Monosodium glutamate administration early in life alters pineal melatonin nocturnal profile in adulthood
MSG alters melatonin nocturnal profile in the pineal glands
Abstract
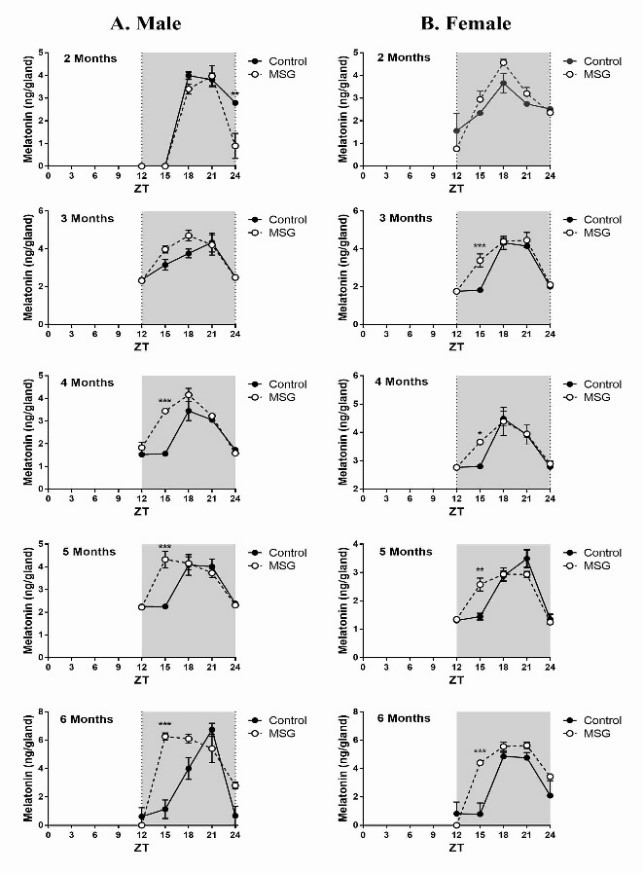
The pineal gland synthesizes melatonin exclusively at night, which gives melatonin the characteristic of a temporal synchronizer of the physiological systems. Melatonin is a regulator of insulin activities centrally and also peripherally and its synthesis is reduced in diabetes. Since monosodium glutamate (MSG) is often used to induce the type 2 diabetic and metabolic syndrome in animal models, the purpose of this work is to evaluate the potential effects of MSG given to neonates on the pineal melatonin synthesis in different aged male and female rats. Wistar rats were subcutaneously injected with MSG (4mg/g/day) or saline solution (0.9%) from the second to eighth post-natal day. The circadian profiles both melatonin levels and AANAT activity were monitored at different ages. Body weight, naso-anal length, adipose tissues weight, GTT, ITT and serum insulin levels were also evaluated. Typical obesity with the neonatal MSG treatment was observed, indicated by a great increase in adipose depots without a concurrent increase in body weight. MSG treatment did not cause hyperglycemia or glucose intolerance, but induced insulin resistance. An increase of melatonin synthesis at ZT 15 with phase advance was observed in in some animals. The AANAT activity was positively parallel to the melatonin circadian profile. It seems that MSG causes hypothalamic obesity which may increase AANAT activity and melatonin production in pineal gland. These effects were not temporally correlated with insulin resistance and hyperinsulinemia indicating the hypothalamic lesions, particularly in arcuate nucleus induced by MSG in early age, as the principal cause of the increase in melatonin production.
References
2. Amaral FGD, Cipolla-Neto J (2018) A brief review about melatonin, a pineal hormone. Arch. Endocrinol. Metab. 62 (4): 472-479. doi: 10.20945/2359-3997000000066.
3. Cipolla-Neto J, Amaral FGD (2018) Melatonin as a Hormone: New Physiological and Clinical Insights. Endocr. Rev. 39 (6): 990-1028. doi: 10.1210/er.2018-00084.
4. Anhê GF, Caperuto LC, Pereira-da-Silva M, Souza LC, Hirata AE, Velloso LA, Cipolla-Neto J, Carvalho CR (2004) In vivo activation of insulin receptor tyrosine kinase by melatonin in the rat hypothalamus. J. Neurochem. 90 (3): 559-566.
5. Cipolla-Neto J, Amaral F G, Afeche S C, Tan D X, Reiter R J (2014) Melatonin, Energy Metabolism and Obesity: a Review. J. Pineal Res. 56 (4): 371-81. doi: 10.1111/jpi.12137.
6. Amaral FGD, Andrade-Silva J, Kuwabara WMT, Cipolla-Neto J (2019) New insights into the function of melatonin and its role in metabolic disturbances. Expert Rev. Endocrinol. Metab. 14 (4): 293-300. doi: 10.1080/17446651.2019.1631158.
7. Amaral FG, Turati AO, Barone M, Scialfa JH, do Carmo Buonfiglio D, Peres R, Peliciari-Garcia RA, Afeche SC, Lima L, Scavone C, Bordin S, Reiter RJ, Menna-Barreto L, Cipolla-Neto J (2014) Melatonin synthesis impairment as a new deleterious outcome of diabetes-derived hyperglycemia. J. Pineal Res. 57 (1): 67-79. doi: 10.1111/jpi.12144.
8. Hardeland R (2013) Chronobiology of Melatonin beyond the Feedback to the Suprachiasmatic Nucleus—Consequences to Melatonin Dysfunction. Int. J. Mol. Sci. 14: 5817-5841. doi:10.3390/ijms14035817.
9. Karamitri A, Renault N, Clement N, Guillaume J-L, Jockers R (2013) Minireview: Toward the Establishment of a Link between Melatonin and Glucose Homeostasis: Association of Melatonin MT2 Receptor Variants with Type 2 Diabetes. Mol. Endocrinol. 27: 1217–1233. doi:10.1210/me.2013-1101.
10. Karamitri A, Jockers R (2019) Melatonin in type 2 diabetes mellitus and obesity. Nat. Rev. Endocrinol. 15: 105–125. doi: 10.1038/s41574-018-0130-1.
11. Lyssenko V, Nagorny C L, Erdos MR (2009) Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat. Genet. 41: 82–88.
12. Das UN, Fams MD (2001) Is obesity an inflammatory condition? Nutrition 17: 953–966.
13. Gregor MF, Hotamisligil GS (2011) Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 29: 415-445.
14. Leguisamo NM, Lehnen AM, Machado UF, Okamoto MM, Markoski MM, Pinto GH, Schaan BD (2012) GLUT4 content decreases along with insulin resistance and high levels of inflammatory markers in rats with metabolic syndrome. Cardiovasc. Diabetol. 11: 100-110.
15. Oda E (2012) Metabolic syndrome: its history, mechanisms, and limitations. Acta Diabetol. 49: 89-95.
16. Bahadoran Z, Mirmiran P, Ghasemi A (2019) Pre-clinical Models: Techniques and Protocols, Methods in Molecular Biology. Monosodium glutamate (MSG)-induced animal model of type 2 diabetes. Methods Mol. Biol. 1916: 49-65.
17. Hernández-Bautista RJ, Mahmoud AM, Königsberg M, Guerrero NELD (2019) Obesity: Pathophysiology, monosodium glutamate-induced model and antiobesity medicinal plants. Biomed. Pharmacother. 111: 503-516.
18. Nagata M, Suzuki W, Lizuka S, Tabuchi M, Maruyama H, Aburada M, Miyamoto K (2006) Type 2 diabetes mellitus in obese mouse model induced by monosodium glutamate. Exp. Anim. 55: 109-115.
19. Morrison JFB, Shehabs S, Sheen R, Dhanasekaran S, Shaffiullah M, Brown EM (2007) Sensory and autonomic nerve changes in the monosodium glutamate-treated rat: a model of type II diabetes. Exp. Physiol. 93 (2): 213-222.
20. Nemeroff CB, Lipton MA, Kizer JS (1978) Models of neuroendocrine regulation: use of monosodium glutamate as an investigational tool. Dev. Neurosci. 1: 102-109.
21. Afifi MM, Abbas AM (2011) Monosodium glutamate versus diet induced obesity in pregnant rats and their offspring. Acta Physiol. Hun. 98 (2): 177-188.
22. He K, Zhao L, Daviglus ML, Dyer AR, Van Horn L, Gerside D, Zhu L, Guo D, Wu Y, Zhou B, Stamler J (2008) Association of Monosodium Glutamate Intake with overweight in Chinese adults: the INTERMAP study. Obesity 16: 1875-1880.
23. He K, Du S, Xun P, Sharma S, Wang H, Zhai F, Popkin B (2011) Consumption of monosodium glutamate in relation to incidence of overweight in Chinese adults: China Health and Nutrition Survey (CHNS). Am. J. Clin. Nutr. 93: 1328-1336.
24. Insawang T, Selmi C, Cha’on U, Pethlert S, Yongvanit P, Areejitranusorn P, Boonsiri P, Khampitak T, Tangrassameeprasert R, Pinitsoontorn C, Prasongwattana V, Gershwin ME, Hammock BD (2012) Monosodium glutamate (MSG) intake is associated with the prevalence of metabolic syndrome in a rural Thai population. Nutr. Metab. 9: 50-56. doi: 10.1186/1743-7075-9-50.
25. Parfitt A, Weller JL, Klein DC (1976) Beta adrenergic-blockers decrease adrenergically stimulated N-acetyltransferase activity in pineal glands in organ culture. Neuropharmacology 15: 353-358.
26. Deguchi T, Axelrod J (1972) Sensitive assay for serotonin N-acetyltransferase activity in rat pineal. Anal. Biochem. 50: 176-179.
27. Ferreira D S, Amaral FG, Mesquita CC, Barbosa APL, Lellis-Santos C, Turati AO, Santos LR, Sollon CS, Gomes PR, Faria JA, Cipolla-Neto J, Bordin S, Anhe GF (2012) Maternal melatonin programs the daily pattern of energy metabolism in adult offspring. PLoS One 7 (6): e.38795.
28. Lehnen AM, Leguisamo NM, Pinto GH, Markoski MM, De Angelis K, Machado U F, Schaan B (2010) The beneficial effects of exercise in rodents are preserved after detraining: a phenomenon unrelated to GLUT4 expression. Cardiovasc. Diabetol. 9: 67. doi: 10.1186/1475-2840-9-67.
29. Nelson W, Tong YL, Lee JK, Halberg F (1979) Methods for cosinor-rhythmometry. Chronobiologia 6 (4): 305-23.
30. Hirata AE, Andrade I S, Vaskevicius P, Dolnikoff MS (1997) Monosodium glutamate (MSG)-obese rats develop glucose intolerance and insulin resistance to peripheral glucose uptake. Braz. J. Med. Biol. Res. 30: 671–674.
31. Macho L, Ficková M, Jezová D, Zórad S (2000) Late effects of postnatal administration of monosodium glutamate on insulin action in adult rats. Physiol. Res. 49 (1): 79-85.
32. Bloch B, Ling N, Benoit R, Wehrenberg W B, Guillemin R (1984) Specific depletion of immunoreactive growth hormone-releasing factor by mono sodium glutamate in rat median eminence. Nature 307: 272–273.
33. Abe M, Saito M, Shimazu T (1990) Neuropeptide Y in the specific hypothalamic nuclei of rats treated neonatally with monosodium glutamate. Brain. Res. Bull. 24: 289–291.
34. Kerkérian L, Pelletier G (1986) Effects of l-glutamate administration on neuropeptide Y-containing neurons in the rat hypothalamus. Brain Res. 369: 388-390.
35. Peschke E, Frese T, Chankiewitz E, Peschke D, Preiss U, Scheneyer U, Spessert R, Muhlbauer E (2006) Diabetic Goto-Kakizaki rats as well as type 2 diabetic patients show a decreased diurnal serum melatonin level and an increased pancreatic melatonin-receptor status. J. Pineal Res. 40: 135-143.
36. Peschke E, Stumpf I, Bazwinsky I, Litvak L, Dralle H, Mühlbauer E (2007) Melatonin and type 2 diabetes - a possible link? J. Pineal Res. 42: 350-358.
37. Beck B (2006) Neuropeptide Y in normal eating and in genetic and dietary-induced obesity. Philos. Trans. R. Soc. B 361 (1471): 1159–1185.
38. Garcia RAP, Afeche SC, Scialfa JH, Amaral FG, Santos SHJ, Lima FB, Young MB, Cipolla-Neto J (2008) Insulin modulates norepinephrine-mediated melatonin synthesis in cultured pineal gland. Life Sci. 82: 108-114. doi: 10.1016/j.lfs.2007.10.016.
39. Garcia RAP, Marçal AC, Andrade JS, Carmo-Buonfiglio D, Amaral FG, Afeche SC, Cipolla-Neto J, Carvalho CRO (2010) Insulin temporal sensitivity and its signaling pathway in the rat pineal gland. Life Sci. 87: 169-174. doi: 10.1016/j.lfs.2010.06.005.
40. Edelstein K, Pfaus JG; Rusak B, Amir S (1995) Neonatal monosodium glutamate treatment prevents effects of constant light on circadian temperature rhythms of adult rats. Brain Res. 675: 135-142.
41. yabo S, Yamamura I, Ooya E, Aoyagi N, Horikawa Y, Hoyashi S (1985) Effects of neonatal treatment with monosodium glutamate on circadian locomotor rhythm in the rat. Brain Res. 339 (2): 201-208.
42. Nemeroff C B, Konkol RJ, Bissette G, Youngblood W, Martin JB, Brazeau P, Rone MS, Prange AJ, Breese GR, Kizer JS (1977) Analysis of the disruption in hypothalamic-pituitary regulation in rats treated neonatally with monosodium L-glutamate (MSG): evidence for the involvement of tuberoinfundibular cholinergic and dopaminergic systems in neuroendocrine regulation. Endocrinology 101 (2): 613-622.
43. Pickard GE, Turek FW, Lamperti AA, Silverman J (1982) The effect of neonatally administered monosodium glutamate (MSG) on the development of retinofugal projections and the entrainment of circadian locomotor activity. Behav. Neural Biol. 34: 449-526.
44. Kizer JS, Nemeroff CB, Youngblood WW (1978) Neurotoxic amino acids and structurally related analogs. Pharmacol. Rev. 29 (4): 301-318.
45. Laakso M-L, Alila A, Hatonen T, Mustanoja SM (1996) Ontogeny of pineal melatonin rhythm in rats under 12: 12-hr and 14: 14-hr light: dark conditions. J. Pineal Res. 21:155-164.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


