Salt stress in Arabidopsis thaliana seedlings: Role of indoleamines in stress alleviation
Role of indoleamines in salt stress
Abstract
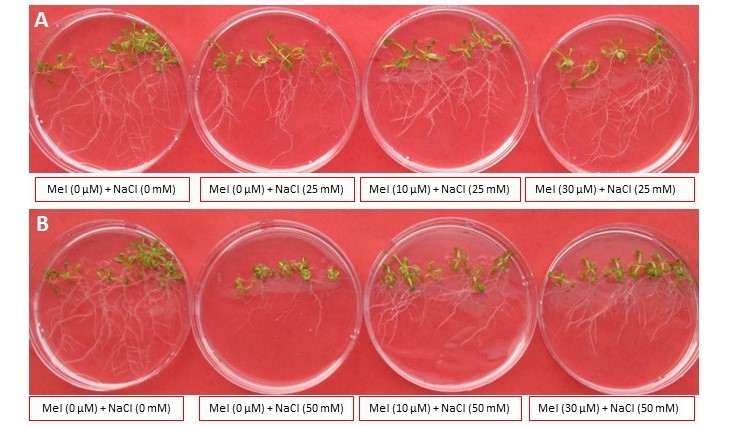
Salinity is a major environmental stress in agriculture with significantly detrimental effects on crop productivity. The development of strategies to enhance salinity stress tolerance in plants is essential to ensure crop production in saline environments. Melatonin (Mel) and serotonin (Ser) accumulate in response to environmental stresses and are presumed to play protective roles and improve growth of tissues during recovery. In this study, the effects of Mel and Ser were investigated in Arabidopsis under NaCl stress. Exogenous Mel (10 µM) and Ser (10 µM) treatment significantly increased fresh weight, lateral root number, and shoot height in A. thaliana seedlings exposed to NaCl stress (25 mM and 50 mM) compared to the non-treated control seedlings. In order to understand the role of these indoleamines in alleviating salt stress, we investigated the effects of Mel and Ser treatments on the expression of salt stress responsive genes including, transcription factors involved in abscisic acid (ABA) signaling pathway, ABA-INSENSITIVE 3 (ABI3)and ABA-INSENSITIVE 5 (ABI5); ABA responsive gene, RESPONSIVE TO DESSICATION 29B (RD29B), ABA-independent gene, RESPONSIVE TO DESSICATION 29A (RD29A) and Arabidopsis trithorax-like gene (ATX1) which function in stress responses via ABA-dependent and ABA-independent manner. Other genes included, ROS-signaling transcription factor ZAT10 and ZAT12, and the genes encoding ion transporters crucial for maintaining ion homeostasis, HIGH AFFINITY K+ TRANSPORTER 5 (HAK5) and SALT OVERLY SENSITIVE 1 (SOS1). Mel (10 µM) pre-treatment for 24 hrs followed by 50 mM salt treatment up-regulated ABI3, RD29B, ZAT12 and HAK5. The Ser (10 µM) pre-treatment significantly up-regulated ZAT12.These results indicate that indoleamine pre-treatment improved plant growth under salt stress with Mel facilitating salt tolerance via upregulation of ABA responsive genes, mediation of antioxidant defense systems to counteract the salt-induced ROS overproduction as well as controlling ion homeostasis. Although Ser displayed no significant effects on ABA signaling, it was found to increase the expression of antioxidant defense gene, ZAT12. This study demonstrates the importance of indoleamine pathway in mediation of salt stress response and provides the first indication of the involvement of Ser in salt stress tolerance.
References
2. Zörb C, Geilfus CM, Dietz KJ (2019) Salinity and crop yield. Plant Biol. 21: 31–38.
3. Jamil A, Riaz S, Ashraf M, Foolad MR (2011) Gene expression profiling of plants under salt stress. CRC. Crit. Rev. Plant Sci. 30: 435–458.
4. Hasanuzzaman M, Nahar K, Fujita M (2013) “Plant Response to salt stress and role of exogenous protectants to mitigate salt-induced damages” in Ecophysiology and Responses of Plants under salt stress, Ahmad P, Azooz M, Prasad M, Eds. (Springer New York, 2013), pp. 28–87.
5. Tilman D, et al. (2001) Forecasting agriculturally driven global environmental change. Science 292: 281–284.
6. Foley JA, DeFries R, Asner GP, Barford C, Bonan G (2005) Global consequences of land use. Science 309: 570–574.
7. Hernández JA (2019) Salinity tolerance in plants: trends and perspectives. Int. J. Mol. Sci. 20: 2408.
8. van Zelm E. Zhang Y, Testerink C (2020) Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 71: 403–433.
9. Reiter RJ (1991) Pineal melatonin: cell biology of its synthesis and of its physiological interactions. Endocr. Rev. 12: 151–180.
10. Murch SJ, Campbell SS, Saxena PK (2001) The role of serotonin and melatonin in plant morphogenesis: regulation of auxin-induced root organogenesis in in vitro-cultured explants of St. John’s wort (Hypericum perforatum L.). In. Vitr. Cell. Dev. Biol. 37: 786–793.
11. Afreen F, Zobayed S, Kozai T (2006) Melatonin in Glycyrrhiza uralensis: response of plant roots to spectral quality of light and UV‐B radiation. J. Pineal Res. 41: 108–115.
12. Tan D-X, Manchester LC, Terron MP, Flores LJ, Reiter RJ (2007) One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 42: 28–42.
13. Li C, et al. (2012) The mitigation effects of exogenous melatonin on salinity-induced stress in Malus hupehensis. J. Pineal Res. 53: 298–306.
14. Tiryaki I, Keles H (2012) Reversal of the inhibitory effect of light and high temperature on germination of Phacelia tanacetifolia seeds by melatonin. J. Pineal Res. 52: 332–339.
15. Wang P, et al. (2012) Delayed senescence of apple leaves by exogenous melatonin treatment: toward regulating the ascorbate-glutathione cycle. J. Pineal Res. 53: 11–20.
16. Yin L, et al. (2013) Exogenous melatonin improves Malus resistance to Marssonina apple blotch. J. Pineal Res. 54: 426–434.
17. Bajwa VS, Shukla MR, Sherif SM, Murch SJ, Saxena PK (2014) Role of melatonin in alleviating cold stress in Arabidopsis thaliana. J. Pineal Res. 56: 238–245.
18. Li H, et al. (2017) Exogenous melatonin confers salt stress tolerance to watermelon by improving photosynthesis and redox homeostasis. Front. Plant Sci. 8: 295.
19. Zhao G, et al. (2018) Nitric oxide is required for melatonin-enhanced tolerance against salinity stress in rapeseed (Brassica napus L.) seedlings. Int. J. Mol. Sci. 19: 1912.
20. Kostopoulou Z, Therios I, Roumeliotis E, Kanellis AK, Molassiotis A (2015) Melatonin combined with ascorbic acid provides salt adaptation in Citrus aurantium L. seedlings. Plant Physiol. Biochem. 86: 155–165.
21. Lee H-J, Back K (2019) 2-Hydroxymelatonin confers tolerance against combined cold and drought stress in tobacco, tomato, and cucumber as a potent anti-stress compound in the evolution of land plants. Melatonin Res. 2: 35–46.
22. Mukherjee S, David A, Yadav S, Baluška F, Bhatla SC (2014) Salt stress-induced seedling growth inhibition coincides with differential distribution of serotonin and melatonin in sunflower seedling roots and cotyledons. Physiol. Plant. 152: 714–728.
23. Erland LAE, Shukla MR, Singh AS, Murch SJ, Saxena PK (2018) Melatonin and serotonin: Mediators in the symphony of plant morphogenesis. J. Pineal Res. 64: e12452.
24. Tuteja N (2007) Abscisic acid and abiotic atress aignaling. Plant Signal. Behav. 2: 135–138.
25. Sah SK, Reddy KR, Li J (2016) Abscisic acid and abiotic stress tolerance in crop plants. Front. Plant Sci. 7:571.
26. Zhang L, et al. (2020) Wheat TabZIP8, 9, 13 participate in ABA biosynthesis in NaCl-stressed roots regulated by TaCDPK9-1. Plant Physiol. Biochem. 151: 650–658.
27. Ding Y, Avramova Z, Fromm M (2011) The Arabidopsis trithorax-like factor ATX1 functions in dehydration stress responses via ABA-dependent and ABA-independent pathways. Plant J. 66: 735–744.
28. Rock CD (2000) Pathways to abscisic acid‐regulated gene expression. New Phytol. 148: 357–396.
29. Yamaguchi T, Blumwald E (2005) Developing salt-tolerant crop plants: challenges and opportunities. Trends Plant Sci. 10: 615–620.
30. Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15: 473–497.
31. Lindsey BE, Rivero L, Calhoun CS, Grotewold E, Brkljacic J (2017) Standardized method for high-throughput sterilization of Arabidopsis seeds. J. Vis. Exp. 128: e56587.
32. Clouse SD, Hall AF, Langford M, McMorris TC, Baker ME (1993) Physiological and molecular effects of brassinosteroids on Arabidopsis thaliana. J. Plant Growth Regul. 12: 61–66.
33. Malboobi MA, Lefebvre DD (1997) A phosphate-starvation inducible β-glucosidase gene (psr3.2) isolated from Arabidopsis thaliana is a member of a distinct subfamily of the BGA family. Plant Mol. Biol. 34: 57–68.
34. Hétu M-F, Tremblay LJ, Lefebvre DD (2005) High root biomass production in anchored Arabidopsis plants grown in axenic sucrose supplemented liquid culture. Biotechniques 39: 345–349.
35. Logemann J, Schell J, Willmitzer L (1987) Improved method for the isolation of RNA from plant tissues. Anal. Biochem. 163: 16–20.
36. Shahbaz M, Ashraf M (2013) Improving salinity tolerance in cereals. CRC. Crit. Rev. Plant Sci. 32: 237–249.
37. Paul D (2013) Osmotic stress adaptations in rhizobacteria. J. Basic Microbiol. 53: 101–110.
38. FAOSTAT database (2013) Food and Agriculture Organization of the United Nations, Statistics Division, Rome. 2013. http://fenix.fao.org/faostat/beta/en/#home. Accessed 11 Jan 2019.
39. Ke Q et al. (2018) Melatonin mitigates salt stress in wheat seedlings by modulating polyamine metabolism. Front. Plant Sci. 9: 914.
40. Meng J-F, et al. (2014) The ameliorative effects of exogenous melatonin on grape cuttings under water-deficient stress: antioxidant metabolites, leaf anatomy, and chloroplast morphology. J. Pineal Res. 57: 200–212.
41. Zhang H-J, et al. (2014) Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA 4 interaction in cucumber (Cucumis sativus L.). J. Pineal Res. 57: 269–279.
42. Dawood MG, El-Awadi ME (2015) Alleviation of salinity stress on Vicia faba L. plants via seed priming with melatonin. Acta Biológica Colomb. 20: 223–235.
43. Liang C et al. (2015) Melatonin delays leaf senescence and enhances salt stress tolerance in rice. J. Pineal Res. 59: 91–101.
44. Wei W et al., (2015) Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J. Exp. Bot. 66: 695–707.
45. Jiang C et al., (2016) Melatonin improves antioxidant capacity and ion homeostasis and enhances salt tolerance in maize seedlings. Acta Physiol. Plant. 38: 82.
46. Mittal A et al., (2014) Related to ABA-Insensitive3(ABI3)/Viviparous1 and AtABI5 transcription factor coexpression in cotton enhances drought stress adaptation. Plant Biotechnol. J. 12: 578–589.
47. Tamminen I, Mäkelä P, Heino P, Palva ET (2001) Ectopic expression of ABI3 gene enhances freezing tolerance in response to abscisic acid and low temperature in Arabidopsis thaliana. Plant J. 25: 8.
48. Msanne J, Lin J, Stone JM, Awada T (2011) Characterization of abiotic stress-responsive Arabidopsis thaliana RD29A and RD29B genes and evaluation of transgenes. Planta 234: 97–107.
49. Fujita Y, et al. (2005) AREB1 is a transcription activator of novel ABRE-dependent ABA signaling that enhances drought stress tolerance in Arabidopsis. Plant Cell 17: 3470–3488.
50. McCarty DR (1995) Genetic control and integration of maturation and germination pathways in seed development. Annu. Rev. Plant Physiol. Plant Mol. Biol. 46: 71–93.
51. Lopez-Molina L, Mongrand S, McLachlin DT, Chait BT, Chua N-H (2002) ABI5 acts downstream of ABI3 to execute an ABA-dependent growth arrest during germination. Plant J. 32: 317–328.
52. Kazuo N et al. (2006) Transcriptional regulation of ABI3-and ABA-responsive genes including RD29B and RD29A in seeds, germinating embryos, and seedlings of Arabidopsis. Plant Mol. Biol. 60: 51–68.
53. Ding Y, Fromm M, Avramova Z (2012) Multiple exposures to drought “train” transcriptional responses in Arabidopsis. Nat. Commun. 3: 740.
54. Weeda S et al., (2014) Arabidopsis transcriptome analysis reveals key roles of melatonin in plant defense systems. PLoS One 9: e93462.
55. Xu L, Yue Q, Xiang G, Bian F, Yao Y (2018) Melatonin promotes ripening of grape berry via increasing the levels of ABA, H2O2, and particularly ethylene. Hortic. Res. 5: 41.
56. Mittler R et al. (2006) Gain- and loss-of-function mutations in Zat10 enhance the tolerance of plants to abiotic stress. FEBS Lett. 580: 6537–6542.
57. Li Y et al. (2010) Overexpression of a Malus vacuolar Na+/H+ antiporter gene (MdNHX1) in apple rootstock M.26 and its influence on salt tolerance. Plant Cell Tissue Organ Cult. 102: 337–345.
58. Nieves-Cordones M, Alemán F, Martínez V, Rubio F (2010) The Arabidopsis thaliana HAK5 K+ transporter is required for plant growth and K+ acquisition from low K+ solutions under saline conditions. Mol. Plant 3: 326–333.
59. Arnao M, Hernández‐Ruiz J (2014) Melatonin: plant growth regulator and/or biostimulator during stress? Trends Plant Sci. 19: 789–797.
60. Erland LAE, Murch SJ, Reiter RJ, Saxena PK (2015) A new balancing act: The many roles of melatonin and serotonin in plant growth and development. Plant Signal. Behav. 10: e1096469.
61. Reiter R, et al. (2015) Phytomelatonin: assisting plants to survive and thrive. Molecules 20: 7396–7437.
62. Erland LAE, Yasunaga A, Li ITS, Murch SJ, P. K. Saxena (2019) Direct visualization of location and uptake of applied melatonin and serotonin in living tissues and their redistribution in plants in response to thermal stress. J. Pineal Res. 66: e12527.
63. Forsyth JA, Erland LAE, Shipley PR, Murch SJ (2020) Plant Perception of Light: The role of indoleamines in Scutellaria species. Melatonin Res. 3: 161–176.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


