Blood melatonin level can serve as a potential biomarker for prostate and hepatocellular carcinomas
Melatonin as biomarker for prostate and hepatocellular carcinomas
Abstract
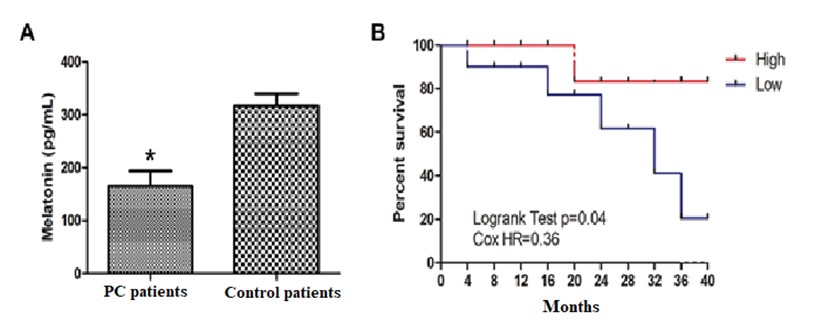
Many systemic functions display circadian rhythms driven by an endogenous mechanism that is regulated by circadian-related genes and these gene expressions control a central clock in the brain and subordinate clocks in peripheral tissues. However, modern life has introduced environmental factors that often interfere with natural circadian rhythms. Importantly, circadian disruption has been identified as an independent risk factor of cancers. Melatonin is a major circadian rhythm regulator. In cancer patients, the rhythm of melatonin is often disrupted and its level is also reduced. These changes of melatonin impair its antioxidant and circadian regulatory functions on cells and tissues making them more susceptible to mutations and cancer initiation. In this context, the objectives of this study are to evaluate the sleep quality and blood levels of melatonin in patients with either prostate cancer (PC) or hepatocarcinoma (HCC) with the intent of using its levels as a potential biomarker of the cancers. The study involved 20 PC and 18 HCC patients, and 26 healthy volunteers. All blood samples were collected in the early morning, at 07:00 hours. Comparative sleep quality between PC, HCC patients and control subjects was acessed with a questionnaire, and melatonin and vitamin D were measured using conventional assays. The results revealed that patients with the worse sleep quality also had lower values of melatonin and vitamin D compared to control subjects. Notably, expression of melatonin-synthesizing enzymes and specific clock genes (PER, CRY and BMAL1) were significantly reduced and associated with worse prognosis in PC and HCC patients. These findings are consistent with the results of previous studies and suggest that disruption of the circadian rhythms, associated with changes in the light:dark cycle, has consequences for the maintenance of systemic health. We suggest that supplementation of melatonin and vitamin D may represent the important therapeutic strategies for patients with solid tumors for the purpose of improving their sleep quality and recuperative capacity.
References
2. Deeb KK, Trump DL, Johnson CS (2007) Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat. Rev. Cancer 7 (9): 684-700. doi:10.1038/nrc2196.
3. Gauger, M. A. & Sancar, A (2005) Cryptochrome, circadian cycle, cell cycle checkpoints, and cancer. Cancer Res. 65: 6828-34. doi: 10.1158/0008-5472.CAN-05-1119.
4. Stevens RG, Hansen J, Costa G, Haus E, Kauppinen T, Aronson KJ, Castaño-Vinyals G, Davis S, Frings-Dresen MH, Fritschi L, Kogevinas M, Kogi K, Lie JA, Lowden A, Peplonska B, Pesch B, Pukkala E, Schernhammer E, Travis RC, Vermeulen R, Zheng T, Cogliano V, Straif K (2011) Considerations of circadian impact for defining 'shift work' in cancer studies: IARC Working Group Report. Occup. Environ. Med. 68 (2):154-62. doi: 10.1136/oem.2009.053512.
5. Reiter RJ, Rosales-Corral S, Sharma R (2020) Circadian disruption, melatonin rhythm perturbations and their contributions to chaotic physiology. Adv. Med. Sci. 65 (2): 394-402. doi: 10.1016/j.advms.2020.07.001.
6. Feldman, D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ (2014) The role of vitamin D in reducing cancer risk and progression. Nature reviews Cancer 14 (5): 342–357. https://doi.org/10.1038/nrc3691.
7. Kiss Z, Ghosh PM (2016) Women in cancer thematic review: Circadian rhythmicity and the influence of ‘clock’genes on prostate cancer. Endocrine-Related Cancer 23 (11): T123- T34. doi: 10.1530/ERC-16-0366.
8. Touitou Y, Reinberg A, Touitou D (2017) Association between light at night, melatonin secretion, sleep deprivation, and the internal clock: health impacts and mechanisms of circadian disruption. Life Sci. 173: 94–106. doi: 10.1016/j.lfs.2017.02.008.
9. Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z (2017) GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 45 (W1): W98–W102. doi:10.1093/nar/gkx247.
10. Tomczak K, Czerwinska P, Wiznerowicz M (2015) The Cancer Genome Atlas (TCGA): an immeasurable source of knowledge. Contemp. Oncol. (Pozn) 19 (1A): A68-77. doi: 10.5114/wo.2014.47136.
11. Robinson MD, Oshlack A (2010) A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 11 (3): R25. doi: 10.1186/gb-2010-11-3-r25.
12. Kuleshov MV, Jones MR, Rouillard AD, Fernandez NF, Duan Q, Wang Z, Koplev S, Jenkins SL, Jagodnik KM, Lachmann A, McDermott MG, Monteiro CD, Gundersen GW, Ma'ayan A (2016) Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 44 (W1):W90-97. doi: 10.1093/nar/gkw377.
13. Starruss J, de Back W, Brusch L, Deutsch A (2014) Morpheus: a user-friendly modeling environment for multiscale and multicellular systems biology. Bioinformatics 30 (9):1331-1332. doi: 10.1093/bioinformatics/btt772.
14. Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork P, Jensen LJ, Mering CV (2019) STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 47 (D1): D607-D613. doi: 10.1093/nar/gky1131.
15. Truong T, Liquet B, Menegaux F, Plancoulaine S, Laurent-Puig P, Mulot C, Cordina-Duverger E, Sanchez M, Arveux P, Kerbrat P, Richardson S, Guénel P (2014) Breast cancer risk, nightwork, and circadian clock gene polymorphisms. Endocrine-Related Cancer 21 (4): 629-38. doi: 10.1530/ERC-14-0121.
16. You S, Wood PA, Xiong Y, Kobayashi M, Du-Quiton J, Hrushesky WJ (2005) Daily coordination of cancer growth and circadian clock gene expression. Breast Cancer Res. Treat. 91: 47–60. doi: 10.1007/s10549-004-6603-z.
17. Reiter, R J (2000) Melatonin: lowering the high price of free radicals. Physiology 15 (5): 246-250. doi: 10.1152/physiologyonline.2000.15.5.246.
18. Kaneko I, Sabir M S, Dussik C M, Whitfield G K, Karrys A, Hsieh J C, Haussler M R, Meyer M B, Pike J W, Jurutka P W (2015) 1,25-Dihydroxyvitamin D regulates expression of the tryptophan hydroxylase 2 and leptin genes: implication for behavioral influences of vitamin D. FASEB journal: official publication of the Federation of American Societies for Experimental Biology 29 (9): 4023–4035. doi.org/10.1096/fj.14-269811.
19. Jung J, Kang J, Kim T (2019) Changes in sleep and circadian rhythm in an animal model of vitamin D deficiency. Sleep 42 (1): A29. doi: 10.1093/sleep/zsz067.069.
20. Masood T, Kushwaha RS, Singh R, Sailwal S, Pandey H, Varma A, Singh RK, Cornelissen G (2015) Circadian rhythm of serum 25 (OH) vitamin D, calcium and phosphorus levels in the treatment and management of type-2 diabetic patients. Drug Discov. Ther. 9 (1):70-4. doi: 10.5582/ddt.2015.01002.
21. Gutierrez-Monreal MA, Cuevas-Diaz Duran R, Moreno-Cuevas JE, Scott SP (2014) A role for 1α,25-dihydroxyvitamin D3 in the expression of circadian genes. J. Biol. Rhythms 29 (5): 384-8. doi: 10.1177/0748730414549239.
22. Han B, Zhu FX, Shi C, Wu H L, Gu XH (2017) Association between serum vitamin d levels and sleep disturbance in hemodialysis Patients. Nutrients 9 (2): 139. doi: org/10.3390/nu9020139.
23. Jung YS, Chae CH, Kim YO, Son JS, Kim CW, Park HO, Lee JH, Shin YH, Kwak HS (2017) The relationship between serum vitamin D levels and sleep quality in fixed day indoor field workers in the electronics manufacturing industry in Korea. Ann. Occup. Environ. Med. 29: 25. doi: 10.1186/s40557-017-0187-7.
24. Golan D, Staun-Ram E, Glass-Marmor L, Lavi I, Rozenberg O, Dishon S, Barak M, Ish-Shalom S, Miller A (2013) The influence of vitamin D supplementation on melatonin status in patients with multiple sclerosis. Brain Behav Immun. 32: 180-185. doi: 10.1016/j.bbi.2013.04.010.
25. Petrou S, Mamais I, Lavranos G, P Tzanetakou I, Chrysostomou S (2018) Effect of vitamin D supplementation in prostate cancer: A systematic review of randomized control trials. Int J. Vitam. Nutr. Res. 88 (1-2): 100-112. doi: 10.1024/0300-9831/a000494.
26. Fleet JC, Kovalenko PL, Li Y, Smolinski J, Spees C, Yu JG, Thomas-Ahner JM, Cui M, Neme A, Carlberg C, Clinton SK (2019) Vitamin D signaling suppresses early prostate carcinogenesis in TgAPT121 mice. Cancer Prev. Res. 12 (6): 343-356. doi: 10.1158/1940-6207.
27. Krishnan AV, Trump DL, Johnson CS, Feldman D (2010) The role of vitamin D in cancer prevention and treatment. Rheum. Dis. Clin. North Am. 38 (1): 161-78. doi: 10.1016/j.rdc.2012.03.014.
28. Woloszynska-Read A, Johnson CS, Trump DL (2011) Vitamin D and cancer: clinical aspects. Best Pract. Res. Clin. Endocrinol. Metab. 25 (4): 605-615. doi:10.1016/j.beem.2011.06.006.
29. Reiter RJ, Rosales-Corral SA, Tan DX, Acuna-Castroviejo D, Qin L, Yang SF, Xu K (2017) Melatonin, a full service anti-cancer agent: inhibition of initiation, progression and metastasis. Int. J. Mol. Sci. 18 (4): 843. doi: 10.3390/ijms18040843.
30. Antoch MP, Gorbacheva VY, Vykhovanets O, Toshkov IA, Kondratov RV, Kondratova AA, Lee C, Nikitin AY (2008) Disruption of the circadian clock due to the clock mutation has discrete effects on aging and carcinogenesis. Cell Cycle 7 (9): 1197-204. doi: 10.4161/cc.7.9.5886.
31. Stevens RG, Brainard GC, Blask DE, Lockley SW, Motta ME (2014) Breast cancer and circadian disruption from electric lighting in the modern world. CA. Cancer J. Clin. 64 (3): 207-18. doi: 10.3322/caac.21218.
32. De Castro TB, Bordin-Junior NA, de Almeida EA, de Campos Zuccari DAP (2018) Evaluation of melatonin and AFMK levels in women with breast cancer. Endocrine 62 (1): 242-249. doi: 10.1007/s12020-018-1624-2.
33. Sigurdardottir LG, Markt SC, Rider JR, Haneuse S, Fall K, Schernhammer ES, Tamimi RM, Flynn-Evans E, Batista JL, Launer L, Harris T, Aspelund T, Stampfer MJ, Gudnason V, Czeisler CA, Lockley SW, Valdimarsdottir UA, Mucci LA (2015) Urinary melatonin levels, sleep disruption, and risk of prostate cancer in elderly men. Eur. Urol. 67 (2): 191-194. doi: 10.1016/j.eururo.2014.07.008.
34. Erren TC, Slanger TE, Groß JV, Reiter RJ (2015) Melatonin, sleep, and prostate cancer in elderly men: study, hypothesis development, and icelandic options. Eur. Urol. 67 (2): 195-197. doi: 10.1016/j.eururo.2014.09.033.
35. Hevia D, González-Menéndez P, Quiros-González I, Miar A, Rodríguez-García A, Tan DX, Reiter RJ, Mayo JC, Sainz RM (2015) Melatonin uptake through glucose transporters: a new target for melatonin inhibition of cancer. J. Pineal Res. 58: 234–250. doi: 10.1111/jpi.12210.
36. De Almeida Chuffa LG, Seiva FRF, Cucielo MS, Silveira HS, Reiter RJ, Lupi LA (2019) Mitochondrial functions and melatonin: a tour of the reproductive cancers. Cell Mol. Life Sci. 76 (5): 837-863. doi: 10.1007/s00018-018-2963-0.
37. Fang N, Hu C, Sun W, Xu Y, Gu Y, Wu L, Peng Q, Reiter RJ, Liu L (2020) Identification of a novel melatonin‐binding nuclear receptor: Vitamin D receptor. J. Pineal Res. 68 (1): e12618. doi: 10.1111/jpi.12618.
38. Tan DX and Reiter R.J (2019) Mitochondria: the birth place, battle ground and the site of melatonin metabolism in cells. Melatonin Res. 2 (1): 44-66. doi: org/10.32794/mr11250011.
39. Hill SM, Belancio VP, Dauchy RT, Xiang S, Brimer S, Mao L, Hauch A, Lundberg PW, Summers W, Yuan L, Frasch T, Blask DE (2015) Melatonin: an inhibitor of breast cancer. Endocr. Relat. Cancer 22 (3): R183-R204. doi:10.1530/ERC-15-0030.
40. Brzozowski T & Jaworek J (2014) Editorial (Thematic issues: Basic and clinical aspects of melatonin in the gastrointestinal tract. new advancements and future perspectives). Cur. Pharmaceut. Design 20 (30): 4785-4787. doi: 10.2174/1381612819666131119111201.
41. Tan DX, Manchester LC, Esteban-Zubero E, Zhou Z, Reiter RJ (2015) Melatonin as a potent and inducible endogenous antioxidant: synthesis and metabolism. Molecules 20 (10): 18886-18906. doi: 10.3390/molecules201018886.
42. Reiter RJ, Rosales-Corral SA, Manchester LC, Liu X, Tan DX (2014) Melatonin in the biliary tract and liver: health implications. Cur. Pharmaceut. Design 20 (30): 4788-4801. doi: 10.2174/1381612819666131119105826.
43. Hu C, Zhao L, Tao J, Li L (2019) Protective role of melatonin in early-stage and end-stage liver cirrhosis. J. Cell. Mol. Med. 23 (11): 7151-7162. doi:10.1111/jcmm.14634.
44. Chuffa L, Seiva F, Cucielo M, Silveira H, Reiter RJ, Lupi LA (2019) Clock genes and the role of melatonin in cancer cells: an overview. Melatonin Res. 2 (2):133-157. doi: 10.32794/mr11250026.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


