M, Collado PS, González‐Gallego J (2006) Melatonin inhibits the expression of the inducible isoform of nitric oxide synthase and nuclear factor kappa B activation in rat skeletal muscle. J. Pineal Res. 41: 8-14. DOI: 10.1111/j.1600-079X.2006.00323.x.
Wernerman J (2012) Micronutrients against oxidative stress-time for clinical recommendations? Crit. Care 16: 124. DOI: 10.1186/cc11319.
Braakhuis AJ, Hopkins WG (2015) Impact of dietary antioxidants on sport performance: a review. Sports Med. 45: 939-955. DOI: 10.1007/s40279-015-0323-x.
Kreher JB, Schwartz JB (2012) Overtraining syndrome: a practical guide. Sports Health 4: 128-138. DOI: 10.1177/1941738111434406.
Gomez-Cabrera MC, Domenech E, Romagnoli M, Arduini A, Borras C, Pallardo FV, Sastre J, Vina J (2008) Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am. J. Clin. Nutr. 87: 142-149. DOI: 10.1093/ajcn/87.1.142.
Ristow M, Zarse K, Oberbach A, Klöting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Blüher M (2009) Antioxidants prevent health-promoting effects of physical exercise in humans. Proc. Natl. Acad. Sci. USA 106: 8665-8670. DOI: 10.1073/pnas.0903485106.
Novelli GP, Bracciotti G, Falsini S (1990) Spin-trappers and vitamin E prolong endurance to muscle fatigue in mice. Free Radic. Biol. Med. 8: 9-13. DOI: https://doi.org/10.1016/0891-5849(90)90138-9.
Lawrence JD, Bower RC, Riehl WP, Smith JL (1975) Effects of alpha-tocopherol acetate on the swimming endurance of trained swimmers. Am. J. Clin. Nutr. 28: 205-208. DOI: 10.1093/ajcn/28.3.205.
Romano-Ely BC, Todd MK, Saunders MJ, Laurent TS (2006) Effect of an isocaloric carbohydrate-protein-antioxidant drink on cycling performance. Med. Sci. Sports Exerc. 38: 1608-1616. DOI: 10.1249/01.mss.0000229458.11452.e9.
Paulsen G, Cumming KT, Holden G, Hallén J, Rønnestad BR, Sveen O, Skaug A, Paur I, Bastani NE, Østgaard HN, Buer C (2014) Vitamin C and E supplementation hampers cellular adaptation to endurance training in humans: a double‐blind, randomised, controlled trial. J. Physiol. 592: 1887-1901. DOI: 10.1113/jphysiol.2013.267419.
Kobayaski, Y (1974) Effect of vitamin E on aerobic work performance in man during acute exposure to hypoxic hypoxia (Doctoral dissertation, University of New Mexico).
Ilavazhagan G, Bansal A, Prasad D, Thomas P, Sharma SK, Kain AK, Kumar D, Selvamurthy W (2001) Effect of vitamin E supplementation on hypoxia-induced oxidative damage in male albino rats. Aviat. Space Environ. Med. 72: 899-903.
Ulusoy HG, Sanlier N (2019) A minireview of quercetin: from its metabolism to possible mechanisms of its biological activities. Crit. Rev. Food Sci. Nutr. 1: 1-4. DOI: 10.1080/10408398.2019.1683810.
Davis JM, Murphy EA, Carmichael MD, Davis B (2009) Quercetin increases brain and muscle mitochondrial biogenesis and exercise tolerance. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296: R1071-1077. DOI: 10.1152/ajpregu.90925.2008.
Nieman DC (2007) Marathon training and immune function. Sports Med. 37: 412-415. DOI: 10.2165/00007256-200737040-00036.
Murase T, Haramizu S, Ota N, Hase T (2009) Suppression of the aging-associated decline in physical performance by a combination of resveratrol intake and habitual exercise in senescence-accelerated mice. Biogerontology 10: 423-434. DOI: 10.1007/s10522-008-9177-z.
Xiao NN (2015) Effects of resveratrol supplementation on oxidative damage and lipid peroxidation induced by strenuous exercise in rats. Biomol. Ther. 23: 374-378. DOI: 10.4062/biomolther.2015.015.
Hart N, Sarga L, Csende Z, Koch LG, Britton SL, Davies KJ, Radak Z (2014) Resveratrol attenuates exercise-induced adaptive responses in rats selectively bred for low running performance. Dose-Response. 12: 57-71. DOI: 10.2203/dose-response.13-010.Radak.
Gliemann L, Schmidt JF, Olesen J, Biensø RS, Peronard SL, Grandjean SU, Mortensen SP, Nyberg M, Bangsbo J, Pilegaard H, Hellsten Y (2013) Resveratrol blunts the positive effects of exercise training on cardiovascular health in aged men. J. Physiol. 591: 5047-5059. DOI: 10.1113/jphysiol.2013.258061.
Motterlini R, Foresti R, Bassi R, Green CJ (2000) Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic. Biol. Med. 28: 1303-1312. DOI: 10.1016/s0891-5849(00)00294-x.
Sharma OP (1976) Antioxidant activity of curcumin and related compounds. Biochem. Pharmacol. 25: 1811. DOI: 10.1016/0006-2952(76)90421-4.
Abe Y, Hashimoto SH, Horie T (1999) Curcumin inhibition of inflammatory cytokine production by human peripheral blood monocytes and alveolar macrophages. Pharmacol. Res. 39: 41-47. DOI: 10.1006/phrs.1998.0404.
Roohi BN, Moradlou AN, Bolboli L (2017) Influence of curcumin supplementation on exercise-induced oxidative stress. Asian J. Sports Med. 8: e35776. DOI: 10.5812/asjsm.35776.
Davis JM, Murphy EA, Carmichael MD, Zielinski MR, Groschwitz CM, Brown AS, Gangemi JD, Ghaffar A, Mayer EP (2007) Curcumin effects on inflammation and performance recovery following eccentric exercise-induced muscle damage. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292: R2168-2173. DOI: 10.1152/ajpregu.00858.2006.
Takahashi M, Suzuki K, Kim HK, Otsuka Y, Imaizumi A, Miyashita M, Sakamoto S (2014) Effects of curcumin supplementation on exercise-induced oxidative stress in humans. Int. J. Sports Med. 35: 469-475. DOI: 10.1055/s-0033-1357185.
Basham SA, Waldman HS, Krings BM, Lamberth J, Smith JW, McAllister MJ (2019) Effect of Curcumin Supplementation on Exercise-Induced Oxidative Stress, Inflammation, Muscle Damage, and Muscle Soreness. J. Diet. Suppl. 26: 1-4. DOI: 10.1080/19390211.2019.1604604.
Medved I, Brown MJ, Bjorksten AR, Murphy KT, Petersen AC, Sostaric S, Gong X, McKenna MJ (2004) N-acetylcysteine enhances muscle cysteine and glutathione availability and attenuates fatigue during prolonged exercise in endurance-trained individuals. J. Appl. Physiol. 97: 1477-1485. DOI: 10.1152/japplphysiol.00371.2004.
McKenna MJ, Medved I, Goodman CA, Brown MJ, Bjorksten AR, Murphy KT, Petersen AC, Sostaric S, Gong X (2006) N‐acetylcysteine attenuates the decline in muscle Na+, K+‐pump activity and delays fatigue during prolonged exercise in humans. J. Physiol. 576: 279-288. DOI: 10.1113/jphysiol.2006.115352.
Kawamura T, Muraoka I (2018) Exercise-induced oxidative stress and the effects of antioxidant intake from a physiological viewpoint. Antioxidants 7: 119. DOI: 10.3390/antiox7090119.
Lerner AB, Case JD, Takahashi Y, Lee TH, Mori W (1958) Isolation of melatonin, the pineal gland factor that lightens melanocytes. J. Am. Chem. Soc. 80: 2587. DOI: https://doi.org/10.1021/ja01543a060.
Lerner AB, Case JD, Heinzelmann RV (1959) Structure of melatonin. J. Am. Chem. Soc. 81: 6084-6085. DOI: https://doi.org/10.1021/ja01531a060.
Cardinali, D. P., Pandi-Perumal, S. R., & Niles, L. P (2008) Melatonin and its receptors: biological function in circadian sleep-wake regulation (Cambridge University Press, Cambridge), pp 283-314. DOI: 10.1017/CBO9780511541674.011.
Korkmaz A, Topal T, Tan DX, Reiter RJ (2009) Role of melatonin in metabolic regulation. Rev. Endocr. Metab. Disord. 10: 261-270. DOI: 10.1007/s11154-009-9117-5.
Reiter RJ, Tan DX, Manchester LC, Paredes SD, Mayo JC, Sainz RM (2009) Melatonin and reproduction revisited. Biol. Reprod. 81: 445-456. DOI: 10.1095/biolreprod.108.075655.
Pal PK, Bhattacharjee B, Chattopadhyay A, Bandyopadhyay D (2019) Pleiotropic roles of melatonin against oxidative stress mediated tissue injury in the gastrointestinal tract: An overview. Melatonin Res.2: 158-184. DOI: 10.32794/mr11250027.
Pal PK, Sarkar S, Chattopadhyay A, Tan DX, Bandyopadhyay D (2019) Enterochromaffin cells as the source of melatonin: key findings and functional relevance in mammals. Melatonin Res.2: 61-82. DOI: 10.32794/mr11250041.
Reiter RJ, Tan DX, Paredes SD, Fuentes-Broto L (2010) Beneficial effects of melatonin in cardiovascular disease. Ann. Med. 42: 276-285. DOI: 10.3109/07853890903485748.
Lee JG, Woo YS, Park SW, Seog DH, Seo MK, Bahk WM (2019) The neuroprotective effects of melatonin: Possible role in the pathophysiology of neuropsychiatric disease. Brain Sci. 9: 285. DOI: 10.3390/brainsci9100285.
Carrillo-Vico A, Reiter RJ, Lardone PJ, Herrera JL, Fernández-Montesinos R, Guerrero JM, Pozo D (2006) The modulatory role of melatonin on immune responsiveness. Curr. Opin. Investig. Drugs.7: 423.
Reiter RJ, Rosales-Corral SA, Tan DX, Acuna-Castroviejo D, Qin L, Yang SF, Xu K (2017) Melatonin, a full service anti-cancer agent: inhibition of initiation, progression and metastasis. Int. J. Mol. Sci. 18: 843. DOI: 10.3390/ijms18040843.
Tan DX, Reiter RJ, Manchester LC, Yan MT, El-Sawi M, Sainz RM, Mayo JC, Kohen R, Allegra MC, Hardeland R (2002) Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr. Top. Med. Chem. 2: 181-197. DOI: 10.2174/1568026023394443.
Tan DX, Manchester LC, Hardeland R, Lopez‐Burillo S, Mayo JC, Sainz RM, Reiter RJ (2003) Melatonin: a hormone, a tissue factor, an autocoid, a paracoid, and an antioxidant vitamin. J. Pineal Res .34: 75-78. DOI: 10.1034/j.1600-079x.2003.02111.x.
Cipolla-Neto J, Amaral FG (2018) Melatonin as a hormone: new physiological and clinical insights. Endocr. Rev. 39: 990-1028. DOI: 10.1210/er.2018-00084.
Tan DX, Manchester LC, Terron MP, Flores LJ, Reiter RJ (2007) One molecule, many derivatives: a never‐ending interaction of melatonin with reactive oxygen and nitrogen species?. J. Pineal Res. 42: 28-42. DOI: 10.1111/j.1600-079X.2006.00407.x.
Galano A, Medina ME, Tan DX, Reiter RJ (2015) Melatonin and its metabolites as copper chelating agents and their role in inhibiting oxidative stress: a physicochemical analysis. J. Pineal Res. 58: 107-116. DOI: 10.1111/jpi.12196.
Pariente R, Bejarano I, Espino J, Rodríguez AB, Pariente JA (2017) Participation of MT3 melatonin receptors in the synergistic effect of melatonin on cytotoxic and apoptotic actions evoked by chemotherapeutics. Cancer Chemother. Pharmacol. 80: 985-998. DOI: 10.1007/s00280-017-3441-3.
Reiter RJ, Tan DX, Manchester LC, Terron MP, Flores LJ, Koppisepi S (2007) Medical implications of melatonin: receptor-mediated and receptor-independent actions. Adv. Med. Sci. 52: 11-28.
Ekmekcioglu C, Thalhammer T, Humpeler S, Mehrabi MR, Glogar HD, Hölzenbein T, Markovic O, Leibetseder VJ, Strauss‐Blasche G, Marktl W (2003) The melatonin receptor subtype MT2 is present in the human cardiovascular system. J. Pineal Res. 35: 40-44. DOI: 10.1034/j.1600-079x.2003.00051.x.
Dominguez-Rodriguez A, Abreu-Gonzalez P, Reiter RJ (2012) Melatonin and cardiovascular disease: myth or reality? Rev. Esp. Cardiol.(English Edition). 65: 215-218. DOI: 10.1016/j.recesp.2011.10.009.
Pandi-Perumal SR, BaHammam AS, Ojike NI, Akinseye OA, Kendzerska T, Buttoo K, Dhandapany PS, Brown GM, Cardinali DP (2017) Melatonin and human cardiovascular disease. J. Cardiovasc. Pharmacol. Ther. 22: 122-132. DOI: 10.1177/1074248416660622.
Krause DN, Geary GG, Doolen S, Duckles SP (2002) Melatonin and Cardiovascular Function. In Melatonin after Four Decades. (Springer, Boston, MA), pp 299-310. DOI: https://doi.org/10.1007/0-306-46814-X_32.
Xia CM, Shao CH, Xin L, Wang YR, Ding CN, Wang J, Shen LL, Li L, Cao YX, Zhu DN (2008) Effects of melatonin on blood pressure in stress‐induced hypertension in rats. Clin. Exp. Pharmacol. Physiol. 35: 1258-1264. DOI: 10.1111/j.1440-1681.2008.05000.x.
Prado NJ, Ferder L, Manucha W, Diez ER (2018) Anti-inflammatory effects of melatonin in obesity and hypertension. Curr. Hypertens. Rep. 20: 45. DOI: 10.1007/s11906-018-0842-6.
Simko F, Baka T, Krajcirovicova K, Repova K, Aziriova S, Zorad S, Poglitsch M, Adamcova M, Reiter RJ, Paulis L (2018) Effect of melatonin on the renin-angiotensin-aldosterone system in L-NAME-induced hypertension. Molecules 23: 265. DOI: 10.3390/molecules23020265.
Diez ER, Renna NF, Prado NJ, Lembo C, Ponce Zumino AZ, Vazquez‐Prieto M, Miatello RM (2013) Melatonin, given at the time of reperfusion, prevents ventricular arrhythmias in isolated hearts from fructose-fed rats and spontaneously hypertensive rats. J. Pineal Res. 55: 166-173. DOI: 10.1111/jpi.12059.
Benova T, Viczenczova C, Radosinska J, Bacova B, Knezl V, Dosenko V, Weismann P, Zeman M, Navarova J, Tribulova N (2013) Melatonin attenuates hypertension-related proarrhythmic myocardial maladaptation of connexin-43 and propensity of the heart to lethal arrhythmias. Can. J. Physiol. Pharmacol. 91: 633-639. DOI: 10.1139/cjpp-2012-0393.
Sedova KA, Bernikova OG, Cuprova JI, Ivanova AD, Kutaeva GA, Pliss MG, Lopatina EV, Vaykshnorayte MA, Diez ER, Azarov JE (2019) Association between antiarrhythmic, electrophysiological, and antioxidative effects of melatonin in ischemia/reperfusion. Int. J. Mol. Sci. 20: 6331. DOI: 10.3390/ijms20246331.
Prado NJ, Muñoz EM, Farias Altamirano LE, Aguiar F, Ponce Zumino AZ, Sánchez FJ, Miatello RM, Pueyo E, Diez ER (2020) Reperfusion arrhythmias increase after superior cervical ganglionectomy due to conduction disorders and changes in repolarization. Int. J. Mol. Sci. 21: 1804. DOI: 10.3390/ijms21051804.
Dominguez-Rodriguez A, Abreu-Gonzalez P, Jose M, Consuegra-Sanchez L, Piccolo R, Gonzalez-Gonzalez J, Garcia-Camarero T, del Mar Garcia-Saiz M, Aldea-Perona A, Reiter RJ, Caballero-Estevez N (2017) Usefulness of early treatment with melatonin to reduce infarct size in patients with ST-segment elevation myocardial infarction receiving percutaneous coronary intervention (From the Melatonin Adjunct in the Acute Myocardial Infarction Treated With Angioplasty Trial). Am. J. Cardiol. 120: 522-526. DOI:10.1016/j.amjcard.2017.05.018.
Dominguez-Rodriguez A, Abreu-Gonzalez P, Chen Y (2019) Cardioprotection and effects of melatonin administration on cardiac ischemia reperfusion: Insight from clinical studies. Melatonin Res. 2: 100-105. DOI:https://doi.org/https://doi.org/10.32794/mr11250024.
Bojkowski CJ, Arendt J (1990) Factors influencing urinary 6‐sulphatoxymelatonin, a major melatonin metabolite, in normal human subjects. Clin. Endocrinol. 33: 435-444. DOI: 10.1111/j.1365-2265.1990.tb03882.x.
Sakotnik A, Liebmann PM, Stoschitzky K, Lercher P, Schauenstein K, Klein W, Eber B (1999) Decreased melatonin synthesis in patients with coronary artery disease. Eur. Heart J. 20: 1314-1317. DOI: 10.1053/euhj.1999.1527.
Girotti L, Lago M, Ianovsky O, Carbajales J, Elizari MV, Brusco LI, Cardinali DP (2000) Low urinary 6‐sulphatoxymelatonin levels in patients with coronary artery disease. J. Pineal Res.29: 138-142. DOI: 10.1034/j.1600-079x.2000.290302.x.
Dominguez-Rodriguez A, Abreu-Gonzalez P, Piccolo R, Galasso G, Reiter RJ (2016) Melatonin is associated with reverse remodeling after cardiac resynchronization therapy in patients with heart failure and ventricular dyssynchrony. Int. J. Cardiol. 221: 359-363. DOI:10.1016/j.ijcard.2016.07.056.
Tan DX, Hardeland R, Manchester LC, Paredes SD, Korkmaz A, Sainz RM, Mayo JC, Fuentes-Broto L, Reiter RJ (2010). The changing biological roles of melatonin during evolution: from an antioxidant to signals of darkness, sexual selection and fitness. Biol. Rev. Camb. Philos. Soc. 85 (3): 607-23. doi: 10.1111/j.1469-185X.2009.00118.
Zhao D, Yu Y, Shen Y, Liu Q, Zhao Z, Sharma R, Reiter RJ (2019) Melatonin synthesis and function: evolutionary history in animals and plants. Front. Endocrinol.10: 249. DOI: 10.3389/fendo.2019.00249.
Mastorakos G, Pavlatou M, Diamanti-Kandarakis E, Chrousos GP (2005) Exercise and the stress system. Hormones (Athens) 4: 73-89.
Barchas J, Dacosta F, Spector S (1967) Acute pharmacology of melatonin. Nature 214: 919-920. DOI: https://doi.org/10.1038/214919a0.
Erland LA, Saxena PK (2017) Melatonin natural health products and supplements: presence of serotonin and significant variability of melatonin content. J. Clin. Sleep Med..13: 275-281. DOI: 10.5664/jcsm.6462.
Kaya O, Gokdemir K, Kilic M, Baltaci AK (2006) Melatonin supplementation to rats subjected to acute swimming exercise: Its effect on plasma lactate levels and relation with zinc. Neuro. Endocrinol. Lett. 27: 263-266.
Trionfante CP, Davis GR, Farney TM, Miskowiec RW, Nelson AG (2017) A pre-exercise dose of melatonin can alter substrate use during exercise. Int. J. Exerc. Sci.10: 1029.
Farjallah MA, Hammouda O, Ben Mahmoud L, Graja A, Ghattassi K, Boudaya M, Jammoussi K, Sahnoun Z, Souissi N (2018) Melatonin supplementation ameliorates oxidative stress, antioxidant status and physical performances recovery during a soccer training camp. Biol. Rhythm Res. 51: 441-452. DOI: https://doi.org/10.1080/09291016.2018.1533749.
Stratos I, Richter N, Rotter R, Li Z, Zechner D, Mittlmeier T, Vollmar B (2012) Melatonin restores muscle regeneration and enhances muscle function after crush injury in rats. J. Pineal Res. 52: 62-70. DOI: 10.1111/j.1600-079X.2011.00919.x.
Maarman GJ, Reiter RJ (2018) Melatonin therapy for blunt trauma and strenuous exercise: A mechanism involving cytokines, NFκB, Akt, MAFBX and MURF-1. J. Sports Sci. 36: 1897-1901. DOI: 10.1080/02640414.2018.1424491.
Hara M, Iigo M, Ohtani-Kaneko R, Nakamura N, Suzuki T, Reiter RJ, Hirata K (1997) Administration of melatonin and related indoles prevents exercise-induced cellular oxidative changes in rats. Neurosignals 6: 90-100. DOI: 10.1159/000109113.
Bicer M, Baltaci SB, Patlar S, Mogulkoc R, Baltaci AK (2018) Melatonin has a protective effect against lipid peroxidation in the bone tissue of diabetic rats subjected to acute swimming exercise. Horm. Mol. Biol. Clin. Investig. 34. DOI: 10.1515/hmbci-2017-0079.
Costa VM, Carvalho F, Duarte JA, Bastos MD, Remião F (2013) The heart as a target for xenobiotic toxicity: the cardiac susceptibility to oxidative stress. Chem. Res. Toxicol. 26: 1285-1311. DOI: 10.1021/tx400130v.
Tsutsui H, Kinugawa S, Matsushima S (2011) Oxidative stress and heart failure. Am. J. Physiol. Heart Circ. Physiol. 301: H2181-2190. DOI: 10.1152/ajpheart.00554.2011.
Hallqvist J, Möller J, Ahlbom A, Diderichsen F, Reuterwall C, Faire UD (2000) Does heavy physical exertion trigger myocardial infarction? A case-crossover analysis nested in a population-based case-referent study. Am. J. Epidemiol. 151: 459-467. DOI: 10.1093/oxfordjournals.aje.a010231.
Rowell LB (1974) Human cardiovascular adjustments to exercise and thermal stress. Physiol. Rev. 54: 75-159. DOI: 10.1152/physrev.1974.54.1.75.
Kindermann W, Schnabel A, Schmitt WM, Biro G, Cassens J, Weber F (1982) Catecholamines, growth hormone, cortisol, insulin, and sex hormones in anaerobic and aerobic exercise. Eur. J. Appl. Physiol. Occup. Physiol. 49: 389-399. DOI: 10.1007/BF00441300.
Brooks S, Cheetham M, Williams C (1985) Endurance training and the catecholamine response to brief maximum exercise in man. J. Physiol. 361: 81.
Baker JS, Buchan D (2017) Metabolic stress and high intensity exercise. Phys. Med. Rehabil. Res .2: 1-2. DOI: 10.15761/PMRR.1000136.
Zhang DY, Anderson AS (2014) The sympathetic nervous system and heart failure. Cardiol. Clin. 32: 33-45. doi: 10.1016/j.ccl.2013.09.010.
Viswanathan M, Hissa R, George JC (1986) Suppression of sympathetic nervous system by short photoperiod and melatonin in the Syrian hamster. Life Sci. 38: 73-79. DOI: 10.1016/0024-3205(86)90277-8.
Behonick GS, Novak MJ, Nealley EW, Baskin SI (2001) Toxicology update: the cardiotoxicity of the oxidative stress metabolites of catecholamines (aminochromes). J. Appl. Toxicol. 21: S15-22. DOI: 10.1002/jat.793.
Neri M, Cerretani D, Fiaschi AI, Laghi PF, Lazzerini PE, Maffione AB, Micheli L, Bruni G, Nencini C, Giorgi G, D'Errico S (2007) Correlation between cardiac oxidative stress and myocardial pathology due to acute and chronic norepinephrine administration in rats. J. Cell Mol. Med. 11: 156-170. DOI: 10.1111/j.1582-4934.2007.00009.x.
Costa, VM, Silva R, Ferreira LM, Branco PS, Carvalho F, Bastos ML, Carvalho RA, Carvalho M, Remião F (2007) Oxidation process of adrenaline in freshly isolated rat cardiomyocytes: formation of adrenochrome, quinoproteins, and GSH adduct. Chem. Res. Toxicol. 20: 1183-1191. DOI: 10.1021/tx7000916.
Rudra S, Mukherjee D, Dutta M, Ghosh AK, Dey M, Basu A, Pattari SK, Chattopadhyay A, Bandyopadhyay D (2014) Orally administered melatonin protects against adrenaline-induced oxidative stress in rat liver and heart: Involvement of antioxidant mechanism (s). J. Pharm. Res. 8: 303-320. DOI: http://jprsolutions.info/files/final-file56bff9adee9ad8.28733292.pdf.
Paulus WJ (2000) Cytokines and heart failure. Heart Fail. Monit. 1:50-56.
Gullestad L, Aukrust P (2001) The cytokine network in heart failure: pathogenetic importance and potential therapeutic targets. Heart Fail. Monit. 2: 8-13. DOI: 10.1159/000338166.
Belcastro AN, Arthur GD, Albisser TA, Raj DA (1996) Heart, liver, and skeletal muscle myeloperoxidase activity during exercise. J. Appl. Physiol. 80: 1331-1335. DOI: 10.1152/jappl.1996.80.4.1331.
Torres SH, De Sanctis JB, de Briceno LM, Hernandez N, Finol HJ (2004) Inflammation and nitric oxide production in skeletal muscle of type 2 diabetic patients. J. Endocrinol. 181: 419-427. DOI: 10.1677/joe.0.1810419.
Tunon MJ, Garcia-Mediavilla MV, Sanchez-Campos S, Gonzalez-Gallego J (2009) Potential of flavonoids as anti-inflammatory agents: modulation of pro-inflammatory gene expression and signal transduction pathways. Curr. Drug Metab. 10: 256-271. DOI: 10.2174/138920009787846369.
Tan DX, Manchester LC, Reiter RJ, Qi W, Kim SJ, El‐Sokkary GH (1998) Ischemia/reperfusion‐induced arrhythmias in the isolated rat heart: prevention by melatonin. J. Pineal Res. 25: 184-191. DOI: 10.1111/j.1600-079x.1998.tb00558.x.
Pialoux V, Mounier R, Rock E, Mazur A, Schmitt L, Richalet JP, Robach P, Coudert J, Fellmann N (2009) Effects of acute hypoxic exposure on prooxidant/antioxidant balance in elite endurance athletes. Int. J. Sports Med. 30: 87-93. DOI: 10.1055/s-0028-1103284.
Accattato F, Greco M, Pullano SA, Carè I, Fiorillo AS, Pujia A, Montalcini T, Foti DP, Brunetti A, Gulletta E (2017) Effects of acute physical exercise on oxidative stress and inflammatory status in young, sedentary obese subjects. PLoS One 12: e0178900. DOI: 10.1371/journal.pone.0178900.
Noakes TD (2000) Physiological models to understand exercise fatigue and the adaptations that predict or enhance athletic performance. Scand. J. Med. Sci. Spor. 10: 123-145. DOI: 10.1034/j.1600-0838.2000.010003123.x.
Brown DA, Jew KN, Sparagna GC, Musch TI, Moore RL (2003) Exercise training preserves coronary flow and reduces infarct size after ischemia-reperfusion in rat heart. J. Appl. Physiol. 95: 2510-2518. DOI: 10.1152/japplphysiol.00487.2003.
Ding YH, Luan XD, Li J, Rafols JA, Guthinkonda M, Diaz FG, Ding Y (2004) Exercise-induced overexpression of angiogenic factors and reduction of ischemia/reperfusion injury in stroke. Curr. Neurovasc. Res. 1: 411-420. DOI: 10.2174/1567202043361875.
Quindry J, French J, Hamilton K, Lee Y, Mehta JL, Powers S (2005) Exercise training provides cardioprotection against ischemia–reperfusion induced apoptosis in young and old animals. Exp. Gerontol. 40: 416-425. DOI: 10.1016/j.exger.2005.03.010.
Powers SK, Quindry JC, Kavazis AN (2008) Exercise-induced cardioprotection against myocardial ischemia–reperfusion injury. Free Radic. Biol. Med. 44: 193-201. DOI: 10.1016/j.freeradbiomed.2007.02.006.
Lee Y, Min K, Talbert EE, Kavazis AN, Smuder AJ, Willis WT, Powers SK (2012) Exercise protects cardiac mitochondria against ischemia-reperfusion injury. Med. Sci. Sports Exerc. 44: 397-405. DOI: 10.1249/MSS.0b013e318231c037.
Thompson PD, Franklin BA, Balady GJ, Blair SN, Corrado D, Estes NA 3rd, Fulton JE, Gordon NF, Haskell WL, Link MS, Maron BJ, Mittleman MA, Pelliccia A, Wenger NK, Willich SN, Costa F (2007) Exercise and acute cardiovascular events: placing the risks into perspective: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism and the Council on Clinical Cardiology, American College of Sports Medicine. Circulation 115: 2358-2368. DOI: 10.1161/CIRCULATIONAHA.107.181485.
Lagneux C, Joyeux M, Demenge P, Ribuot C, Godin-Ribuot D (2000) Protective effects of melatonin against ischemia-reperfusion injury in the isolated rat heart. Life Sci. 66: 503-509. DOI: 10.1016/s0024-3205(99)00620-7.
Sahna E, Olmez E, Acet A (2002) Effects of physiological and pharmacological concentrations of melatonin on ischemia–reperfusion arrhythmias in rats: can the incidence of sudden cardiac death be reduced? J. Pineal Res. 32: 194-198.DOI: 10.1034/j.1600-079x.2002.1o853.x.
Mukherjee D, Roy SG, Bandyopadhyay A, Chattopadhyay A, Basu A, Mitra E, Ghosh AK, Reiter RJ, Bandyopadhyay D (2010) Melatonin protects against isoproterenol‐induced myocardial injury in the rat: antioxidative mechanisms. J. Pineal Res. 48: 251-262. DOI: 10.1111/j.1600-079X.2010.00749.x.
Yeung HM, Hung MW, Lau CF, Fung ML (2015) Cardioprotective effects of melatonin against myocardial injuries induced by chronic intermittent hypoxia in rats. J. Pineal Res. 58: 12-25. DOI: 10.1111/jpi.12190.
Vazan R, Ravingerova T (2015) Protective effect of melatonin against myocardial injury induced by epinephrine. J. Physiol. Biochem. 71: 43-49. DOI: 10.1007/s13105-014-0377-5.
Mukherjee D, Ghosh AK, Bandyopadhyay A, Basu A, Datta S, Pattari SK, Reiter RJ, Bandyopadhyay D (2012) Melatonin protects against isoproterenol‐induced alterations in cardiac mitochondrial energy‐metabolizing enzymes, apoptotic proteins, and assists in complete recovery from myocardial injury in rats. J. Pineal Res. 53: 166-179. DOI: 10.1111/j.1600-079x.2012.00984.x.
Nduhirabandi F, Maarman GJ (2018) Melatonin in heart failure: a promising therapeutic strategy? Molecules 23: 1819. DOI: 10.3390/molecules23071819.
![]()
This work is licensed under a Creative Commons Attribution 4.0 International License