Melatonin: an ancient note in a contemporary wrap
Functional evolution of melatonin
Abstract
At the beginning of life, natural selection is and still the principal driving force for the evolution of all organisms to adapt in the particular environments of the earth. As a result, ultimately neither the strongest, nor the supreme intelligent but the most adaptable species win the race. Not only the organisms, but also the elements which are necessary for survival of them also undergo extreme evolution. These include DNA, proteins and other biochemical molecules
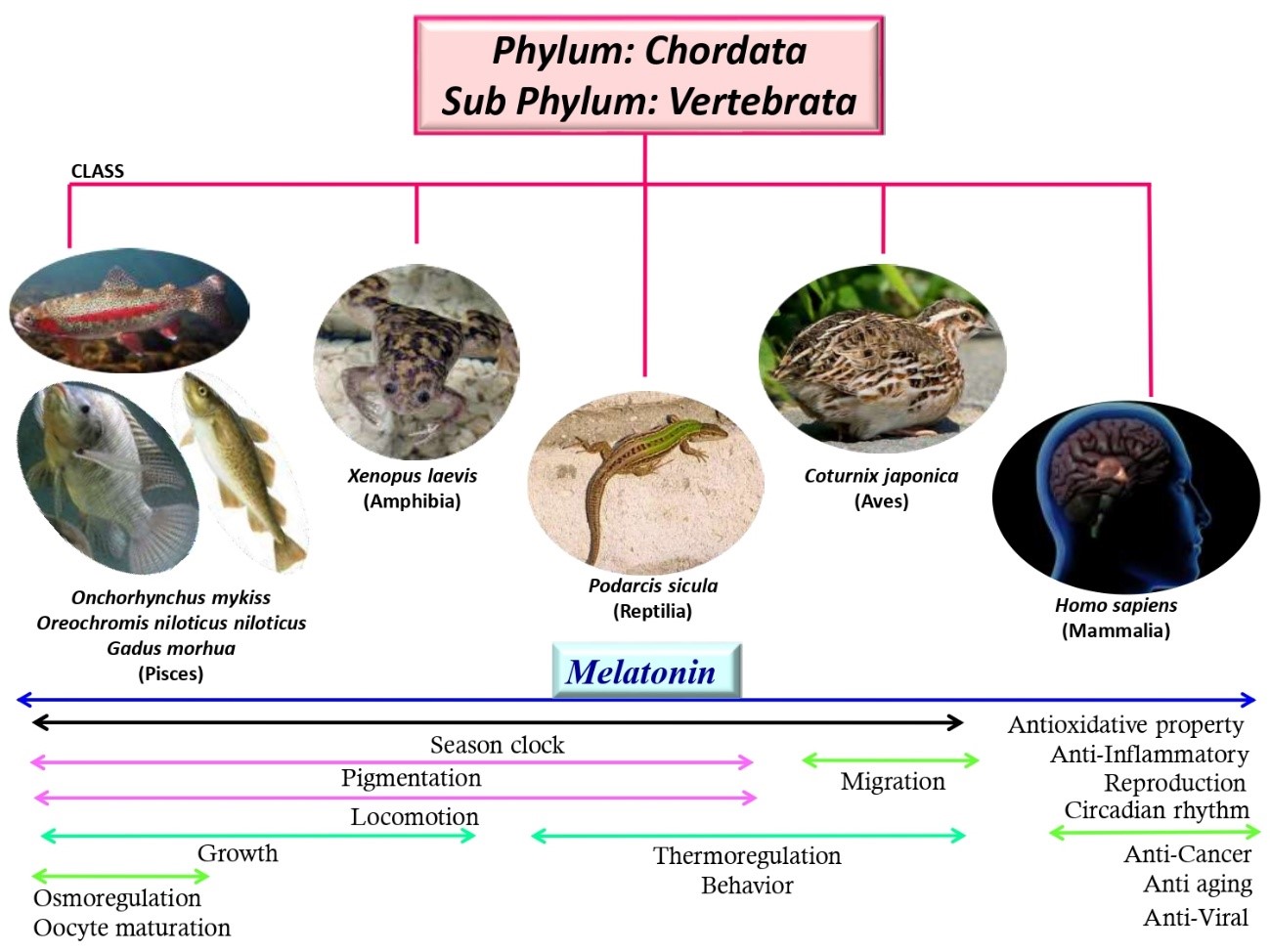
At the beginning of life, natural selection is and still the principal driving force for the evolution of all organisms to adapt in the particular environments of the earth. As a result, ultimately neither the strongest, nor the supreme intelligent but the most adaptable species win the race. Not only the organisms, but also the elements which are necessary for survival of them also undergo extreme evolution. These include DNA, proteins and other biochemical molecules. However, melatonin, an indoleamine, presents in the early life form remains unchanged in its structure from unicellular organisms to mammals. When it was discovered, it was considered to be a neuronal hormone produced exclusively in the pineal gland of vertebrates. The latter discovery of its presence in primitive bacteria drives the melatonin research in different directions. Its primary function is serving as an antioxidant in all organisms. Its chemical structure is perfect to scavenge free radicals and thus, this molecule is preserved from bacteria to mammals. However, this molecule acquired many additional functions during evolution. These include circadian regulation, immuno-enhancement, oncostatic, anti-inflammatory and anti-aging activities. In the review, we are trying to present hypothetical and most plausible chronological events in the functional evolvements of melatonin during the process of evolution.
References
2. Kump LR, Barley ME (2007) Increased subaerial volcanism and the rise of atmospheric oxygen 2.5 billion years ago. Nature 448 (7157): 1033-1036. DOI: 10.1038/nature06058.
3. Kasting JF (1993) Earth's early atmosphere. Science 259 (5097): 920-926. DOI: 10.1126/science.11536547
4. Shaw GH (2008) Earth's atmosphere–Hadean to early Proterozoic. Geochemistry 68 (3): 235-264. DOI: 10.1016/j.chemer.2008.05.001.
5. Ślesak I, Ślesak H, Kruk J (2012) Oxygen and hydrogen peroxide in the early evolution of life on earth: in silico comparative analysis of biochemical pathways. Astrobiology 12 (8): 775-784. DOI: 10.1089/ast.2011.0704.
6. Selsis F, Despois D, Parisot JP (2002) Signature of life on exoplanets: Can Darwin produce false positive detections?. Astron. Astrophysi. 388 (3): 985-1003. DOI: 10.1051/0004-6361:20020527.
7. Borda MJ, Elsetinow AR, Schoonen MA, Strongin DR (2001) Pyrite-induced hydrogen peroxide formation as a driving force in the evolution of photosynthetic organisms on an early Earth. Astrobiology 1 (3): 283-288. DOI: 10.1089/15311070152757474.
8. Schirrmeister BE, Gugger M, Donoghue PC (2015) Cyanobacteria and the Great Oxidation Event: evidence from genes and fossils. Palaeontology 58 (5): 769-785. DOI: 10.1111/pala.12178.
9. Embley TM, Williams TA (2015) Steps on the road to eukaryotes. Nature 521 (7551): 169-170. DOI: 10.1038/nature14522.
10. Embley TM, Martin W (2006) Eukaryotic evolution, changes and challenges. Nature 440 (7084): 623-630. DOI: 10.1038/nature04546.
11. Holland HD (2006) The oxygenation of the atmosphere and oceans. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 361 (1470): 903-915. DOI: 10.1098/rstb.2006.1838.
12. Van Kranendonk MJ, Altermann W, Beard BL, Hoffman PF, Johnson CM, Kasting JF, Melezhik VA, Nutman AP, Papineau D, Pirajno, F (2012) Chapter 16. A chronostratigraphic division of the Precambrian: possibilities and challenges. In: F. M. Gradstein, J. G. Ogg, M. D. Schmitz and G. M. Ogg (eds). The Geologic Time Scale 2012. pp. 299– 392. Elsevier, Boston, MA. DOI: 10.1016/B978-0-444-59425-9.00016-0.
13. Fridovich I (2013) Oxygen: how do we stand it?. Med. Princ. Pract. 22 (2): 131-137. DOI: 10.1159/000339212.
14. Margulis L (1975) Symbiotic theory of the origin of eukaryotic organelles; criteria for proof. In: Symposia of the Society for Experimental Biology 29: 21-38. PMID: 822529.
15. Rockwell NC, Lagarias JC, Bhattacharya D (2014) Primary endosymbiosis and the evolution of light and oxygen sensing in photosynthetic eukaryotes. Front. Ecol. Evol. 2: 66. DOI: 10.3389/fevo.2014.00066.
16. Wernegreen JJ (2012) Endosymbiosis. Curr. Biol. 22 (14): R555-R561. DOI: 10.1016/j.cub.2012.06.010.
17. Gray MW, Burger G, Lang BF (1999) Mitochondrial evolution. Science 283 (5407): 1476-1481. DOI: 10.1126/science.283.5407.1476.
18. Koonin EV (2010) The origin and early evolution of eukaryotes in the light of phylogenomics. Genome Biol. 11 (5): 1-2. DOI: 10.1186/gb-2010-11-5-209.
19. Tan DX, Manchester LC, Liu X, Rosales‐Corral SA, Acuna‐Castroviejo D, Reiter RJ (2013) Mitochondria and chloroplasts as the original sites of melatonin synthesis: a hypothesis related to melatonin's primary function and evolution in eukaryotes. J. Pineal Res. 54 (2): 127-138. DOI: 10.1111/jpi.12026.
20. Tan DX et al. (1993) Melatonin: a potent, endogenous hydroxyl radical scavenger. Endocr. J. 1:57-60.
21. Galano A, Tan DX, Reiter RJ (2011) Melatonin as a natural ally against oxidative stress: a physicochemical examination. J. Pineal Res. 51 (1): 1-6.DOI: 10.1111/j.1600-079X.2011.00916.x.
22. Fischer TW,Kleszczyński K, Hardkop LH, Kruse N, Zillikens D (2013) Melatonin enhances antioxidative enzyme gene expression (CAT, GPx, SOD), prevents their UVR-induced depletion, and protects against the formation of DNA damage (8-hydroxy-2' deoxyguanosine) in ex vivo human skin. J. Pineal Res. 54: 303–312. DOI: 10.1111/jpi.12018.
23. Lerner AB, Case JD, Takahashi Y, Lee TH, Mori W (1958) Isolation of melatonin, the pineal gland factor that lightens melanocyte. J. Am. Chem. Soc. 80 (10): 2587-2587. DOI: 10.1021/ja01543a060.
24. Manchester LC, Poeggeler B, Alvares FL, Ogden GB, Reiter RJ (1995) Melatonin immunoreactivity in the photosynthetic prokaryote Rhodospirillum rubrum: implications for an ancient antioxidant system. Cell Mol. Biol. Res. 41 (5): 391-395. PMID: 8867786.
25. Lurie-Weinberger MN, Peeri M, Tuller T, Gophna U (2012) Extensive inter-domain lateral gene transfer in the evolution of the human commensal Methanosphaerastadtmanae. Front. Genet. 3: 182. DOI: 10.3389/fgene.2012.00182.
26. Tan DX, Zheng X, Kong J, Manchester LC, Hardeland R, Kim SJ, Xu X, Reiter RJ (2014) Fundamental issues related to the origin of melatonin and melatonin isomers during evolution: relation to their biological functions. Int. J. Mol. Sci.15 (9): 15858-15890. DOI: 10.3390/ijms150915858. DOI: 10.3390/ijms150915858.
27. Spang A, Martijn J, Saw JH, Lind AE, Guy L, Ettema TJ (2013) Close encounters of the third domain: the emerging genomic view of archaeal diversity and evolution. Archaea 2013: 202358. DOI: 10.1155/2013/202358.
28. Williams TA, Foster PG, Cox CJ, Embley TM (2013) An archaeal origin of eukaryotes supports only two primary domains of life. Nature 504 (7479): 231-236. DOI: 10.1038/nature12779.
29. Tan DX, Hardeland R, Back K, Manchester LC, Alatorre‐Jimenez MA, Reiter RJ (2016) On the significance of an alternate pathway of melatonin synthesis via 5‐methoxytryptamine: comparisons across species. J. Pineal Res. 61 (1): 27-40. DOI: 10.1111/jpi.12336.
30. Klein DC (2007) Arylalkylamine N-acetyltransferase: “the Timezyme” J. Biol. Chem. 282 (7): 4233-4237. DOI: 10.1074/jbc.R600036200.
31. Poeggeler B, Balzer I, Hardeland R, Lerchl A (1991) Pineal hormone melatonin oscillates also in the dinoflagellate Gonyaulaxpolyedra. Naturwissenschaften 78 (6): 268-269. DOI: 10.1007/BF01134354.
32. Balzer, I., Poeggeler,Hardeland, R. (1993) Circadian rhythms of indoleamines in a dinoflagellate, Gonyaulaxpolyedra: Persistence of melatonin rhythm in constant darkness and relationship to 5- methoxytryptamine. In: Y. Touitou, j. Arendt and P. Pevet, ed., Melatonin and the Pineal Gland. From Basic Science to Clinical Application. 183-186. Elsevier, Amsterdam.
33. Kolář J, Macháčková I (2005) Melatonin in higher plants: occurrence and possible functions. J. Pineal Res. 39 (4): 333-341. DOI: 10.1111/j.1600-079X.2005.00276.x.
34. Kolár J, Johnson CH, Machácková I (1999) Presence and possible role of melatonin in a short-day flowering plant, Chenopodium rubrum. Adv. Exp. Med. Biol. 460: 391-393. DOI: 10.1007/0-306-46814-x_46.
35. Hardeland R, Pandi-Perumal SR, Poeggeler B (2007) Melatonin in plants–Focus on a vertebrate night hormone with cytoprotective properties. Funct. Plant Sci. Biotechnol. 1 (1): 32-45.
36. Pandi-Perumal SR, Zisapel N, Srinivasan V, Cardinali DP (2005) Melatonin and sleep in aging population. Exp. Gerontol. 40 (12): 911-925. DOI: 10.1016/j.exger.2005.08.009.
37. Luboshizsky R, Lavie P (1998) Sleep-inducing effects of exogenous melatonin administration. Sleep Med. Rev. 2 (3): 191-202. DOI: 10.1016/s1087-0792(98)90021-1.
38. Hardeland R, Cardinali DP, Srinivasan V, Spence DW, Brown GM, Pandi-Perumal SR (2011) Melatonin—A pleiotropic, orchestrating regulator molecule. Prog. Neurobiol. 93 (3): 350-384. DOI: 10.1016/j.pneurobio.2010.12.004.
39. Reiter RJ, Tan DX, Sharma R (2018) Historical perspective and evaluation of the mechanisms by which melatonin mediates seasonal reproduction in mammals. Melatonin Res.1 (1): 59-77. DOI: 10.32794/mr11250004.
40. Kriegsfeld LJ, Ubuka T, Bentley GE, Tsutsui K (2015) Seasonal control of gonadotropin-inhibitory hormone (GnIH) in birds and mammals. Front. Neuroendocrinol. 37: 65-75. DOI: 10.1016/j.yfrne.2014.12.001.
41. Gillette MU, McArthur AJ (1995) Circadian actions of melatonin at the suprachiasmatic nucleus. Behav. Brain Res.73 (1-2): 135-139. DOI: 10.1016/0166-4328(96)00085-x.
42. Zhao D, Yu Y, Shen Y, Liu Q, Zhao Z, Sharma R, Reiter RJ (2019) Melatonin synthesis and function: evolutionary history in animals and plants. Front. Endocrinol. 10: 249. DOI: 10.3389/fendo.2019.00249.
43. Najafi M, Shirazi A, Motevaseli E, Rezaeyan AH, Salajegheh A, Rezapoor S (2017) Melatonin as an anti-inflammatory agent in radiotherapy. Inflammopharmacology 25 (4): 403-413. DOI: 10.1007/s10787-017-0332-5.
44. Bondy SC, Campbell A (2018) Mechanisms underlying tumor suppressive properties of melatonin. Int. J. Mol. Sci. 19 (8): 2205. DOI: 10.3390/ijms19082205.
45. Luo G, Ono S, Beukes NJ, Wang DT, Xie S, Summons RE (2016) Rapid oxygenation of Earth’s atmosphere 2.33 billion years ago. Sci. Adv. 2 (5): e1600134. DOI: 10.1126/sciadv.1600134.
46. Lyons TW, Reinhard CT, Planavsky NJ (2014) The rise of oxygen in Earth’s early ocean and atmosphere. Nature 506 (7488): 307-315. DOI: 10.1038/nature13068.
47. Papa S, Skulachev VP (1997) Reactive oxygen species, mitochondria, apoptosis and aging. In: Detection of mitochondrial diseases. 305-319. Springer, Boston, MA. PMID: 9309704.
48. Halliwell B (1996) Free radicals, proteins and DNA: oxidative damage versus redox regulation. Biochem. Soc. Trans 24 (4):1023-1027. DOI: 10.1042/bst0241023.
49. Reiter RJ (1996) Functional aspects of the pineal hormone melatonin in combating cell and tissue damage induced by free radicals. Eur. J. Endocrinol. 134 (4): 412-420. DOI: 10.1530/eje.0.1340412.
50. Hardeland R, Fuhrberg B, Uria H, Behrmann G, Meyer TJ, Burkhardt S, Poeggeler B (1996) Chronobiology of indoleamines in the dinoflagellate Gonyaulaxpolyedra: metabolism and effects related to circadian rhythmicity and photoperiodism. Braz. J. Med. Biol. Res. 29 (1): 119-123. PMID: 8731341.
51. Poeggeler B, Balzer I, Fischer J, Behrmann G, Hardeland R (1989) A role of melatonin in dinoflagellates?. Eur. J Endocrinol. 120 (3 Suppl): S97. DOI: 10.1530/acta.0.120S097.
52. Antolín I, Obst B, Burkhardt S, Hardeland R (1997) Antioxidative protection in a high‐melatonin organism: the dinoflagellate Gonyaulaxpolyedra is rescued from lethal oxidative stress by strongly elevated, but physiologically possible concentrations of melatonin. J. Pineal Res. 23 (4): 182-190. DOI: 10.1111/j.1600-079x.1997.tb00353.x.
53. Fuhrberg B, Hardeland R, Poeggeler B, Behrmann C (1997) Dramatic rises of melatonin and 5-methoxytryptamine in Gonyaulax exposed to decreased temperature. Biol. Rhythm Res. 28 (1): 144-150. DOI: 10.1076/brhm.28.1.144.12978.
54. Hardeland R (1996) Ubiquitous melatonin-presence and effects in unicells, plants and animals. Trends Comp. Biochem. Physiol. 2: 25-45.
55. Macias M, Rodrigueez‐Cabezas MN, Reiter RJ, Osuna A, Acuña‐Castrovejo D (1999) Presence and effects of melatonin in Trypanosomacruzi. J. Pineal Res. 27 (2): 86-94. DOI: 10.1111/j.1600-079x.1999.tb00601.x.
56. Balzer I, Höcker B, Kapp H, Bartolomaeus B (2000) Occurrence and comparative physiology of melatonin in evolutionary diverse organisms. In: Driessche TV, Guisset JL, Petiau-de Vries GM ed., The Redox State and Circadian Rhythms. 95-119. Springer, Dordrecht. DOI: 10.1007/978-94-015-9556-8_6.
57. Dubbels R, Reiter RJ, Klenke E, Goebel A, Schnakenberg E, Ehlers C, Schiwara HW, Schloot W (1995) Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography‐mass spectrometry. J. Pineal Res. 18 (1): 28-31. DOI: 10.1111/j.1600-079x.1995.tb00136.x.
58. Hattori A, Migitaka H, Iigo M, Itoh M, Yamamoto K, Ohtani-Kaneko R, Hara M, Suzuki T, Reiter RJ (1995) Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem. Mol. Biol. Int. 35 (3): 627. PMID: 7773197.
59. Reiter RJ, Tan DX, Manchester LC, Simopoulos AP, Maldonado MD, Flores LJ, Terron MP (2007) Melatonin in edible plants (phytomelatonin): identification, concentrations, bioavailability and proposed functions. World Rev. Nutr. Diet. 97: 211-230. DOI: 10.1159/000097917.
60. Paredes SD, Korkmaz A, Manchester LC, Tan DX, Reiter RJ (2009) Phytomelatonin: a review. J. Exp. Bot. 60 (1): 57-69. DOI: 10.1093/jxb/ern284.
61. Reed CJ, Lewis H, Trejo E, Winston V, Evilia C (2013) Protein adaptations in archaeal extremophiles. Archaea 2013: 373275. DOI: 10.1155/2013/373275.
62. Hardeland R (2019) Melatonin in the evolution of plants and other phototrophs. Meletonin Res. 2 (3): 10-36. DOI: 10.32794/mr11250029.
63. Manchester LC, Tan DX, Reiter RJ, Park W, Monis K, Qi W (2000) High levels of melatonin in the seeds of edible plants: possible function in germ tissue protection. Life Sci. 67 (25): 3023-3029. DOI: 10.1016/s0024-3205(00)00896-1.
64. Conti A, Tettamanti C, Singaravel M, Haldar C, Pandi-Perumal RS, Maestroni GJ (2002) Melatonin: An ubiquitous and evolutionary hormone. In: Haldar C, Singaravel M, Maitra SK ed., Treatise on pineal gland and melatonin. 105-143. Enfield, NH: Science Publishers.
65. Van Tassel DL, Roberts N, Lewy A, O'Neill SD (2001) Melatonin in plant organs. J. Pineal Res. 31 (1): 8-15. DOI: 10.1034/j.1600-079x.2001.310102.x.
66. Tan DX, Manchester LC, Helton P, Reiter RJ (2007) Phytoremediative capacity of plants enriched with melatonin. Plant Signal Behav. 2 (6): 514-516. DOI: 10.4161/psb.2.6.4639.
67. Reiter RJ, Rosales-Corral S, Tan DX, Jou MJ, Galano A, Xu B (2017) Melatonin as a mitochondria-targeted antioxidant: one of evolution’s best ideas. Cell Mol. Life Sci. 74 (21): 3863-3881. DOI: 10.1007/s00018-017-2609-7.
68. Rodriguez C, Mayo JC, Sainz RM, Antolín I, Herrera F, Martín V, Reiter RJ (2004) Regulation of antioxidant enzymes: a significant role for melatonin. J. Pineal Res. 36 (1): 1-9. DOI: 10.1046/j.1600-079x.2003.00092.x.
69. Ghosh AK, Naaz S, Bhattacharjee B, Ghosal N, Chattopadhyay A, Roy S, Reiter RJ, Bandyopadhyay D (2017) Mechanism of melatonin protection against copper-ascorbate-induced oxidative damage in vitro through isothermal titration calorimetry. Life Sci. 180: 123-136. DOI: 10.1016/j.lfs.2017.05.022.
70. Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre‐Jimenez M, Qin L (2016) Melatonin as an antioxidant: under promises but over delivers. J. Pineal Res. 61 (3): 253-278. DOI: 10.1111/jpi.12360.
71. Hardeland R (2015) Melatonin in plants and other phototrophs: advances and gaps concerning the diversity of functions. J. Exp. Bot. 66 (3): 627-646. DOI: 10.1093/jxb/eru386.
72. Bochkov DV, Sysolyatin SV, Kalashnikov AI, Surmacheva IA (2012) Shikimic acid: review of its analytical, isolation, and purification techniques from plant and microbial sources. J. Chem. Biol. 5 (1): 5-17. DOI: 10.1007/s12154-011-0064-8.
73. Back K, Tan DX, Reiter RJ (2016) Melatonin biosynthesis in plants: multiple pathways catalyze tryptophan to melatonin in the cytoplasm or chloroplasts. J. Pineal Res. 61 (4): 426-437. DOI: 10.1111/jpi.12364.
74. De Luca V, Marineau C, Brisson N (1989) Molecular cloning and analysis of cDNA encoding a plant tryptophan decarboxylase: comparison with animal dopa decarboxylases. Proc. Nat. Acad. Sci. 86 (8): 2582-2586. DOI: 10.1073/pnas.86.8.2582.
75. Park M, Kang K, Park S, Back K (2008) Conversion of 5-hydroxytryptophan into serotonin by tryptophan decarboxylase in plants, Escherichia coli, and yeast. Biosci. Biotechnol. Biochem. 72 (9): 2456-2458. DOI: 10.1271/bbb.80220.
76. Axelrod J, Weissbach H (1960) Enzymatic O-methylation of N-acetylserotonin to melatonin. Science 131 (3409): 1312. DOI: 10.1126/science.131.3409.1312.
77. Kang K, Lee K, Park S, Byeon Y, Back K (2013) Molecular cloning of rice serotonin N‐acetyltransferase, the penultimate gene in plant melatonin biosynthesis. J. Pineal Res. 55 (1): 7-13. DOI: 10.1111/jpi.12011.
78. Weissbach H (1960) Biosynthesis of melatonin: enzymic conversion of serotonin to N-acetylserotonin. Biochim. Biophys. Acta 43: 352-353. DOI: 10.1016/0006-3002(60)90453-4.
79. Byeon Y, Lee HJ, Lee HY, Back K (2016) Cloning and functional characterization of the Arabidopsis N‐acetylserotonin O‐methyltransferase responsible for melatonin synthesis. J. Pineal Res. 60 (1): 65-73. DOI: 10.1111/jpi.12289.
80. Isorna E, El M’Rabet A, Confente F, Falcón J, Muñoz-Cueto JA (2009) Cloning and expression of arylalkylamine N-acetyltranferase-2 during early development and metamorphosis in the soleSoleasenegalensis. Gen. Comp. Endocrinol. 161 (1): 97-102. DOI: 10.1016/j.ygcen.2008.10.007.
81. Isorna E, Aliaga‐Guerrero M, M’Rabet AE, Servili A, Falcón J, Muñoz‐Cueto JA (2011) Identification of two arylalkylamine N‐acetyltranferase 1 genes with different developmental expression profiles in the flatfish Soleasenegalensis. J. Pineal Res. 51 (4): 434-444. DOI: 10.1111/j.1600-079X.2011.00907.x.
82. Byeon Y, Lee HY, Back K (2016) Cloning and characterization of the serotonin N‐acetyltransferase‐2 gene (SNAT2) in rice (Oryza sativa). J. Pineal Res. 61 (2): 198-207. DOI: 10.1111/jpi.12339.
83. Tan DX, Manchester LC, Esteban-Zubero E, Zhou Z, Reiter RJ (2015) Melatonin as a potent and inducible endogenous antioxidant: synthesis and metabolism. Molecules 20 (10): 18886-18906. DOI: 10.3390/molecules201018886.
84. Amherd R, HintermannE, Walz D, Affolter M, Meyer UA (2000) Purification, cloning, and characterization of a second arylalkylamine N-acetyltransferase from Drosophila melanogaster. DNA Cell Biol. 19 (11): 697-705. DOI: 10.1089/10445490050199081.
85. Coon SL, Klein DC (2006) Evolution of arylalkylamine N-acetyltransferase: emergence and divergence. Mol. Cell Endocrinol. 252 (1-2): 2-10. DOI: 10.1016/j.mce.2006.03.039.
86. Ganguly S, Mummaneni P, Steinbach PJ, Klein DC, Coon SL (2001) Characterization of the Saccharomyces cerevisiae homolog of the melatonin rhythm enzyme arylalkylamine N-acetyltransferase (EC 2.3. 1.87). J. Biol. Chem. 276 (50): 47239-47247. DOI: 10.1074/jbc.M107222200.
87. Iyer LM, AravindL, Coon SL, Klein DC, Koonin EV (2004) Evolution of cell–cell signaling in animals: did late horizontal gene transfer from bacteria have a role?. Trends Genet. 20 (7): 292-299. DOI: 10.1016/j.tig.2004.05.007.
88. Pavlicek J, Sauzet S, Besseau L, Coon SL, Weller JL, Boeuf G, Gaildrat P, Omelchenko MV, Koonin EV, Falcón J, Klein DC (2010) Evolution of AANAT: expansion of the gene family in the cephalochordate amphioxus. BMC Evol. Biol. 10 (1): 154. DOI: 10.1186/1471-2148-10-154.
89. Han Q, Robinson H, Ding H, Christensen BM, Li J (2012) Evolution of insect arylalkylamine N-acetyltransferases: structural evidence from the yellow fever mosquito, Aedes aegypti. Proc. Natl. Acad. Sci. 109 (29): 11669-11674. DOI: 10.1073/pnas.1206828109.
90. Tan DX, Manchester LC, Reiter RJ, Qi WB, Karbownik M, Calvo JR (2000) Significance of melatonin in antioxidative defense system: reactions and products. Biol. Signals Recept. 9 (3-4): 137-159. DOI: 10.1159/000014635.
91. Byeon Y, Lee HY, Lee K, Park S, Back K (2014) Cellular localization and kinetics of the rice melatonin biosynthetic enzymes SNAT and ASMT. J. Pineal Res. 56 (1): 107-114. DOI: 10.1111/jpi.12103.
92. Wang L, Feng C, Zheng X, Guo Y, Zhou F, Shan D, Liu X, Kong J (2017) Plant mitochondria synthesize melatonin and enhance the tolerance of plants to drought stress. J. Pineal Res. 63 (3): e12429. DOI: 10.1111/jpi.12429.
93. Yu Y, Bian L, Jiao Z, Yu K, Wan Y, Zhang G, Guo D (2019) Molecular cloning and characterization of a grapevine (Vitis vinifera L.) serotonin N-acetyltransferase (VvSNAT2) gene involved in plant defense. BMC genomics 20 (1): 1-3. DOI: 10.1186/s12864-019-6085-3.
94. Reiter RJ, Tan DX, Zhou Z, Cruz MH, Fuentes-Broto L, Galano A (2015) Phytomelatonin: assisting plants to survive and thrive. Molecules 20 (4): 7396-437. DOI: 10.3390/molecules20047396.
95. Gaudet SJ, Hayden BJ, Chader GJ, Namboodiri MA (1993) Differential regulation of arylamine and arylalkylamine N-acetyltransferases in human retinoblastoma (Y-79) cells. Neurochem. Int. 22 (3): 271-275. DOI: 10.1016/0197-0186(93)90055-a.
96. Gaudet SJ, Tsilou E, Chader GJ (1993) Identification and characterization of arylamine N-acetyltransferase activity from the bovine retinal pigment epithelium. Curr. Eye Res. 12 (3): 271-278. DOI: 10.3109/02713689308999473.
97. Slominski A, Fischer TW, Zmijewski MA, Wortsman J, Semak I, Zbytek B, Slominski RM, Tobin DJ (2005) On the role of melatonin in skin physiology and pathology. Endocrine 27 (2): 137-147. DOI: 10.1385/ENDO:27:2:137.
98. Slominski A, Pisarchik A, Semak I, Sweatman T, Wortsman J (2003) Characterization of the serotoninergic system in the C57BL/6 mouse skin. Eur. J. Biochem. 270 (16): 3335-3344. DOI: 10.1046/j.1432-1033.2003.03708.x.
99. Gaudet SJ, Slominski A, Etminan M, Pruski D, Paus R, Namboodiri MA (1993) Identification and characterization of two isozymic forms of arylamine N-acetyltransferase in Syrian hamster skin. J. Invest. Dermatol. 101 (5): 660-665. DOI: 10.1111/1523-1747.ep12371672.
100. Biarrotte-Sorin S, Mayer C (2005) Cloning, purification, crystallization and preliminary crystallographic analysis of a hypothetical acetyltransferase from Pyrococcusfuriosus ActaCrystallogr. Sect. F. Struct. Biol. Cryst. Commun. 61 (3): 269-270. DOI: 10.1107/S174430910500223X.
101. Ma C, Pathak C, Jang S, Lee SJ, Nam M, Kim SJ, Im H, Lee BJ (2014) Structure of Thermoplasma volcanium Ard1 belongs to N-acetyltransferase family member suggesting multiple ligand binding modes with acetyl coenzyme A and coenzyme A. Biochim. Biophys. Acta. 1844 (10): 1790-1797. DOI: 10.1016/j.bbapap.2014.07.011.
102. Byeon Y, Lee K, Park YI, Park S, Back K (2013) Molecular cloning and functional analysis of serotonin N‐acetyltransferase from the cyanobacterium Synechocystis sp. PCC 6803. J. Pineal Res. 55 (4): 371-376. DOI: 10.1111/jpi.12080.
103. Zheng X, Xie G, Zhao A, Zhao L, Yao C, Chiu NH, Zhou Z, Bao Y, Jia W (2011) Nicholson JK, Jia W. The footprints of gut microbial–mammalian co-metabolism. J. Proteome Res. 10 (12): 5512-22.DOI: 10.1021/pr2007945.
104. Tan DX, Reiter RJ (2020) An evolutionary view of melatonin synthesis and metabolism related to its biological functions in plants. J. Exp. Bot. 71 (16): 4677-4689. DOI: 10.1093/jxb/eraa235.
105. Choi GH, Lee HY, Back K (2017) Chloroplast overexpression of rice caffeic acid O‐methyltransferase increases melatonin production in chloroplasts via the 5‐methoxytryptamine pathway in transgenic rice plants. J. Pineal Res. 63 (1): e12412. DOI: 10.1111/jpi.12412.
106. Zheng X, Tan DX, Allan AC, Zuo B, Zhao Y, Reiter RJ, Wang L, Wang Z, Guo Y, Zhou J, Shan D (2017) Chloroplastic biosynthesis of melatonin and its involvement in protection of plants from salt stress. Sci. Rep. 7 (1): 1-2. DOI: 10.1038/srep41236.
107. Lee HY,Byeon Y, Lee K, Lee HJ, Back K (2014) Cloning of A rabidopsis serotonin N‐acetyltransferase and its role with caffeic acid O‐methyltransferase in the biosynthesis of melatonin in vitro despite their different subcellular localizations. J. Pineal Res. 57 (4): 418-426. DOI: 10.1111/jpi.12181.
108. Del Río LA (2015) ROS and RNS in plant physiology: an overview. J. Exp. Bot. 66 (10): 2827-2837. DOI: 10.1093/jxb/erv099.
109. Hardeland R (2016) Melatonin in plants–diversity of levels and multiplicity of functions. Front. Plant Sci. 7: 198. DOI: 10.3389/fpls.2016.00198.
110. RahantaniainaMS, Tuzet A, Mhamdi A, Noctor G (2013) Missing links in understanding redox signaling via thiol/disulfide modulation: how is glutathione oxidized in plants?. Front. Plant Sci. 4: 477. DOI: 10.3389/fpls.2013.00477.
111. Takahashi S, Murata N (2008) How do environmental stresses accelerate photoinhibition?. Trends Plant Sci. 13 (4): 178-182. DOI: 10.1016/j.tplants.2008.01.005.
112. Tan DX, Reiter RJ, Manchester LC, Yan MT, El-Sawi M, Sainz RM, Mayo JC, Kohen R, Allegra MC, Hardeland R (2002) Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr. Top. Med. Chem. 2 (2): 181-197. DOI: 10.2174/1568026023394443.
113. Tan DX, Manchester LC, Reiter RJ, Plummer BF, Limson J, Weintraub ST, Qi W (2000) Melatonin directly scavenges hydrogen peroxide: a potentially new metabolic pathway of melatonin biotransformation. Free Radic. Biol. Med. 29 (11): 1177-1185. DOI: 10.1016/s0891-5849(00)00435-4.
114. Stasica P, Paneth P, Rosiak JM (2000) Hydroxyl radical reaction with melatonin molecule: a computational study. J. Pineal Res. 29 (2): 125-127. DOI: 10.1034/j.1600-079x.2000.290209.x.
115. Wang P, Sun X, Li C, Wei Z, Liang D, Ma F (2013) Long‐term exogenous application of melatonin delays drought‐induced leaf senescence in apple. J. Pineal Res. 54 (3): 292-302. DOI: 10.1111/jpi.12017.
116. Xiang-dong X, Yan S, Bo S, Jian Z, Xiao-qin G (2010) Effects of exogenous melatonin on active oxygen metabolism of cucumber seedlings under high temperature stress. Ying Yong Sheng Tai XueBao. 21 (5): 1295-1300. PMID: 20707116.
117. Uchendu EE, Shukla MR, Reed BM, Saxena PK (2013) Melatonin enhances the recovery of cryopreserved shoot tips of A merican elm (U lmus americana L.). J. Pineal Res. 55 (4): 435-442. DOI: 10.1111/jpi.12094.
118. Bajwa VS, Shukla MR, Sherif SM, Murch SJ, Saxena PK (2014) Role of melatonin in alleviating cold stress in Arabidopsis thaliana. J. Pineal Res. 56 (3): 238-245. DOI: 10.1111/jpi.12115.
119. Zhang N, Zhao B, Zhang HJ, Weeda S, Yang C, Yang ZC, Ren S, Guo YD (2013) Melatonin promotes water‐stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.). J. Pineal Res. 54 (1): 15-23. DOI: 10.1111/j.1600-079X.2012.01015.x.
120. Wang P, Yin L, Liang D, Li C, Ma F, Yue Z (2012) Delayed senescence of apple leaves by exogenous melatonin treatment: toward regulating the ascorbate–glutathione cycle. J. Pineal Res. 53 (1): 11-20. DOI: 10.1111/j.1600-079X.2011.00966.x.
121. Park S, Lee DE, Jang H, Byeon Y, Kim YS, Back K (2013) Melatonin‐rich transgenic rice plants exhibit resistance to herbicide‐induced oxidative stress. J. Pineal Res. 54 (3): 258-263. DOI: 10.1111/j.1600-079X.2012.01029.x.
122. Arnao MB, Hernández‐Ruiz J (2009) Chemical stress by different agents affects the melatonin content of barley roots. J. Pineal Res. 46 (3): 295-299. DOI: 10.1111/j.1600-079X.2008.00660.x.
123. Oladi E, Mohamadi M, Shamspur T, Mostafavi A (2014) Spectrofluorimetric determination of melatonin in kernels of four different Pistacia varieties after ultrasound-assisted solid–liquid extraction. Spectrochim. Acta A Mol. Biomol. Spectrosc. 132: 326-329. DOI: 10.1016/j.saa.2019.04.006.
124. Murch SJ, Simmons CB (1997) Melatonin in feverfew and other medicinal plants. Lancet 350 (9091): 1598-1599. DOI: 10.1016/S0140-6736(05)64014-7.
125. Brown PN, Turi CE, Shipley PR, Murch SJ (2012) Comparisons of large (Vaccinium macrocarpon Ait.) and small (Vacciniumoxycoccos L., Vaccinium vitis-idaea L.) cranberry in British Columbia by phytochemical determination, antioxidant potential, and metabolomic profiling with chemometric analysis. Planta med. 78 (06): 630-640. DOI: 10.1055/s-0031-1298239.
126. Arnao MB, Hernández-Ruiz J (2013) Growth conditions influence the melatonin content of tomato plants. Food chem. 138 (2-3): 1212-1214. DOI: 10.1016/j.foodchem.2012.10.077.
127. Arnao MB, Hernández‐Ruiz J (2013) Growth conditions determine different melatonin levels in L upinusalbus L. J. Pineal Res. 55 (2): 149-155. DOI: 10.1111/jpi.12055.
128. Reiter RJ, Tan DX, Galano A (2014) Melatonin: exceeding expectations. Physiology 29 (5): 325-33. DOI: 10.1152/physiol.00011.2014. DOI: 10.1152/physiol.00011.2014.
129. Venegas C, García JA, Escames G, Ortiz F, López A, Doerrier C, García‐Corzo L, López LC, Reiter RJ, Acuña‐Castroviejo D (2012) Extrapineal melatonin: analysis of its subcellular distribution and daily fluctuations. J. Pineal Res. 52 (2): 217-227. DOI: 10.1111/j.1600-079X.2011.00931.x.
130. Yamamoto HA, Tang HW (1996) Preventive effect of melatonin against cyanide-induced seizures and lipid peroxidation in mice. Neuroscilett. 207 (2): 89-92. DOI: 10.1016/0304-3940(96)12493-9.
131. Dabbeni-Sala FE, DI Santo ST, Franceschini D, D. Skaper ST, Pietro Giusti AN (2001) Melatonin protects against 6‐OHDA‐induced neurotoxicity in rats: a role for mitochondrial complex I activity. FASEB J. 15 (1): 164-170. DOI: 10.1096/fj.00-0129com.
132. Dabbeni-Sala F, Floreani M, Franceschini D, Skaper SD, Giusti P (2001) Kainic acid induces selective mitochondrial oxidative phosphorylation enzyme dysfunction in cerebellar granule neurons: protective effects of melatonin and GSH ethyl ester. FASEB J. 15 (10): 1786-1788. DOI: 10.1096/fj.00-0427fje.
133. Absi E, Ayala A, Machado A, Parrado J (2000) Protective effect of melatonin against the 1‐methyl‐4‐phenylpyridinium‐induced inhibition of complex I of the mitochondrial respiratory chain. J. Pineal Res. 29 (1): 40-47. DOI: 10.1034/j.1600-079x.2000.290106.x.
134. Martin M, Macias M, Escames G, Reiter RJ, Agapito MT, Ortiz GG, Acuña‐Castroviejo D (2000) Melatonin‐induced increased activity of the respiratory chain complexes I and IV can prevent mitochondrial damage induced by ruthenium red in vivo. J. Pineal Res. 28 (4): 242-248. DOI: 10.1034/j.1600-079x.2000.280407.x.
135. Reiter R, Paredes S, Korkmaz A, JouMJ, Tan DX (2008) Melatonin combats molecular terrorism at the mitochondrial level. Interdiscip. Toxicol. 1 (2): 137-149. DOI: 10.2478/v10102-010-0030-2.
136. Hardeland R (2013) Melatonin and the theories of aging: a critical appraisal of melatonin's role in antiaging mechanisms. J. Pineal Res. 55 (4): 325-356. DOI: 10.1111/jpi.12090.
137. Tan DX, Hardeland R (2020) Targeting host defensesystem and rescuing compromised mitochondria to increase tolerance against pathogens by melatonin may impact outcome of deadly virus infection pertinent to COVID-19. Molecules 25 (19): 4410. DOI: 10.3390/molecules25194410.
138. Huether GE (1993) The contribution of extrapineal sites of melatonin synthesis to circulating melatonin levels in higher vertebrates. Experientia 49 (8): 665-670. DOI: 10.1007/BF01923948.
139. Pang SF, Brown GM, Grota LJ, Chambers JW, Rodman RL (1977) Determination of N-acetylserotonin and melatonin activities in the pineal gland, retina, Harderian gland, brain and serum of rats and chickens. Neuroendocrinology 23 (1): 1-3. DOI: 10.1159/000122649. DOI: 10.1159/000122649.
140. Reiter RJ, Richardson BA, Matthews SA, Lane SJ, Ferguson BN (1983) Rhythms in immunoreactive melatonin in the retina and Harderian gland of rats: persistence after pinealectomy. Life sci. 32 (11): 1229-1236. DOI: 10.1016/0024-3205(83)90192-3.
141. Slominski A, Tobin DJ, Zmijewski MA, Wortsman J, Paus R (2008) Melatonin in the skin: synthesis, metabolism and functions. Trends Endocrinol. Metab. 19 (1): 17-24. DOI: 10.1016/j.tem.2007.10.007.
142. Pévet P (2014) The internal time-giver role of melatonin. A key for our health. Rev. Neurol. 170 (11): 646-652. DOI: 10.1016/j.neurol.2014.05.008.
143. Nagle CA, Cardinali DP, Rosner JM (1973) Retinal and pineal hydroxyindole-o-methyl transferases in the rat: changes following cervical sympathectomy, pinealectomy or blinding. Endocrinology 92 (5): 1560-1564. DOI: 10.1210/endo-92-5-1560. DOI: 10.1210/endo-92-5-1560.
144. Quay WB (1965) Indole derivatives of pineal and related neural and retinal tissues. Pharmacol. Rev. 17 (3): 321-345. PMID: 5318083.
145. Cardinali DP, Rosner JM (1971) Retinal localization of the hydroxyindole-O-methyl transferase (HIOMT) in the rat. Endocrinology 89 (1): 301-303. DOI: 10.1210/endo-89-1-301.
146. Eakin RM, Westfall JA (1959) Fine structure of the retina in the reptilian third eye. J. Biophys. Biochem. Cytol. 6 (1): 133. DOI: 10.1083/jcb.6.1.133.
147. Szafrańska K, Reiter RJ, Posmyk MM (2017) Melatonin improves the photosynthetic apparatus in pea leaves stressed by paraquat via chlorophyll breakdown regulation and its accelerated de novo synthesis. Front. Plant Sci. 8: 878. DOI: 10.3389/fpls.2017.00878.
148. Balzer I, Fuhrberg B, Hardeland R, Elsner N, Schnitzler HU (1996) The neurohormone melatonin oscillates in a circadian fashion already in unicells. In: Brain and Evolution. Elsner N, Schnitzler HU, eds., pp. 228. Thieme, Stuttgart, New York.
149. Sprenger J, Hardeland R, Fuhrberg B, Han SZ (1999) Melatonin and other 5-methoxylated indoles in yeast: presence in high concentrations and dependence on tryptophan availability. Cytologia 64 (2): 209-213. DOI: 10.1508/cytologia.64.209.
150. Mechawar N, Anctil M (1997) Melatonin in a primitive metazoan: seasonal changes of levels and immunohistochemical visualization in neurons. J. Comp. Neurol. 387 (2): 243-54. PMID: 9336226.
151. Roopin M, Levy O (2012) Melatonin distribution reveals clues to its biological significance in basal metazoans. PLoS one 7 (12): e52266. DOI: 10.1371/journal.pone.0052266.
152. Morita M, Best JB (1984) Effects of photoperiods and melatonin on planarian asexual reproduction. J. Exp. Zool. 231 (2): 273-282. DOI: 10.1002/jez.1402310212.
153. Migliori ML, Romanowski A, Simonetta SH, Valdez D, Guido M, Golombek DA (2012) Daily variation in melatonin synthesis and arylalkylamine N‐acetyltransferase activity in the nematode Caenorhabditis elegans. J. Pineal Res. 53 (1): 38-46. DOI: 10.1111/j.1600-079X.2011.00969.x.
154. Tosches MA, Bucher D, Vopalensky P, Arendt D (2014) Melatonin signaling controls circadian swimming behavior in marine zooplankton. Cell 159 (1): 46-57. DOI: 10.1016/j.cell.2014.07.042.
155. Munoz JL, Patino MA, Hermosilla C, Conde-Sieira M,Soengas JL, Rocha F, Míguez JM (2011) Melatonin in octopus (Octopus vulgaris): tissue distribution, daily changes and relation with serotonin and its acid metabolite. J. Comp. Physiol. A. Neuroethol. Sens. Neural. Behav. Physiol. 197 (8): 789-797. DOI: 10.1007/s00359-011-0641-x.
156. Wang Q, Egi Y, Takeda M, Oishi K, Sakamoto K (2015) Melatonin pathway transmits information to terminate pupal diapause in the Chinese oak silkmoth A ntheraeapernyi and through reciprocated inhibition of dopamine pathway functions as a photoperiodic counter. Entomologic. Sci. 18 (1): 74-84. DOI: 10.1111/ens.12083.
157. Ding K, Zhang L, Zhang T, Yang H, Brinkman R (2019) The Effect of melatonin on locomotor behavior and muscle physiology in the sea cucumber apostichopusjaponicus. Front. Physiol. 10: 221. DOI: 10.3389/fphys.2019.00221.
158. Hardeland R, Poeggeler B (2003) Non‐vertebrate melatonin. J. Pineal Res. 34 (4): 233-241. DOI: 10.1034/j.1600-079x.2003.00040.x.
159. Tan DX, Hardeland R, Manchester LC, Paredes SD, Korkmaz A, Sainz RM, Mayo JC, Fuentes‐Broto L, Reiter RJ (2010) The changing biological roles of melatonin during evolution: from an antioxidant to signals of darkness, sexual selection and fitness. Biol. Rev.Camb. Philos. Soc. 85 (3): 607-623. DOI: 10.1111/j.1469-185X.2009.00118.x.
160. AfreenF, Zobayed SM, Kozai T (2006) Melatonin in Glycyrrhiza uralensis: response of plant roots to spectral quality of light and UV‐B radiation. J. Pineal Res. 41 (2): 108-115. DOI: 10.1111/j.1600-079X.2006.00337.x.
161. Arnao MB, Hernández‐Ruiz J (2009) Protective effect of melatonin against chlorophyll degradation during the senescence of barley leaves. J. Pineal Res. 46 (1): 58-63. DOI: 10.1111/j.1600-079X.2008.00625.x.
162. Ullah A, Manghwar H, Shaban M, Khan AH, Akbar A, Ali U, Ali E, Fahad S (2018) Phytohormones enhanced drought tolerance in plants: a coping strategy. Environ. Sci. Pollut. Res. Int. 25 (33): 33103-33118. DOI: 10.1007/s11356-018-3364-5.
163. Ku YS, Sintaha M, Cheung MY, Lam HM (2018) Plant hormone signaling crosstalks between biotic and abiotic stress responses. Int. J. Mol. Sci. 19 (10): 3206. DOI: 10.3390/ijms19103206.
164. Erland LA, Shukla MR, Singh AS, Murch SJ, Saxena PK (2018) Melatonin and serotonin: mediators in the symphony of plant morphogenesis. J. Pineal Res. 64 (2): e12452. DOI: 10.1111/jpi.12452.
165. Hardeland R, Pandi-Perumal SR (2005) Melatonin, a potent agent in antioxidative defense: actions as a natural food constituent, gastrointestinal factor, drug and prodrug. Nutr. Metab. 2 (1): 2 2. DOI: 10.1186/1743-7075-2-22.
166. Burkhardt S, Tan DX, Manchester LC, Hardeland R, Reiter RJ (2001) Detection and quantification of the antioxidant melatonin in Montmorency and Balaton tart cherries (Prunus cerasus). J. Agric. Food Chem. 49 (10): 4898-4902. DOI: 10.1021/jf010321+.
167. Arnao MB, Hernández-Ruiz J (2018) Melatonin and its relationship to plant hormones. Ann. Bot. 121 (2): 195-207. DOI: 10.1093/aob/mcx114.
168. Arnao MB, Hernández-Ruiz J (2019) Melatonin: a new plant hormone and/or a plant master regulator?. Trends Plant Sci. 24 (1): 38-48. DOI: 10.1016/j.tplants.2018.10.010.
169. Hernandez-Ruiz J, Cano A, Arnao MB (2004) Melatonin: a growth-stimulating compound present in lupin tissues. Planta 220 (1): 140-144. DOI: 10.1007/s00425-004-1317-3.
170. Liang C, Li A, Yu H, Li W, Liang C, Guo S, Zhang R, Chu C (2017) Melatonin regulates root architecture by modulating auxin response in rice. Front. Plant Sci. 8: 134. DOI: 10.3389/fpls.2017.00134.
171. Arnao MB, Hernández‐Ruiz J (2007) Melatonin promotes adventitious‐and lateral root regeneration in etiolated hypocotyls of Lupinus albus L. J. Pineal Res. 42 (2): 147-152. DOI: 10.1111/j.1600-079X.2006.00396.x.
172. Mao J, Niu C, Li K, Chen S, Tahir MM, Han M, Zhang D (2020) Melatonin promotes adventitious root formation in apple by promoting the function of MdWOX11. BMC Plant Biol. 20 (1): 1-1. DOI: 10.1186/s12870-020-02747-z.
173. Murch SJ, Saxena PK (2002) Mammalian neurohormones: potential significance in reproductive physiology of St. John's wort (Hypericum perforatum L.)?. Naturwissenschaften 89 (12): 555-560. DOI: 10.1007/s00114-002-0376-1.
174. Kolář J, Johnson CH, Macháčková I (2003) Exogenously applied melatonin (N‐acetyl‐5‐methoxytryptamine) affects flowering of the short‐day plant Chenopodium rubrum. Physiologia Plantarum 118 (4): 605-612. DOI: 10.1034/j.1399-3054.2003.00114.x.
175. Murch SJ, KrishnaRaj S, Saxena PK (2000) Tryptophan is a precursor for melatonin and serotonin biosynthesis in in vitro regenerated St. John's wort (Hypericum perforatum L. cv. Anthos) plants. Plant Cell Rep. 19 (7): 698-704. DOI: 10.1007/s002990000206.
176. Maitra SK, Hasan KN (2016) The role of melatonin as a hormone and an antioxidant in the control of fish reproduction. Front. Endocrinol. 7: 38. DOI: 10.3389/fendo.2016.00038.
177. Axelrod J, QUAY WB, BAKER PC (1965) Enzymatic synthesis of the skin-lightening agent, melatonin, in amphibians. Nature 208 (5008): 386. DOI: 10.1038/208386a0.
178. Foa A, Janik D, Minutini L (1992) Circadian rhythms of plasma melatonin in the ruin lizard Podarcissicula: effects of pinealectomy. J. Pineal Res. 12 (3): 109-113. DOI: 10.1111/j.1600-079x.1992.tb00036.x.
179. Cassone VM, Westneat DF (2012) The bird of time: cognition and the avian biological clock. Front. Mol. Neurosci. 5: 32. DOI: 10.3389/fnmol.2012.00032.
180. Ubuka T, Bentley GE, Ukena K, Wingfield JC, Tsutsui K (2005) Melatonin induces the expression of gonadotropin-inhibitory hormone in the avian brain. Proc. Nat. Acad. Sci. 102 (8): 3052-3057. DOI: 10.1073/pnas.0403840102.
181. Grivas TB, Savvidou OD (2007) Melatonin the" light of night" in human biology and adolescent idiopathic scoliosis. Scoliosis 2 (1): 6. DOI: 10.1186/1748-7161-2-6.
182. Mayo JC, Sainz RM, Tan DX, Hardeland R, Leon J, Rodriguez C, Reiter RJ (2005) Anti-inflammatory actions of melatonin and its metabolites, N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) and N1-acetyl-5-methoxykynuramine (AMK), in macrophages. J. Neuroimmunol. 165 (1-2): 139-149. DOI: 10.1016/j.jneuroim.2005.05.002.
183. Nabavi SM, Nabavi SF, Sureda A, Xiao J, Dehpour AR, Shirooie S, Silva AS, Baldi A, Khan H, Daglia M (2019) Anti-inflammatory effects of Melatonin: A mechanistic review. Crit. Rev. Food Sci. Nutr. 59 (sup1): S4-S16. DOI: 10.1080/10408398.2018.1487927.
184. Pal PK, Sarkar S, Mishra S, Chattopadhyay S, Chattopadhyay A, Bandyopadhyay D (2020) Amelioration of adrenaline induced oxidative gastrointestinal damages in rat by melatonin through SIRT1-NFκB and PGC1α-AMPKα cascades. Meletonin Res. 3 (4): 482-502. DOI: 10.32794/mr11250074
185. Crespo E, Macías M, Pozo D, Escames G, Martín M, Vives F, Guerrero JM, Acuña‐Castroviejo D (1999) Melatonin inhibits expression of the inducible NO synthase II in liver and lung and prevents endotoxemia in lipopolysaccharide‐induced multiple organ dysfunction syndrome in rats. FASEB J. 13 (12): 1537-1546. PMID: 10463945.
186. Hardeland R (2019) Aging, melatonin, and the pro-and anti-inflammatory networks. Int. J. Mol. Sci. 20 (5): 1223. DOI: 10.3390/ijms20051223.
187. Prado NJ, Ferder L, Manucha W, Diez ER (2018) Anti-inflammatory effects of melatonin in obesity and hypertension. Curr. Hypertens Rep. 20 (5): 45. DOI: 10.1007/s11906-018-0842-6.
188. Lu T, Galijasevic S, Abdulhamid I, Abu‐Soud HM (2008) Analysis of the mechanism by which melatonin inhibits human eosinophil peroxidase. Br. J. Pharmacol. 154 (6): 1308-1317. DOI: 10.1038/bjp.2008.173.
189. Zhang ZW, Cheng J, Xu F, Chen YE, Du JB, Yuan M, Zhu F, Xu XC, Yuan S (2011) Red blood cell extrudes nucleus and mitochondria against oxidative stress. IUBMB life 63 (7): 560-565. DOI: 10.1002/iub.490.
190. Banerjee A, Chattopadhyay A, Pal PK, Bandyopadhyay D (2020) Melatonin is a potential therapeutic molecule for oxidative stress induced red blood cell (RBC) injury: A review. Melatonin Res. 3 (1): 1-31. DOI: 10.32794/mr11250045.
191. Cassone VM, Warren WS, Brooks DS, Lu J (1993) Melatonin, the pineal gland, and circadian rhythms. J. Biol. Rhythms 8: S73-S81.PMID: 8274765.
192. Pozdeyev N, Taylor C, Haque R, Chaurasia SS, Visser A, Thazyeen A, Du Y, Fu H, Weller J, Klein DC, Iuvone PM (2006) Photic regulation of arylalkylamine N-acetyltransferase binding to 14-3-3 proteins in retinal photoreceptor cells. J. Neurosci. 26 (36): 9153-9161. DOI: 10.1523/JNEUROSCI.1384-06.2006.
193. Hardeland R (2013) Chronobiology of melatonin beyond the feedback to the suprachiasmatic nucleus—consequences to melatonin dysfunction. Int. J. Mol. Sci. 14 (3): 5817-5841. DOI: 10.3390/ijms14035817.
194. Green CB (2003) Molecular control of Xenopus retinal circadian rhythms. J. Neuroendocrinol. 15 (4): 350-354. DOI: 0.1046/j.1365-2826.2003.00999.x.
195. Tosini G, Baba K, Hwang CK, Iuvone PM (2012) Melatonin: an underappreciated player in retinal physiology and pathophysiology. Exp. Eye Res. 103: 82-89. DOI: 10.1016/j.exer.2012.08.009.
196. Underwood H, Binkley S, Siopes T, Mosher K (1984) Melatonin rhythms in the eyes, pineal bodies, and blood of Japanese quail (Coturnixcoturnix japonica). Gen. Comp. Endocrinol. 56 (1): 70-81. DOI: 10.1016/0016-6480(84)90063-7.
197. Menaker M (1985) Eyes-the second (and third) pineal glands. Ciba. Found. Symp.117: 78-92. DOI: 10.1002/9780470720981.ch6.
198. Isorna E, Besseau L, Boeuf G, Desdevises Y, Vuilleumier R, Alonso-Gómez AL, Delgado MJ, Falcón J (2006) Retinal, pineal and diencephalic expression of frog arylalkylamine N-acetyltransferase-1. Mol. Cell Endocrinol. 252 (1-2): 11-18. DOI: 10.1016/j.mce.2006.03.032.
199. Falcon J, Migaud H, Munoz-Cueto JA, Carrillo M (2010) Current knowledge on the melatonin system in teleost fish. Gen. Comp. Endocrinol. 165 (3): 469-482. DOI: 10.1016/j.ygcen.2009.04.026.
200. Acuña-Castroviejo D, Escames G, Venegas C, Díaz-Casado ME, Lima-Cabello E, López LC, Rosales-Corral S, Tan DX, Reiter RJ (2014) Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol. Life Sci. 71 (16): 2997-3025. DOI: 10.1007/s00018-014-1579-2.
201. Gern WA (1981) Evolution of melatonin function: a hypothesis. In: N. Birau W. Schloot ed., Melatonin: Current status and perspectives. 85-87. Pergamon.
202. Aulinas A (2019) Physiology of the pineal gland and melatonin. Endotext [Internet]. PMID: 31841296.
203. AschoffJ (1960) Exogenous and endogenous components in circadian rhythms. In: Cold Spring Harbor symposia on quantitative biology. 25: 11-28. Cold Spring Harbor Laboratory Press.
204. Kramm CM, De Grip WJ, Korf HW (1993) Rod-opsin immunoreaction in the pineal organ of the pigmented mouse does not indicate the presence of a functional photopigment. Cell Tissue Res. 274 (1): 71-78. DOI: 10.1007/BF00327987.
205. Oksche A (1991) The development of the concept of photoneuroendocrine systems: historical perspective. In: Klein DC, Moor RY, Reppert SM. ed., Suprachiasmatic Nucleus. New York, NY: Oxford University Press pp. 5–11.
206. Menaker M, Roberts R, Elliott J, Underwood H (1970) Extraretinal light perception in the sparrow, III: the eyes do not participate in photoperiodic photoreception. Proc. Natl. Acad. Sci. 67 (1): 320-325. DOI: 10.1073/pnas.67.1.320.
207. Binkley SA (1980) In: RJ Reiter ed., The Pineal Gland: Its Anatomy and Biochemistry. CRC Press, West Palm Beach, Florida.
208. Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, Berson DM (2006) Central projections of melanopsin‐expressing retinal ganglion cells in the mouse. J. Comp. Neurol. 497 (3): 326-349. DOI: 10.1002/cne.20970.
209. Banerjee A, Chattopadhyay A, Bandyopadhyay D (2020) Biorhythmic and receptor mediated interplay between melatonin and insulin: its consequences on diabetic erythrocytes. Melatonin Res. 3 (2): 243-263. DOI: 10.32794/mr12250060.
210. Reiter RJ (1993) The melatonin rhythm: both a clock and a calendar. Experientia 49 (8): 654-664. DOI: 10.1007/BF01923947.
211. Lincoln GA (1998) Photoperiod-melatonin relay in deer. Acta Vet. Hung. 46 (3): 341-356. PMID: 9704533.
212. Chemineau P, Pelletier J, Guérin Y, Colas G, Ravault JP, Toure G, Almeida G, Thimonier J, Ortavant R, Daveau A, Maurice F (1988) Photoperiodic and melatonin treatments for the control of seasonal reproduction in sheep and goats. Reprod. Nutr. Dev. 28 (2B): 409-422. DOI: 10.1051/rnd:19880307.
213. Powers JB, Jetton AE, Mangels RA, Bittman EL (1997) Effects of photoperiod duration and melatonin signal characteristics on the reproductive system of male syrian hamsters. J. Neuroendocrinol. 9 (6): 451-466. DOI: 10.1046/j.1365-2826.1997.t01-1-00602.x.
214. Reiter RJ (1974) Influence of pinealectomy on the breeding capability of hamsters maintained under natural photoperiodic and temperature conditions. Neuroendocrinology 13 (6): 366-370. DOI: 10.1159/000122222.
215. Reiter RJ (1973) Pineal control of a seasonal reproductive rhythm in male golden hamsters exposed to natural daylight and temperature. Endocrinology 92 (2): 423-430. DOI: 10.1210/endo-92-2-423.
216. Haldar C, Vidhu S (1997) Effect of pinealectomy and testosterone on gonadal regression and accessory sex organs in Indian palm squirrel, Funambuluspennanti. Indian J. Exp. Biol. 35 (6): 594-596. PMID: 9357162
217. Chen HJ (1981) Spontaneous and melatonin-induced testicular regression in male golden hamsters: augmented sensitivity of the old male to melatonin inhibition. Neuroendocrinology 33 (1): 43-46. DOI: 10.1159/000123198.
218. Reiter RJ (1980) The pineal and its hormones in the control of reproduction in mammals. Endocr. Rev. 1 (2): 109-131. DOI: 10.1210/edrv-1-2-109.
219. Samejima M, Shavali S, Tamotsu S, Uchida K, Morita Y, Fukuda A (2000) Light-and temperature-dependence of the melatonin secretion rhythm in the pineal organ of the lamprey, Lampetra japonica. Jpn. J. Physiol. 50 (4): 437-442. DOI: 10.2170/jjphysiol.50.437.
220. Fisher SP, Sugden D (2010) Endogenous melatonin is not obligatory for the regulation of the rat sleep-wake cycle. Sleep 33 (6): 833-840. DOI: 10.1093/sleep/33.6.833.
221. Mueller U, Hardeland R (1999) Transient accumulations of exogenous melatonin indicate binding sites in the dinoflagellate Gonyaulaxpolyedra. In: Studies on Antioxidants and Their Metabolites. Hardeland, R., Ed. pp. 140-147Cuvillier: Göttingen, Germany.
222. Nosjean O, Ferro M, Cogé F, Beauverger P, Henlin JM, Lefoulon F, Fauchère JL, Delagrange P, Canet E, Boutin JA (2000) Identification of the melatonin-binding site MT 3 as the quinone reductase 2. J. Biol. Chem. 275 (40): 31311-31317. DOI: 10.1074/jbc.M005141200.
223. Reppert SM, Godson C, Mahle CD, Weaver DR, Slaugenhaupt SA, Gusella JF (1995) Molecular characterization of a second melatonin receptor expressed in human retina and brain: the Mel1b melatonin receptor. Proc. Nat. Acad. Sci. 92 (19): 8734-8738. DOI: 10.1073/pnas.92.19.8734.
224. Dubocovich ML, Yun K, Al‐Ghoul WM, Benloucif S, Masana MI (1998) Selective MT2 melatonin receptor antagonists block melatonin‐mediated phase advances of circadian rhythms. FASEB J. 12 (12): 1211-1220. DOI: 10.1096/fasebj.12.12.1211.
225. Liu J, Clough SJ, Hutchinson AJ, Adamah-Biassi EB, Popovska-Gorevski M, Dubocovich ML (2016) MT1 and MT2 melatonin receptors: a therapeutic perspective. Annu. Rev. Pharmacol. Toxicol. 56: 361-383. DOI: 10.1146/annurev-pharmtox-010814-124742.
226. Mayo JC, Cernuda R, Quiros I, Rodriguez P, Garcia JI, Hevia D, Sainz RM (2019) Understanding the role of melatonin in cancer metabolism. Melatonin Res. 2 (3): 76-104. DOI: 10.32794/11250032.
227. Mazzucchelli C, Pannacci M, Nonno R, Lucini V, Fraschini F, Stankov BM (1996) The melatonin receptor in the human brain: cloning experiments and distribution studies. Mol. Brain Res. 39 (1-2): 117-126. DOI: 10.1016/0169-328x(96)00017-4.
228. Dubocovich ML (2007) Melatonin receptors: role on sleep and circadian rhythm regulation. Sleep med. 8: 34-42. DOI: 10.1016/j.sleep.2007.10.007.
229. Reppart SM, Weaver DR, Godson C (1996) Melatonin receptors step into the light: cloning and classification of subtypes. Trends Pharmacol. Sci. 17 (3): 100-102. DOI: 10.1016/0165-6147(96)10005-5.
230. Slaugenhaupt SA, Roca AL, Liebert CB, Altherr MR, Gusella JF, Reppert SM (1995) Mapping of the gene for the Mel1a-melatonin receptor to human chromosome 4 (MTNR1A) and mouse chromosome 8 (Mtnr1a). Genomics 27 (2): 355-357. DOI: 10.1006/geno.1995.1056.
231. Audinot V, Mailliet F, Lahaye-Brasseur C, Bonnaud A, Le Gall A, Amossé C, Dromaint S, Rodriguez M, Nagel N, Galizzi JP, Malpaux B (2003) New selective ligands of human cloned melatonin MT 1 and MT 2 receptors. NaunynSchmiedebergs Arch. Pharmacol. 367 (6): 553-561. DOI: 10.1007/s00210-003-0751-2.
232. Dubocovich ML, Masana MI, Iacob S, Sauri DM (1997) Melatonin receptor antagonists that differentiate between the human Mel1a and Mel1b recombinant subtypes are used to assess the pharmacological profile of the rabbit retina ML1 presynaptic heteroreceptor. NaunynSchmiedebergs Arch. Pharmacol. 355 (3): 365-375. DOI: 10.1007/pl00004956.
233. Ayoub MA, Levoye A, Delagrange P, Jockers R (2004) Preferential formation of MT1/MT2 melatonin receptor heterodimers with distinct ligand interaction properties compared with MT2 homodimers. Mol. Pharmacol. 66 (2): 312-321. DOI: 10.1124/mol.104.000398.
234. Wang LM, Suthana NA, Chaudhury D, Weaver DR, Colwell CS (2005) Melatonin inhibits hippocampal long‐term potentiation. Eur. J. Neurosci. 22 (9): 2231-2237. DOI: 10.1111/j.1460-9568.2005.04408.x.
235. O’Neal-Moffitt G, Pilli J, Kumar SS, Olcese J (2014) Genetic deletion of MT1/MT2 melatonin receptors enhances murine cognitive and motor performance. Neuroscience 277: 506-521. DOI: 10.1016/j.neuroscience.2014.07.018.
236. Chern CM, Liao JF, Wang YH, Shen YC (2012) Melatonin ameliorates neural function by promoting endogenous neurogenesis through the MT2 melatonin receptor in ischemic-stroke mice. Free Radic. Biol. Med. 52 (9): 1634-1647. DOI: 10.1016/j.freeradbiomed.2012.01.030.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


