Could melatonin be an adjunct therapy for post-TB lung disease?
The potential roles of melatonin in post-TB lung disease
Abstract
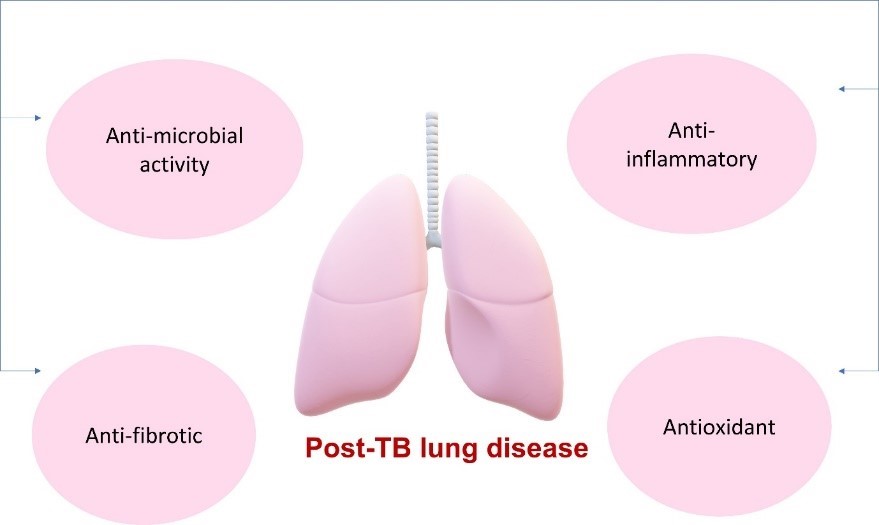
Post-tuberculosis (post-TB) lung disease is a complex interplay between organism, host, and environmental factors, and it affects long-term respiratory health. It associates with underlying processes such as inflammation, fibrosis, and oxidative stress. Decades of research has demonstrated melatonin as a potent anti-inflammatory, anti-fibrotic, antioxidant, and vasodilatory agent. These effects have been observed in numerous experimental and clinical models of lung diseases. Moreover, melatonin has significant anti-microbial activity, which has also been observed in the context of TB bacterial growth. It is worth pointing out that these effects of melatonin are a reminder of the pathologic processes that underpin post-TB lung disease. Based on the intriguing evidence presented and discussed in this paper, melatonin could be considered a safe, affordable, and adjunct therapy against post-TB lung disease. Melatonin may provide health benefits in this context, mediated via its anti-inflammatory, anti-fibrotic, vasodilatory, antimicrobial and antioxidant properties.
References
2. WHO (2020) World Health Organization. Global Tuberculosis Report 2020. (Geneva, Switzerland).
3. Urbanowski ME, Ordonez AA, Ruiz-Bedoya CA, Jain SK, Bishai WR (2020) Cavitary tuberculosis: the gateway of disease transmission. Lancet Infect. Dis. 20: e117-e128.
4. Allwood BW, Maarman GJ, Kyriakakis CG, Doubell AF (2018) Post-pulmonary tuberculosis complications in South Africa and a potential link with pulmonary hypertension: Premise for clinical and scientific investigations. S. Afr. Med. J. 108: 12339.
5. Allwood BW et al. (2021) Post-tuberculosis lung disease: clinical review of an under-recognised global challenge. Respir. 100: 751-76.
6. Chaves TNM, Quijano Rodríguez JJ, Porras Andrade PS, Arriaga MB, Netto EM (2019) Factors predictive of the success of tuberculosis treatment: A systematic review with meta-analysis. PLOS ONE 14: e0226507.
7. Rayasam GV, Balganesh TS (2015) Exploring the potential of adjunct therapy in tuberculosis. Trends Pharmacol. Sci. 36: 506-513.
8. Worthington RJ, Melander C (2013) Combination approaches to combat multidrug-resistant bacteria. Trends Biotechnol. 31: 177-184.
9. Karamchand S, Williams M, Naidoo P, Decloedt E, Allwood B, (2021) Post-tuberculous lung disease: should we be using Theophylline? J. Thorac. Dis. 13: 1230-1238.
10. Ordonez AA et al. (2014) Novel adjunctive therapies for the treatment of tuberculosis. Curr. Mol. Med. 14: 385-395.
11. Zumla A et al. (2015) Inflammation and tuberculosis: host-directed therapies. J. Intern. Med. 277: 373-387.
12. Ravimohan S, Kornfeld H, Weissman D, Bisson GP (2018) Tuberculosis and lung damage: from epidemiology to pathophysiology. Eur. Respir. Rev. 27.
13. Shastri MD et al., (2018) Role of oxidative stress in the pathology and management of human tuberculosis. Oxid. Med. Cell Longev. 2018: 7695364.
14. de Matos Cavalcante AG et al. (2012) Melatonin reduces lung oxidative stress in patients with chronic obstructive pulmonary disease: a randomized, double-blind, placebo-controlled study. J. Pineal Res. 53: 238-244.
15. Inci I, Inci D, Dutly A, Boehler A, Weder W (2002) Melatonin attenuates posttransplant lung ischemia-reperfusion injury. Ann. Thorac. Surg. 73: 220-225.
16. Wang W, Gao J (2021) Effects of melatonin on protecting against lung injury (Review). Exp. Ther. Med. 21: 228.
17. Wu WS et al., (2012) Melatonin reduces acute lung inflammation, edema, and hemorrhage in heatstroke rats. Acta Pharmacol. Sin. 33: 775-782.
18. Wu X et al. (2019) Melatonin Alleviates radiation-induced lung injury via regulation of miR-30e/NLRP3 axis. Oxid. Med. Cell Longev. 2019: 4087298.
19. Zhao X et al. (2018) Melatonin protects against lung fibrosis by regulating the Hippo/YAP pathway. Int. J. Mol. Sci. 19 (4): 1118. doi: 10.3390/ijms19041118.
20. Scheer FAJL, Montfrans GAV, Someren EJWv, Mairuhu G, Buijs RM (2004) Daily nighttime melatonin reduces blood pressure in male patients with essential hypertension. Hyperten. 43: 192-197.
21. Zisapel N, (2009) Controlled release melatonin (Circadin) in the treatment of insomnia in older patients: efficacy and safety in patients with history of use and non-use of hypnotic drugs. Harefuah. 148: 337-341, 348.
22. Robinson JE, Karsch FJ, (1987) Photoperiodic history and a changing melatonin pattern can determine the neuroendocrine response of the ewe to daylength. J. Reprod. Fertil. 80: 159-165.
23. Meng X et al. (2017) Dietary sources and bioactivities of melatonin. Nutri. 9 (4): 367. doi: 10.3390/nu9040367.
24. Kennaway DJ (2019) A critical review of melatonin assays: Past and present. J. Pineal. Res. 67: e12572.
25. Venegas C et al. (2012) Extrapineal melatonin: analysis of its subcellular distribution and daily fluctuations. J. Pineal Res. 52: 217-227.
26. Jiki Z, Lecour S, Nduhirabandi F (2018) Cardiovascular benefits of dietary melatonin: a myth or a reality? Front. Physiol. 9: 528.
27. Slominski RM, Reiter RJ, Schlabritz-Loutsevitch N, Ostrom RS, Slominski AT (2012) Melatonin membrane receptors in peripheral tissues: distribution and functions. Mol. Cell Endocrinol. 351: 152-166.
28. Tan DX, Manchester LC, Sanchez-Barcelo E, Mediavilla MD, Reiter RJ, (2010) Significance of high levels of endogenous melatonin in Mammalian cerebrospinal fluid and in the central nervous system. Curr. Neuropharmacol. 8: 162-167.
29. Opie LH, Lecour S (2016) Melatonin has multiorgan effects. Eur. Heart J. Cardiovasc. Pharmacother. 2: 258-265.
30. Hardeland R et al. (2011) Melatonin--a pleiotropic, orchestrating regulator molecule. Prog. Neurobiol. 93: 350-384.
31. Lamont KT, Somers S, Lacerda L, Opie LH, Lecour S (2011) Is red wine a SAFE sip away from cardioprotection? Mechanisms involved in resveratrol- and melatonin-induced cardioprotection. J. Pineal Res. 50: 374-380.
32. Maarman G et al. (2015) Melatonin as a preventive and curative therapy against pulmonary hypertension. J. Pineal Res. 59: 343-353.
33. Andersen LPH, Gögenur I, Rosenberg J, Reiter RJ (2016) The safety of melatonin in humans. Clin. Drug Invest. 36: 169-175.
34. Shiu SY, Reiter RJ, Tan DX, Pang SF (2003) Urgent search for safe and effective treatments of severe acute respiratory syndrome: is melatonin a promising candidate drug? J. Pineal Res. 35: 69-70.
35. Armstrong L, Millar AB, (1997) Relative production of tumour necrosis factor alpha and interleukin 10 in adult respiratory distress syndrome. Thorax. 52: 442-446.
36. Park WY et al. (2001) Cytokine balance in the lungs of patients with acute respiratory distress syndrome. Am. J. Respir. Crit. Care. Med. 164: 1896-1903.
37. Wiedermann, FJ et al. (2004) Alveolar granulocyte colony-stimulating factor and alpha-chemokines in relation to serum levels, pulmonary neutrophilia, and severity of lung injury in ARDS. Chest 125: 212-219.
38. Kang DD, Lin Y, Moreno J-R, Randall TD, Khader SA (2011) Profiling early lung immune responses in the mouse model of tuberculosis. PLOS ONE 6: e16161.
39. Cho JH, Bhutani S, Kim CH, Irwin MR, (2021) Anti-inflammatory effects of melatonin: A systematic review and meta-analysis of clinical trials. Brain. Behav. Immun. 93: 245-253.
40. Ahmed M, Hassanein K (2014) Effect of melatonin in a rat model of allergic lung inflammation. Bull. Egypt Soc. Physiol. Sci. 34: 237-248.
41. Plantier L et al. (2018) Physiology of the lung in idiopathic pulmonary fibrosis. Eur. Resp. Rev. 27: 170062.
42. Wijsenbeek M, Cottin V (2020) Spectrum of fibrotic lung diseases. New Engl. J. Med. 383: 958-968.
43. Moore BB et al. (2013) Animal models of fibrotic lung disease. Am. J. Respir. Cell Mol. Biol. 49: 167-179.
44. Yildirim Z et al. (2006) Preventive effect of melatonin on bleomycin-induced lung fibrosis in rats. J. Pineal Res. 40: 27-33.
45. Pizzino G et al. (2017) Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell Longev. 2017: 8416763.
46. Niyazov DM, Kahler SG, Frye RE (2016) Primary mitochondrial disease and secondary mitochondrial dysfunction: Importance of distinction for diagnosis and treatment. Mol. Syndromol. 7: 122-137.
47. Zhao RZ, Jiang S, Zhang L, Yu ZB, (2019) Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int. J. Mol. Med. 44: 3-15.
48. Zabłocka-Słowińska K et al. (2019) Oxidative stress in lung cancer patients is associated with altered serum markers of lipid metabolism. PLOS ONE. 14: e0215246.
49. Kleme ML, Levy E, (2015) Cystic fibrosis-related oxidative stress and intestinal lipid disorders. Antioxid. Redox. Signal 22: 614-631.
50. Boukhenouna S, Wilson MA, Bahmed K, Kosmider B (2018) Reactive oxygen species in chronic obstructive pulmonary disease. Oxid. Med. Cell Longev. 2018: 5730395.
51. He B, Zhang W, Qiao J, Peng Z, Chai X, (2019) Melatonin protects against COPD by attenuating apoptosis and endoplasmic reticulum stress via upregulating SIRT1 expression in rats. Can. J. Physiol. Pharmacol. 97: 386-391.
52. Voelkel NF, Gomez-Arroyo J, Mizuno S (2011) COPD/emphysema: The vascular story. Pulm. Circ. 1: 320-326.
53. Luks AM, Swenson ER, Bärtsch P (2017) Acute high-altitude sickness. Eur. Respir. Rev. 26: 160096.
54. Rey-Parra GJ et al. (2008) Blunted hypoxic pulmonary vasoconstriction in experimental neonatal chronic lung disease. Am. J. Resp. Crit. Car. Med. 178: 399-406.
55. Cook JS, Sauder CL, Ray CA, (2011) Melatonin differentially affects vascular blood flow in humans. Am. J. Physiol. Heart Circ. Physiol. 300: H670-674.
56. Zhao T et al. (2017) Melatonin mediates vasodilation through both direct and indirect activation of BK(Ca) channels. J. Mol. Endocrinol. 59: 219-233.
57. Klimentova J et al. (2016) Effect of melatonin on blood pressure and nitric oxide generation in rats with metabolic syndrome. Physiol. Res. 65: S373-S380.
58. Tain Y-L, Huang L-T, Hsu C-N, Lee C-T (2014) Melatonin therapy prevents programmed hypertension and nitric oxide deficiency in offspring exposed to maternal caloric restriction. Oxid. Med. Cell Longev. 2014: 283180.
59. Shao G, Zhang S, Nie J, Li J, Tong J (2017) Effects of melatonin on mechanisms involved in hypertension using human umbilical vein endothelial cells. J. Toxicol. Environ. Health 80: 1342-1348.
60. Gonzaléz-Candia A et al. (2020) Melatonin long-lasting beneficial effects on pulmonary vascular reactivity and redox balance in chronic hypoxic ovine neonates. J. Pineal Res. 68: e12613.
61. Astorga CR et al. (2018) Melatonin decreases pulmonary vascular remodeling and oxygen sensitivity in pulmonary hypertensive newborn lambs. Front Physiol. 9: 185.
62. Hung MW et al. (2017) Melatonin attenuates pulmonary hypertension in chronically hypoxic rats. Int. J. Mol. Sci. 18 (6): 1125. doi: 10.3390/ijms18061125.
63. Almuqdadi SFH, Al-abbassi MG, Jasim TM (2010) Anti-bacterial properties of melatonin against mycobacterium tuberculosis in vitro. Iraq. J. Pharmaceut. Sci. 19: 59-63.
64. Xu L et al. (2019) Protective effect of melatonin against polymicrobial sepsis is mediated by the anti-bacterial effect of neutrophils. Front Immunol. 10: 1371. doi: 10.3389/fimmu.2019.01371.
65. Srinivasan V, Mohamed M, Kato H (2012) Melatonin in bacterial and viral infections with focus on sepsis: a review. Recent Pat. Endocr. Metab. Immune Drug Discov. 6: 30-39.
66. Ozkan E et al. (2012) Plasma melatonin and urinary 6-hydroxymelatonin levels in patients with pulmonary tuberculosis. Inflam. 35: 1429-1434.
67. Wiid I, Hoal-van Helden E, Hon D, Lombard C, van Helden P (1999) Potentiation of isoniazid activity against Mycobacterium tuberculosis by melatonin. Antimicrob. Agents Chemother. 43: 975-977.
68. Li J et al. (2020) Melatonin attenuates sepsis-induced acute lung injury through improvement of epithelial sodium channel-mediated alveolar fluid clearance via activation of SIRT1/SGK1/Nedd4-2 signaling pathway. Front Pharmacol. 11: 590652. doi: 10.3389/fphar.2020.590652.
69. Dheda K et al. (2005) Lung remodeling in pulmonary tuberculosis. J. Infect. Dis. 192: 1201-1210.
70. Marquis J-F et al. (2008) Fibrotic response as a distinguishing feature of resistance and susceptibility to pulmonary infection with Mycobacterium tuberculosis in Mice. Infect. Immun. 76: 78-88.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


