Study assessing the efficacy of herbal teas on bone health and quality of life in a population with osteopenia: rooibos actions on melatonin and tulsi actions on quality of life.
Herbal teas on bone health
Abstract
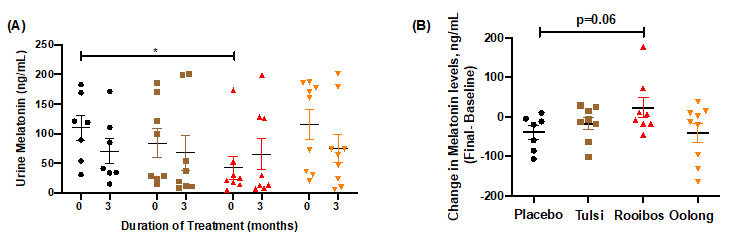
The purpose of the OsTea translational study was to assess the efficacy of teas (tulsi, rooibos, oolong) compared to placebo (coriander) on markers of bone health and quality of life (QOL) in those with osteopenia and on human mesenchymal stem cell (hMSC) differentiation into osteoblasts to identify potential mechanisms of action. Following consumption of tea (3 times/day; 90 days), participants collected a urine sample during the night (10pm-6am) and filled in questionnaires before and after the study. Rooibos consumption demonstrated a significant decrease in urinary CTX levels vs placebo; trended towards increases in nocturnal melatonin levels (p=0.06); significantly decreased serotonin-producing microbes in the gut; and demonstrated trends towards improvements (p=0.09) in QUALIOST emotional parameters. Tulsi consumption primarily affected subjective measures, such as significantly improved scores for PSS, STAI-trait anxiety, and osteoporosis/osteopenia-related parameters in the QUALIOST. To further identify potential mechanisms underlying these actions of rooibos on CTX and melatonin (urinary and gut), rooibos and melatonin effects on human osteoblastogenesis were carried out for 21 days under oxidative stress conditions to mimic osteopenia. Although both rooibos and melatonin protected against oxidative stress-induced loss of osteoblasts in vitro, their underlying mechanisms were different. Melatonin, like tulsi and oolong, demonstrated the greatest protection against oxidative stress at days 10-11 of exposure, which was due to effects on hMSC viability and through melatonin receptors. Rooibos, on the other hand, demonstrated protection at days 10-11 and 20-21, which was through signaling mechanisms involved in differentiation processes and not on cell viability. These findings suggest that the clinical actions of rooibos on decreasing CTX levels in a population with osteopenia may be through a cooperative effort between melatonin and rooibos by protecting hMSC viability against oxidative stress-induced loss and by promoting osteoblast differentiation, respectively. This study also supports the use of tulsi for improving quality of life in a population susceptible to osteoporosis.
References
2. Lassila H, O’Neil CK, Johns JR, Balk JL, Witt-Enderby PA. (2014) Alternative options to manage menopausal symptoms with a focus on melatonin and osteoporosis. Clin. Pharmacol. Biopharm. 3: 115.
3. Shen CL, Chyu MC, Wang JS (2013) Tea and bone health: steps forward in translational nutrition. Am. J. Clin. Nutr. 98, 1694S-1699S.
4. Al-Azzawi F, Palacios S (2009) Hormonal changes during menopause. Maturitas 63: 135-137.
5. Iguichi H, Kato KI, Ibayashi H (1982) Age-dependent reduction in serum melatonin concentrations in healthy human subjects. J. Clin. Endocrinol. Metab. 55: 27-29.
6. Yiallouris A, et al. (2019) Adrenal aging and its implications on stress responsiveness in humans. Front. Endocrinol. (Lausanne) 10: 54.
7. Domazetovic V, Marcucci G, Iantomasi T, Brandi ML, Vincenzini MT (2017) Oxidative stress in bone remodeling: role of antioxidants. Clin. Cases Miner. Bone Metab. 14: 209-216.
8. Wauquier F, Leotoing L, Coxam V, Guicheux J, Wittrant Y (2009) Oxidative stress in bone remodelling and disease. Trends Mol. Med. 15: 468-477.
9. Altindag O, Erel O, Soran N, Celik H, Selek S. (2008) Total oxidative/anti-oxidative status and relation to bone mineral density in osteoporosis. Rheumatol. Int. 28: 317-321.
10. AK Amstrup, Sikjaer T, Mosekilde L, Rejnmark L. (2013) Melatonin and the skeleton. Osteoporos. Int. 24: 2919-2927.
11. Baek KH, et al. (2010) Association of oxidative stress with postmenopausal osteoporosis and the effects of hydrogen peroxide on osteoclast formation in human bone marrow cell cultures. Calcif. Tissue Int. 87: 226-235.
12. Sheweita SA, Khoshhal KI. (2007) Calcium metabolism and oxidative stress in bone fractures: role of antioxidants. Curr. Drug Metab. 8: 519-525.
13. Mody N, Parhami F, Sarafian TA, Demer LL (2001) Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic. Biol. Med. 31: 509-519.
14. Bai XC, et al. (2004) Oxidative stress inhibits osteoblastic differentiation of bone cells by ERK and NF-kappaB. Biochem. Biophys. Res. Comm. 314: 197-207.
15. Lee NK, et al. (2005) A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood 106: 852-859.
16. Maria S, Witt-Enderby PA. (2014) Melatonin effects on bone: potential use for the prevention and treatment for osteopenia, osteoporosis and periodontal diseases and for use in bone-grafting procedures. J. Pineal Res. 56: 115-125.
17. International Osteoporosis Foundation. Available from: https://www.iofbonehealth.org/.
18. Amstrup AK, Sikjaer T, Mosekilde L, Rejnmark L (2015) The effect of melatonin treatment on postural stability, muscle strength, and quality of life and sleep in postmenopausal women: a randomized controlled trial. Nutr. J. 14: 102.
19. Kotlarczyk MP, et al. (2012) Melatonin osteoporosis prevention study (MOPS): a randomized, double-blind, placebo-controlled study examining the effects of melatonin on bone health and quality of life in perimenopausal women. J. Pineal Res. 52: 414-426.
20. Kronenberg F, Fugh-Berman A. (2002) Complementary and alternative medicine for menopausal symptoms: a review of randomized, controlled trials. Ann. Intern. Med. 137: 805-813.
21. Nash LA, Ward WE. (2017) Tea and bone health: Findings from human studies, potential mechanisms, and identification of knowledge gaps. Crit. Rev. Food Sci. Nutr. 57: 1603-1617.
22. Maria S, et al. (2018) Biological effects of melatonin on osteoblast/osteoclast cocultures, bone, and quality of life: Implications of a role for MT2 melatonin receptors, MEK1/2, and MEK5 in melatonin-mediated osteoblastogenesis. J. Pineal Res. 64: e12465.
23. Burge R, et al. (2007) Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J. Bone Miner Res. 22: 465-475.
24. Lin JT, Lane JM (2004) Osteoporosis: a review. Clin. Orthop. Relat. Res. 425:126-134.
25. Nijveldt RJ, et al. (2001) Flavonoids: a review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 74: 418-425.
26. Wang Y, Ho CT (2009) Polyphenolic chemistry of tea and coffee: a century of progress. J. Agric. Food Chem. 57: 8109-8114.
27. Sharma VK, Bhattacharya A, Kumar A, Sharma HK (2007) Health benefits of tea consumption. Trop. J. Pharmaceu. Res. 6: 785.
28. Shen CL, Chyu MC (2016) Tea flavonoids for bone health: from animals to humans. J. Investig. Med. 64: 1151-1157.
29. Welch AA, Hardcastle AC (2014) The effects of flavonoids on bone. Curr. Osteoporos. Rep. 12: 205-210.
30. Ng KW, et al. (2018) Oolong tea: A critical review of processing methods, chemical composition, health effects, and risk. Crit. Rev. Food Sci. Nutr. 58: 2957-2980.
31. Rumpler W, et al. (2001) Oolong tea increases metabolic rate and fat oxidation in men. J. Nutr. 131: 2848-2852.
32. Duan P, et al. (2020) Oolong tea drinking boosts calcaneus bone mineral density in postmenopausal women: a population-based study in southern China. Arch. Osteoporos. 15: 49.
33. McKay DL, Blumberg JB (2007) A review of the bioactivity of South African herbal teas: rooibos (Aspalathus linearis) and honeybush (Cyclopia intermedia). Phytother. Res. 21: 1-16.
34. Joubert E, de Beer D. (2011) Rooibos (Aspalathus linearis) beyond the farm gate: From herbal tea to potential phytopharmaceutical. South African J. Botany 77: 869.
35. McAlpine MD and Ward WE (2016) Influence of steep time on polyphenol content and antioxidant capacity of black, green, rooibos, and herbal teas. Beverages 2: 17.
36. McAlpine MD, Gittings W, MacNeil AJ, Ward WE (2019) Red rooibos tea stimulates osteoblast mineralization in a dose-dependent manner. Beverages 5: 69.
37. Nash LA, Sullivan PJ, Peters SJ, Ward WE (2015) Rooibos flavonoids, orientin and luteolin, stimulate mineralization in human osteoblasts through the Wnt pathway. Mol. Nutr. Food Res. 59: 443-453.
38. Nash LA, Ward WE. (2016) Comparison of black, green and rooibos tea on osteoblast activity. Food Funct. 7: 1166-1175.
39. Sagara T, Kasonga A, Baschant U, Rauner M, Moosa S, Maraisa S, Kruger M, Coetzee M (2020) Aspalathin from Aspalathus linearis (rooibos) reduces osteoclast activity and T increases osteoblast activity in vitro. J. Functional Foods 64: 103616.
40. Moosa S, et al. (2018) Rooibos tea extracts inhibit osteoclast formation and activity through the attenuation of NF-kappaB activity in RAW264.7 murine macrophages. Food Funct. 9: 3301-3312.
41. Sasaki YF, Yamada H, Shimoi K, Kator K, Kinae N. (1993) The clastogen-suppressing effects of green tea, Po-lei tea and Rooibos tea in CHO cells and mice. Mutat. Res. 286, 221-232.
42. Cohen MM (2014) Tulsi - Ocimum sanctum: A herb for all reasons. J. Ayurveda Integr. Med. 5: 251-259.
43. Pattanayak P, Behera P, Das D, Panda SK (2010) Ocimum sanctum Linn. A reservoir plant for therapeutic applications: An overview. Pharmacogn. Rev. 4: 95-105.
44. Acuna-Castroviejo D, et al. (2001) Melatonin, mitochondria, and cellular bioenergetics. J. Pineal Res. 30: 65-74.
45. Kleszczynski K, et al. (2019) Melatonin exerts oncostatic capacity and decreases melanogenesis in human MNT-1 melanoma cells. J. Pineal Res. 67: e12610.
46. Reiter RJ, Sharma R, Rosales-Corral S. (2021) Anti-Warburg effect of melatonin: a proposed mechanism to explain its inhibition of multiple diseases. Int. J. Mol. Sci. 22: 764.
47. Reiter RJ, Tan DX, Fuentes-Broto L. (2010) Melatonin: a multitasking molecule. Prog. Brain Res. 181: 127-151.
48. Slominski A, et al. (2005) On the role of melatonin in skin physiology and pathology. Endocrine 27: 137-148.
49. Slominski AT, et al. (2018) Melatonin: A cutaneous perspective on its production, metabolism, and functions. J. Invest Dermatol. 138: 490-499.
50. Slominski AT, et al. (2020) Characterization of serotonin and N-acetylserotonin systems in the human epidermis and skin cells. J. Pineal Res. 68: e12626.
51. P. S. O. U. G. O. C. U. For technical support (Pitt+Me). Available from: https://pittplusme.org/.
52. Maria S, et al. (2017) Melatonin-micronutrients Osteopenia Treatment Study (MOTS): a translational study assessing melatonin, strontium (citrate), vitamin D3 and vitamin K2 (MK7) on bone density, bone marker turnover and health related quality of life in postmenopausal osteopenic women following a one-year double-blind RCT and on osteoblast-osteoclast co-cultures. Aging (Albany NY) 9: 256-285.
53. Prachayasittikul V, Prachayasittikul S, Ruchirawat S, Prachayasittikul V (2018) Coriander (Coriandrum sativum): A promising functional food toward the well-being. Food Res. Int. 105: 305-323.
54. de la Loge C, et al. (2005) Cross-cultural validation and analysis of responsiveness of the QUALIOST: QUAlity of Life questionnaire In OSTeoporosis. Health Qual. Life Outcomes 3: 69.
55. Marquis P, Cialdella P, De la Loge C (2001) Development and validation of a specific quality of life module in post-menopausal women with osteoporosis: the QUALIOST. Qual. Life Res. 10: 555-566.
56. QUAlity of Life questionnaire In OSTeoporosis (QUALIOST®) Servier (France); Mapi. Available from: https://eprovide.mapi-trust.org/instruments/quality-of-life-questionnaire-in-osteoporosis.
57. Spielberger CD, Gorsuch RL, Lushene RE (1970) Manual for the state-trait anxiety inventory. Consulting Psychologists press, Palo Alto, CA 20.
58. Ho SC, Ruby Yu (2010) Psychometric evaluation of the perceived stress scale in early postmenopausal chinese women. Psychology 1: 8.
59. Cohen S. (1988) Perceived stress in a probability sample of the United States. The Social Psychology of Health (Eds: S. Spacapan and S. Oskamp)Newbury Park, CA, 1988.
60. Cohen S, Kamarck T, Mermelstein R (1983) A global measure of perceived stress. J. Health Soc. Behav. 24: 12.
61. Radloff LS (1977) The CES-D Scale: A self-report depression scale for research in the general population. Appl. Psychol. Meas. 1: 385-401.
62. Wilkins LW (2013) American College of Sports Medicine. ACSM's guidelines for exercise testing and prescription.
63. Sethi S, et al. (2010) Determination of the minimal melatonin exposure required to induce osteoblast differentiation from human mesenchymal stem cells and these effects on downstream signaling pathways. J. Pineal Res. 49: 222-238.
64. Claus DR, Osmand AP, Gewurz H (1976) Radioimmunoassay of human C-reactive protein and levels in normal sera. J. Lab Clin. Med. 87: 120-128.
65. Oliphant K, Allen-Vercoe E (2019) Macronutrient metabolism by the human gut microbiome: major fermentation by-products and their impact on host health. Microbiome 7: 91.
66. Heeney DD, Gareau MG, Marco ML (2018) Intestinal Lactobacillus in health and disease, a driver or just along for the ride? Curr. Opin. Biotechnol. 49: 140-147.
67. Baba H, et al. (2009) Studies of anti-inflammatory effects of Rooibos tea in rats. Pediatr. Int. 51: 700-704.
68. Espino J, Pariente JA, Rodriguez AB (2012) Oxidative stress and immunosenescence: therapeutic effects of melatonin. Oxid. Med. Cell Longev. 2012: 670294.
69. Tordjman S, et al. (2017) Melatonin: Pharmacology, functions and therapeutic benefits. Curr. Neuropharmacol. 15: 434-443.
70. Bubenik GA. (2002) Gastrointestinal melatonin: localization, function, and clinical relevance. Dig. Dis. Sci. 47: 2336-2348.
71. Krafczyk N, Glomb MA. (2008) Characterization of phenolic compounds in rooibos tea. J. Agric. Food Chem. 56: 3368-3376.
72. Allaband C, et al. (2019) Microbiome 101: studying, analyzing, and interpreting gut microbiome data for clinicians. Clin. Gastroenterol. Hepatol. 17: 218-230.
73. Bond T, Derbyshire E (2019) Tea Compounds and the gut microbiome: findings from trials and mechanistic studies. Nutrients 11: 2364.
74. Ulicna O, et al. (2006) Rooibos tea (Aspalathus linearis) partially prevents oxidative stress in streptozotocin-induced diabetic rats. Physiol. Res. 55: 157-164.
75. Sola VM, Aguilar JJ, Vazquez Mosquera AP, Carpentieri AR (2020) Melatonin is an effective protector of gingival cells damaged by the cytotoxic effect of glutamate and DL-buthionine sulfoximine. J. Periodontal Res. 56 (1):154-161 10.1111/jre.12806.
76. Li L, et al. (2019) Microbial osteoporosis: The interplay between the gut microbiota and bones via host metabolism and immunity. Microbiologyopen 8: e00810.
77. Muhammad SI, Maznah I, Mahmud R, Zuki AB, Imam MU. (2013) Upregulation of genes related to bone formation by gamma-amino butyric acid and gamma-oryzanol in germinated brown rice is via the activation of GABAB-receptors and reduction of serum IL-6 in rats. Clin. Interv. Aging 8: 1259-1271.
78. Gundberg CM, Lian JB, Booth SL. (2012) Vitamin K-dependent carboxylation of osteocalcin: friend or foe? Adv. Nutr. 3: 149-157.
79. Iwamoto J, Takeda T, Sato Y. (2006) Menatetrenone (vitamin K2) and bone quality in the treatment of postmenopausal osteoporosis. Nutr. Rev. 64: 509-517.
80. Blazevic S, Erjavec I, Brizic M, Vukicevic S, Hranilovic D. (2015) Molecular background and physiological consequences of altered peripheral serotonin homeostasis in adult rats perinatally treated with tranylcypromine. J. Physiol. Pharmacol. 66: 529-537.
81. Erjavec I, et al. (2016) Constitutively elevated blood serotonin is associated with bone loss and type 2 diabetes in rats. PLoS One 11: e0150102.
82. Zhu QY, Hackman RM, Ensunsa JL, Holt RR, Keen CL (2002) Antioxidative activities of oolong tea. J. Agric. Food Chem. 50: 6929-6934.
83. Chatterjee M, Verma P, Maurya R, Palit G (2011) Evaluation of ethanol leaf extract of Ocimum sanctum in experimental models of anxiety and depression. Pharm. Biol. 49: 477-483.
84. Tabassum I, Siddiqui ZN, Rizvi SJ. (2010) Effects of Ocimum sanctum and Camellia sinensis on stress-induced anxiety and depression in male albino Rattus norvegicus. Indian J. Pharmacol. 42: 283-288.
85. Bhattacharyya D, Sur TK, Jana U, Debnath PK (2008) Controlled programmed trial of Ocimum sanctum leaf on generalized anxiety disorders. Nepal Med. Coll. J. 10: 176-179.
86. Saxena RC, et al. (2012) Efficacy of an extract of Ocimum tenuiflorum (OciBest) in the management of general stress: a double-blind, placebo-controlled study. Evid. Based Complement. Alternat. Med. 2012: 894509.
87. Maher P, Hanneken A. (2005) The molecular basis of oxidative stress-induced cell death in an immortalized retinal ganglion cell line. Invest. Ophthalmol. Vis. Sci. 46: 749-757.
88. Nayak V, Devi PU (2005) Protection of mouse bone marrow against radiation-induced chromosome damage and stem cell death by the ocimum flavonoids orientin and vicenin. Radiat. Res. 163: 165-171.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


