Melatonin-Index as a biomarker for predicting the distribution of presymptomatic and asymptomatic SARS-CoV-2 carriers
Lung melatonin modulates SARS-CoV-2 infection
Abstract
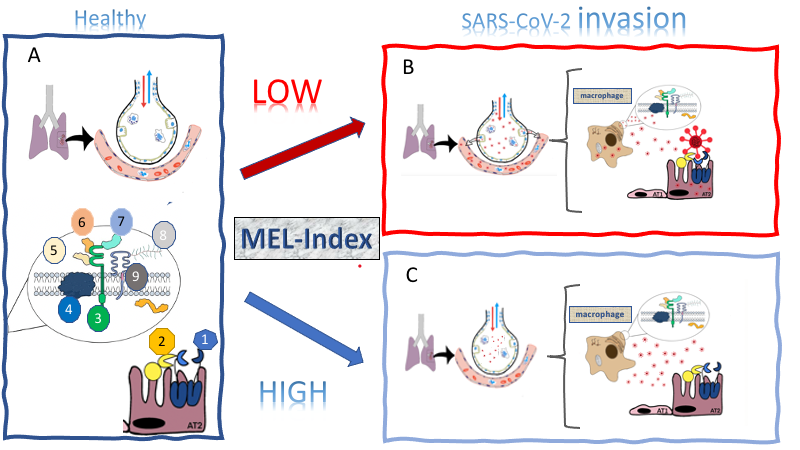
The pandemic dissemination of the SARS-CoV-2 led, on the one hand, to a worldwide effort to develop mechanistic-based therapeutics and vaccines, and on the other hand, the searching for determining the spreaders and the mechanisms of transmission. Melatonin, a multitask molecule, orchestrates defense responses by allowing the proper mounting, duration, and magnitude of innate immune responses. Melatonin is synthesized on demand by immune-competent cells and constitutively by resident macrophages such as alveolar macrophages. Here we investigated whether the expression of genes relevant to virus invasion and infection varies according to a genic index (MEL-Index) that estimates the capacity of the lung to synthesize melatonin. A COVID-19-Signature composed of 455 genes of 288 human lungs (GTEX, UCSD) was correlated with MEL-Index by Pearson correlation test, gene-set enrichment analysis, and networking tool that integrates the connectivity between the most expressed genes, allowing us to compare the same set of genes under different states. The three independent procedures point to a negative relationship between MEL-Index and SARS-CoV-2 infection. The entry in epithelial AT2 cells should be hampered by a positive correlation TMRPSS2 and a negative correlation with the coding gene for furin, suggesting dysfunctional processing in virus spike. Moreover, MEL-Index also negatively correlates with the genes that codify the proteins of multi-molecular receptor complex CD147, the gateway in macrophages, and other immune cells. In summary, the perspective that lung and respiratory tract melatonin could be a natural protective factor opens new epidemiological and pharmacological perspectives, as high MEL-Index scores could be predictive of asymptomatic carriers, and nasal administrated melatonin could prevent evolution of presymptomatic carriers.
References
2. Wijaya I, Andhika R, Huang I (2020) The Use of Therapeutic-Dose Anticoagulation and Its Effect on Mortality in Patients With COVID-19: A Systematic Review. Clin. Appl. Thromb. Hemost. 26: 1076029620960797. doi: 10.1177/1076029620960797.
3. Stone JH, et al. (2020) Efficacy of Tocilizumab in Patients Hospitalized with Covid-19. N. Engl. J. Med. 383 (24): 2333-2344. doi: 10.1056/NEJMoa2028836.
4. Horby P, et al. (2020) Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report. N. Engl. J. Med. 17: NEJMoa2021436. doi: 10.1056/NEJMoa2021436.
5. Rice AM, et al. (2020) Evidence for strong mutation bias towards, and selection against, U content in SARS-CoV-2: implications for vaccine design. Mol. Biol. Evol. 188. doi: 10.1093/molbev/msaa188.
6. Nikolai LA, Meyer CG, Kremsner PG, Velavan TP (2020) Asymptomatic SARS Coronavirus 2 infection: Invisible yet invincible. Int. J. Infect. Dis. 100: 112-116. doi: 10.1016/j.ijid.2020.08.076.
7. Rocklöv J, Sjödin H, Wilder-Smith A (2020) COVID-19 outbreak on the Diamond Princess cruise ship: estimating the epidemic potential and effectiveness of public health countermeasures. J. Travel. Med. 27: taaa030. doi: 10.1093/jtm/taaa030.
8. Keeley AJ, Evans CM, de Silva TI (2020) Asymptomatic SARS-CoV-2 infection: the tip or the iceberg? Thorax. 75: 621-622. doi: 10.1136/thoraxjnl-2020-215337.
9. Noorimotlagh Z, Jaafarzadeh N, Martínez SS, Mirzaee SA (2020) A systematic review of possible airborne transmission of the COVID-19 virus (SARS-CoV-2) in the indoor air environment. Environ. Res. 193: 110612. doi: 10.1016/j.envres.2020.110612.
10. Markus RP, Fernandes PA., Kinker GS, da Silveira Cruz-Machado S, Marçola M (2018) Immune‐pineal axis – acute inflammatory responses coordinate melatonin synthesis by pinealocytes and phagocytes. Br. J. Pharmacol. 175: 3239–3250. doi: 10.1111/bph.14083.
11. Golan K, et al (2018) Daily onset of light and darkness differentially controls hematopoietic stem cell differentiation and maintenance. Cell Stem Cell 23: 572-585. doi:10.1016/j.stem.2018.08.002
12. Hardeland R, Tan DX (2020) Protection by melatonin in respiratory diseases: valuable information for the treatment of COVID-19. Melatonin Res. 3: 264-275. doi:10.32794/mr11250061.
13. Cardinali D, et al. (2020) Elderly as a High-risk Group during COVID-19 Pandemic: Effect of Circadian Misalignment, Sleep Dysregulation and Melatonin Administration. Sleep Vigilance 4: 81–87. doi:10.1007/s41782-020-00111-7.
14. Reiter RJ, et al. (2020) Plasticity of glucose metabolism in activated immune cells: advantages for melatonin inhibition of COVID-19 disease. Melatonin Res. 3: 362-379. doi: 10.32794/mr11250068.
15. García, IG, et al. (2020) A randomized multicenter clinical trial to evaluate the efficacy of melatonin in the prophylaxis of SARS-CoV-2 infection in high-risk contacts (MeCOVID Trial): A structured summary of a study protocol for a randomised controlled trial. Trials 21: 466. doi: 10.1186/s13063-020-04436-6.
16. Acuña‐Castroviejo D, et al. (2020) Clinical trial to test the efficacy of melatonin in COVID‐19. J. Pineal Res. 69: e12683. doi: 10.1111/jpi.12683.
17. Carvalho-Sousa CE, et al. (2020) Immune-pineal axis protects rat lungs exposed to polluted air. J. Pineal Res. 68: e12636. doi: 10.1111/jpi.12636.
18. Kinker GS, et al. (2016) Melatonergic system-based two-gene index is prognostic in human gliomas. J. Pineal Res. 60: 84 – 94. doi: 10.1111/jpi.12293.
19. Lv JW, et al. (2019) Pan-cancer genomic analyses reveal prognostic and immunogenic features of the tumor melatonergic microenvironment across 14 solid cancer types. J. Pineal Res. 66: e12557. doi: 0.1111/jpi.12557.
20. Pinto BGG, et al. (2020) ACE2 Expression Is Increased in the Lungs of Patients With Comorbidities Associated With Severe COVID-19. J. Infect. Dis. 222: 556-563. doi: 10.1093/infdis/jiaa332.
21. Gordon DE, et al. (2020) A SARS-CoV-2-human protein-protein interaction map reveals drug targets and potential drug-repurposing. Nature doi: 10.1101/2020.03.22.002386.
22. Muus C, et al. (2020) Integrated analyses of single-cell atlases reveal age, gender, and smoking status associations with cell type-specific expression of mediators of SARS-CoV-2 viral entry and highlights inflammatory programs in putative target cells. bioRxiv. doi: 10.1101/2020.04.19.049254.
23. Radzikowska U, et al. (2020) Distribution of ACE2, CD147, CD26, and other SARS-CoV-2 associated molecules in tissues and immune cells in health and in asthma, COPD, obesity, hypertension, and COVID-19 risk factors. Allergy 75: 2829-2845. doi: 10.1111/all.14429.
24. Ma X, Idle JR, Krausz KW, Gonzalez FJ (2005) Metabolism of melatonin by human cytochromes P450. Drug Metab. Dispos. 33: 489-494. doi: 10.1124/dmd.104.002410.
25. Subramanian A, et al. (2005) Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 102: 15545–15550. doi: 10.1073/pnas.0506580102.
26. Jardim VC, et al. (2019) BioNetStat: A Tool for Biological Networks Differential Analysis. Front. Genet. 10: 594. doi: 10.3389/fgene.2019.00594.
27. Aguiar, JA et al. (2020) Gene expression and in situ protein profiling of candidate SARS-CoV-2 receptors in human airway epithelial cells and lung tissue. Eur. Respir. J. 56: 2001123. doi:10.1183/13993003.01123-2020.
28. Italiani, P, Boraschi, D (2017) Development and functional differentiation of tissue-resident versus monocyte-derived macrophages in inflammatory reactions. Results Probl. Cell. Differ. 62 23–43.
29. Pontes GN, Cardoso EC, Carneiro-Sampaio MM, Markus RP (2006) Injury switches melatonin production source from endocrine (pineal) to paracrine (phagocytes) - melatonin in human colostrum and colostrum phagocytes. J. Pineal Res. 41: 136-141. doi: 10.1111/j.1600-079X.2006.00345.x.
30. Pires-Lapa MA, Tamura EK, Salustiano EM, Markus RP (2013) Melatonin synthesis in human colostrum mononuclear cells enhances dectin-1-mediated phagocytosis by mononuclear cells. J. Pineal Res. 55: 240-246. doi: 10.1111/jpi.12066.
31. Pires-Lapa MA, Carvalho-Sousa CE, Cecon E, Fernandes PA, Markus RP (2018) β-Adrenoceptors trigger melatonin synthesis in phagocytes. Int. J. Mol. Sci. 19: 2182-2194. doi: 10.3390/ijms19082182.
32. Kitsak M, et al. (2010) Identification of influential spreaders in complex networks. Nature Phys. 6: 888–893. doi: 10.1038/nphys1746.
33. Kinori E, Okamoto, K (2015) PINK1/Parkin-mediated mitophagy in mammalian cells. Curr. Opin. Cell Biol. 33: 95-101, doi: 10.1016/j.ceb.2015.01.002.
34. Wu J, Yang Y, Gao Y, Wang Z, Ma J (2020) Melatonin Attenuates Anoxia/Reoxygenation Injury by Inhibiting Excessive Mitophagy Through the MT2/SIRT3/FoxO3a Signaling Pathway in H9c2 Cells. Drug Des. Devel. Ther. 14: 2047-2060. doi: 10.2147/DDDT.S248628.
35. Ramasamy S, et al. (2016) Tle1 tumor suppressor negatively regulates inflammation in vivo and modulates NF-κB inflammatory pathway. Proc. Natl. Acad. Sci. U S A. 113: 1871-1876. doi: 10.1073/pnas.1511380113.
36. Breda, CNF, et al. (2019) Mitochondria as central hub of the immune system. Redox Biol. 26: 101255, 2019. doi: 10.1016/j.redox.2019.101255.
37. Codo AC, et al. (2020) Elevated glucose levels favor Sars-Cov-2 infection and monocyte response through a Hif-1α/glycolysis dependent axis. Cell Metab. 32 (3): 498-499. doi: 10.1016/j.cmet.2020.07.015.
38. Vriend J, Reiter, RJ (2016) Melatonin and the von Hippel–Lindau/HIF-1 oxygen sensing mechanism: A review. Biochim. Biophys. Acta 1865: 176-183. doi: 10.1016/j.bbcan.2016.02.004.
39. Burrows C, et al. (2010) The RNA binding protein Larp1 regulates cell division, apoptosis and cell migration. Nucleic. Acids Res. 38: 5542–5553, doi: 10.1093/nar/gkq294.
40. Tcherkezian J, et al. (2014) Proteomic analysis of cap-dependent translation identifies LARP1 as a key regulator of 5'TOP mRNA translation. Genes Dev. 28: 357-71. doi: 10.1101/gad.231407.113.
41. Suzuki Y, et al. (2016) Characterization of RyDEN (C19orf66) as an interferon-stimulated cellular inhibitor against dengue virus replication. PLoS Pathog. 12: E1005357-E1005357.
42. Benton DJ, et al. (2020) Receptor binding and priming of the spike protein of SARS-Cov-2 for membrane fusion. Nature 588: 327-330. doi: 10.1038/s41586-020-2772-0.
43. Rabaan AA, et al. (2020) SARS-CoV-2, SARS-CoV, and MERS-COV: A comparative overview. Infez. Med. 28: 174-184.
44. Hoffmann M, et al. (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181: 271-280.e8. doi:10.1016/j.cell.2020.02.052.
45. Matsuyama S, Ujike M, Morikawa S, Tashiro M, Taguchi F (2005) Protease-mediated enhancement of severe acute respiratory syndrome coronavirus infection. Proc. Natl. Acad. Sci. USA 102: 12543-12547. doi: 10.1073/pnas.0503203102.
46. Bestle D, et al. (2020) TMPRSS2 and furin are both essential for proteolytic activation of SARS-CoV-2 in human airway cells. Life Sci. Alliance 3:e202000786. doi: 10.26508/lsa.202000786.
47. Rahman N, et al. (2020) Virtual screening of natural products against type II transmembrane serine protease (TMPRSS2), the priming agent of coronavirus 2 (SARS-CoV-2). Molecules 25: 2271. doi: 10.3390/molecules25102271.
48. Ural BB, et al. (2020) Identification of a nerve-associated, lung-resident interstitial macrophage subset with distinct localization and immunoregulatory properties. Sci. Immunol. 5: eaax8756. doi: 10.1126/sciimmunol.aax8756.
49. Tumpey TM, et al. (2005) Pathogenicity of influenza viruses with genes from the 1918 pandemic virus: functional roles of alveolar macrophages and neutrophils in limiting virus replication and mortality in mice. J. Virol. 79: 14933-14944. doi: 10.1128/JVI.79.23.14933-14944.2005.
50. Pribul PK, et al. (2008) Alveolar macrophages are a major determinant of early responses to viral lung infection but do not influence subsequent disease development. J. Virol. 82: 4441-4448. doi: 10.1128/JVI.02541-07.
51. Schneider C, et al. (2014) Alveolar macrophages are essential for protection from respiratory failure and associated morbidity following influenza virus infection. PLoS Pathog. 10:e1004053. doi: 10.1371/journal.ppat.1004053.
52. Kumagai Y, et al. (2007) Alveolar macrophages are the primary interferon-alpha producer in pulmonary infection with RNA viruses. Immunity 27: 240-252. doi: 10.1016/j.immuni.2007.07.013.
53. Liu C, von Brunn A, Zhu D (2020) Cyclophilin A and CD147: novel therapeutic targets for the treatment of COVID-19. Med. Drug Discov. 7: 100056. doi:10.1016/j.medidd.2020.100056.
54. Trachtenberg A, et al. (2011) The level of CD147 expression correlates with cyclophilin-induced signalling and chemotaxis. BMC Res. Notes 4: 396. https://doi.org/10.1186/1756-0500-4-396.
55. Su H, et al. (2020) Expression of CD147 and cyclophilin A in kidneys of patients with COVID-19. Clin. J. Am. Soc. Nephrol. 2: CJN.09440620. doi: 10.2215/CJN.09440620. Epub ahead of print.
56. Gaymes TJ, Cebrat M, Siemion IZ, Kay JE (1997) Cyclolinopeptide A (CLA) mediates its immunosuppressive activity through cyclophilin-dependent calcineurin inactivation. FEBS Lett. 418: 224-227. doi: 10.1016/s0014-5793(97)01345-8.
57. Liu C, et al. (2016) Cyclophilin A stabilizes the HIV-1 capsid through a novel non-canonical binding site. Nat. Commun. 7: 10714. doi: 10.1038/ncomms10714.
58. Pfefferle S, et al. (2011) The SARS-coronavirus-host interactome: identification of cyclophilins as target for pan-coronavirus inhibitors. PLoS Pathog. 7: e1002331. doi: 10.1371/journal.ppat.1002331.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


