Melatonin and oral squamous cell carcinoma: current knowledge and future perspectives
Melatonin and oral squamous cell carcinomas
Abstract
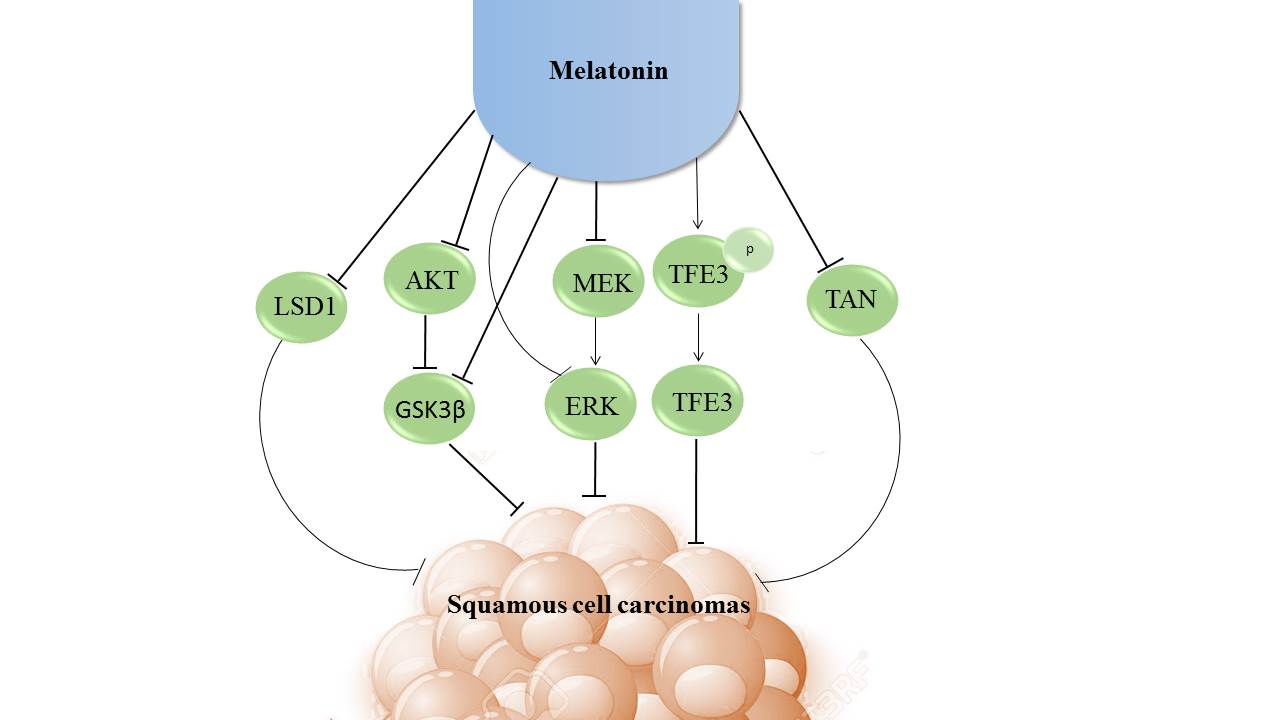
On the basis of worldwide ranking, oral cancer is the eighth most prevalent cancer. Oral squamous cell carcinoma is a cancer that occurs following dysplasia of the mucosa of the oral cavity and oropharynx. There are different inflammatory pathways involved in the pathophysiology of oral squamous cell carcinoma. Melatonin (N-acetyl-5-methoxytryptamine), a well documented anticancer agent, exhibits numerous functions including induction of apoptotic pathways and controlling of oxidative stress. In the in vivo and in vitro studies the results have demonstrated that melatonin supplementation is an appropriate therapeutic approach for oral squamous cell carcinoma. Melatonin might inhibit cancer cells through the regulation of molecular pathways including AKT/mTOR pathway, ERK/AKT signaling, LSD1 expression and tumor-associated neutrophils releasing. Limited clinical studies; however, have evaluated the role of melatonin in oral squamous cell carcinoma. This review summarizes current knowledge and evidence regarding the effects of melatonin on oral squamous cell carcinoma and the mechanisms involved.
References
2. Gillison ML (2007) Current topics in the epidemiology of oral cavity and oropharyngeal cancers. Head & neck 29 (8): 779-792.
3. Scully C & Bagan J (2009) Oral squamous cell carcinoma overview. Oral Oncol. 45 (4/5): 301-308.
4. Gandolfo S, et al. (2004) Risk of oral squamous cell carcinoma in 402 patients with oral lichen planus: a follow-up study in an Italian population. Oral Oncol. 40 (1): 77-83.
5. Miller CS & Johnstone BM (2001) Human papillomavirus as a risk factor for oral squamous cell carcinoma: a meta-analysis, 1982-1997. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 91 (6): 622-635.
6. Fan H, et al. (2017) Nrf2 regulates cellular behaviors and Notch signaling in oral squamous cell carcinoma cells. Biochem. Biophys. Res. Commun. 493 (1): 833-839.
7. Juneja S, Rathore AS, Sharma K, Shetty D, & Jain A (2017) Antioxidant-oxidant index as a biomarker in oral potentially malignant disorders and oral squamous cell carcinoma: a biochemical study. JCDR. 11 (3): Zc05-zc08.
8. Pedro NF, et al. (2018) Candidate biomarkers for oral squamous cell carcinoma: differential expression of oxidative stress-related genes. APJCP. 19 (5): 1343-1349.
9. Sant'Anna-Silva ACB, et al. (2018) Metabolic profile of oral squamous carcinoma cell lines relies on a higher demand of lipid metabolism in metastatic cells. Front Oncol. 8: 13. doi: 10.3389/fonc.2018.00013. eCollection 2018
10. Garley M, et al. (2018) Differences and similarities in the phenomenon of NETs formation in oral inflammation and in oral squamous cell carcinoma. J. Cancer 9 (11): 1958-1965.
11. Goertzen C, et al. (2018) Oral inflammation promotes oral squamous cell carcinoma invasion. Oncotarget 9 (49): 29047-29063.
12. Nasry WHS, Rodriguez-Lecompte JC, & Martin CK (2018) Role of COX-2/PGE2 Mediated Inflammation in Oral Squamous Cell Carcinoma. Cancers (Basel) 10 (10): pii: E348. doi: 10.3390/cancers10100348.
13. Brown DC & Gatter KC (2002) Ki67 protein: the immaculate deception? Histopathology 40 (1): 2-11.
14. Kim SJ, et al. (2007) Prognostic value of carbonic anhydrase IX and Ki-67 expression in squamous cell carcinoma of the tongue. Jpn. J. Clin. Oncol. 37 (11): 812-819.
15. Wang L, et al. (2006) Activation of ERK1/2 and cyclin D1 expression in oral tongue squamous cell carcinomas: relationship between clinicopathological appearances and cell proliferation. Oral Oncol. 42 (6): 625-631.
16. Kunkel M, et al. (2003) Overexpression of Glut‐1 and increased glucose metabolism in tumors are associated with a poor prognosis in patients with oral squamous cell carcinoma. Cancer 97 (4): 1015-1024.
17. Xia W, et al. (1999) Combination of EGFR, HER-2/neu, and HER-3 is a stronger predictor for the outcome of oral squamous cell carcinoma than any individual family members. Clin. Cancer Res. 5 (12): 4164-4174.
18. Lo WL, Kao SY, Chi LY, Wong YK, & Chang RC (2003) Outcomes of oral squamous cell carcinoma in Taiwan after surgical therapy: factors affecting survival. J. Oral Maxillofac. Surg. 61 (7):751-758.
19. Elango N, Samuel S, & Chinnakkannu P (2006) Enzymatic and non-enzymatic antioxidant status in stage (III) human oral squamous cell carcinoma and treated with radical radio therapy: influence of selenium supplementation. Clin. Chim. Acta 373 (1-2): 92-98.
20. Goncalves Ndo N, et al. (2014) Molecular markers of angiogenesis and metastasis in lines of oral carcinoma after treatment with melatonin. Anticancer Agents Med. Chem. 14 (9): 1302-1311.
21. Nakamura E, et al. (2008) Frequent silencing of a putative tumor suppressor gene melatonin receptor 1 A (MTNR1A) in oral squamous-cell carcinoma. Cancer Sci. 99 (7): 1390-1400.
22. Zwirska-Korczala K, et al. (2004) Influence of extremely-low-frequency magnetic field on antioxidative melatonin properties in AT478 murine squamous cell carcinoma culture. Biol. Trace Elem. Res. 102 (1-3): 227-243.
23. Klein DC & Moore RY (1979) Pineal N-acetyltransferase and hydroxyindole-O-methyl-transferase: control by the retinohypothalamic tract and the suprachiasmatic nucleus. Brain Res. 174 (2): 245-262.
24. Tan DX, Manchester LC, Esteban-Zubero E, Zhou Z, & Reiter RJ (2015) Melatonin as a Potent and Inducible Endogenous Antioxidant: Synthesis and Metabolism. Molecules 20 (10): 18886-18906.
25. Reiter RJ, et al. (2017) Melatonin as a mitochondria-targeted antioxidant: one of evolution’s best ideas. Cell. Mol. Life Sci. 74 (21): 3863-3881.
26. Andersen LPH, Gögenur I, Rosenberg J, & Reiter RJ (2016) The safety of melatonin in humans. Clin. Drug Investig. 36 (3): 169-175.
27. Tamtaji OR, et al. (2018) Clinical and metabolic response to probiotic administration in people with Parkinson's disease: a randomized, double-blind, placebo-controlled trial. Clin Nutr. S0261-5614 (18): 30203-6. doi: 10.1016/j.clnu.2018.05.018 Clinical Nutrition.
28. Hardeland R (2018) Melatonin and inflammation - story of a double-edged blade. J Pineal Res. 65 (4): e12525. doi: 10.1111/jpi.12525.
29. Carrascal L, Nunez-Abades P, Ayala A, & Cano M (2018) Role of melatonin in the inflammatory process and its therapeutic potential. Curr. Pharm. Des. 24 (14): 1563-1588. doi:10.2174/1381612824666180426112832.
30. Chen Y, et al. (2018) Melatonin induces anti-inflammatory effects via endoplasmic reticulum stress in RAW264.7 macrophages. Mol. Med. Rep. 17 (4): 6122-6129.
31. El-Bakry HA, Ismail IA, & Soliman SS (2018) Immunosenescence-like state is accelerated by constant light exposure and counteracted by melatonin or turmeric administration through DJ-1/Nrf2 and P53/Bax pathways. J. Photochem. Photobiol. B. 186: 69-80. doi: 10.1016/j.jphotobiol.2018.07.003.
32. Abadi S, et al. (2018) The effect of melatonin on Superoxide dismutase and Glutathione peroxidase activity, and Malondialdehyde levels in the targeted and the non-targeted lung and heart tissues after irradiation in xenograft mice colon cancer. Curr. Mol. Pharmacol. 11 (4): 326-335.
33. Chang CC, et al. (2018) Protective Effect of melatonin against oxidative stress-induced apoptosis and enhanced autophagy in human retinal pigment epithelium cells. Oxid. Med. Cell Longev. 2018: 9015765.
34. Tamtaji OR, Mirhosseini N, Reiter RJ, Behnamfar M, & Asemi Z (2019) Melatonin and pancreatic cancer: Current knowledge and future perspectives. J. Cell Physiol. 234 (5): 5372-5378. doi: 10.1002/jcp.27372.
35. Chen CC, et al. (2018) Melatonin sensitizes hepatocellular carcinoma cells to chemotherapy through long non-coding RNA RAD51-AS1-mediated suppression of DNA repair. Cancers. 10 (9): pii: E320.
36. El-Sokkary GH, Ismail IA, & Saber SH (2019) Melatonin inhibits breast cancer cell invasion through modulating DJ-1/KLF17/ID-1 signaling pathway. J. Cell Biochem. 120 (3): 3945-3957. doi: 10.1002/jcb.27678.
37. Yang CY, et al. (2017) Melatonin exerts anti-oral cancer effect via suppressing LSD1 in patient-derived tumor xenograft models. Oncotarget. 8 (20): 33756-33769.
38. Lu H, et al. (2017) Melatonin represses oral squamous cell carcinoma metastasis by inhibiting tumor-associated neutrophils. Am. J. Transl. Res. 9 (12): 5361.
39. Tan M, et al. (2018) Inhibiting ROS-TFE3-dependent autophagy enhances the therapeutic response to metformin in breast cancer. Free Radic. Res. 52 (8): 872-886.
40. Deng F, Xu Q, Long J, & Xie H (2018) Suppressing ROS‐TFE3‐dependent autophagy enhances ivermectin‐induced apoptosis in human melanoma cells. J. Cell Biochem. doi: 10.1002/jcb.27490.
41. Fan T, et al. (2018) Inhibiting MT2-TFE3-dependent autophagy enhances melatonin-induced apoptosis in tongue squamous cell carcinoma. J. Pineal Res. 64 (2): e12457. doi: 10.1111/jpi.12457.
42. Datta SR, Brunet A, & Greenberg ME (1999) Cellular survival: a play in three Akts. Genes Dev. 13 (22): 2905-2927.
43. Porta C, Paglino C, & Mosca A (2014) Targeting PI3K/Akt/mTOR signaling in cancer. Front Oncol. 4: 64. doi: 10.3389/fonc.2014.00064.
44. Fruman DA, Meyers RE, & Cantley LC (1998) Phosphoinositide kinases. Annu. Rev. Biochem. 67: 481-507.
45. Kumar CC & Madison V (2005) AKT crystal structure and AKT-specific inhibitors. Oncogene. 24 (50): 7493-7501.
46. Burris HA (2013) Overcoming acquired resistance to anticancer therapy: focus on the PI3K/AKT/mTOR pathway. Cancer Chemother. Pharmacol. 71 (4): 829-842.
47. Polivka J, Jr. & Janku F (2014) Molecular targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol. Ther. 142 (2): 164-175.
48. Janku F, et al. (2011) PIK3CA mutations in patients with advanced cancers treated with PI3K/AKT/mTOR axis inhibitor. Mol. Cancer Ther. 10 (3): 558-565.
49. Morgensztern D & McLeod HL (2005) PI3K/Akt/mTOR pathway as a target for cancer therapy. Anticancer Drugs. 16 (8): 797-803.
50. Shimizu T, et al. (2012) The clinical effect of the dual-targeting strategy involving PI3K/AKT/mTOR and RAS/MEK/ERK pathways in patients with advanced cancer. Clin. Cancer Res. 18 (8): 2316-2325.
51. Li X, et al. (2018) Efficacy of PI3K/AKT/mTOR pathway inhibitors for the treatment of advanced solid cancers: A literature-based meta-analysis of 46 randomised control trials. PloS one 13 (2): e0192464. doi: 10.1371/journal.pone.0192464.
52. Munoz-Cordero MG, et al. (2019) Predictive value of EGFR-PI3K-pAKT-mTOR-pS6 pathway in sinonasal squamous cell carcinomas. Acta Otorrinolaringol. Esp. 70 (1):16-24. doi: 10.1016/j.otorri.2017.10.005.
53. Wang Q, Zhang X, Song X, & Zhang L (2018) Overexpression of T-cadherin inhibits the proliferation of oral squamous cell carcinoma through the PI3K/AKT/mTOR intracellular signalling pathway. Arch. Oral Biol. 96: 74-79.
54. Wu N, et al. (2018) The expression and prognostic impact of the PI3K/AKT/mTOR signaling pathway in advanced esophageal squamous cell carcinoma. Technol. Ccancer Res. Treat. 17: 1533033818758772. doi: 10.1177/1533033818758772.
55. Cao J, et al. (2018) Ricolinostat (ACY-1215) suppresses proliferation and promotes apoptosis in esophageal squamous cell carcinoma via miR-30d/PI3K/AKT/mTOR and ERK pathways. Cell death Dis. 9 (8): 817.
56. Choi MS, et al. (2018) Adenosine induces intrinsic apoptosis via the PI3K/Akt/mTOR signaling pathway in human pharyngeal squamous carcinoma FaDu cells. Oncol. Lett. 15 (5): 6489-6496.
57. Zhu X & Zhu R (2018) Curcumin suppresses the progression of laryngeal squamous cell carcinoma through the upregulation of miR-145 and inhibition of the PI3K/Akt/mTOR pathway. OncoTargets Ther. 11: 3521-3531.
58. Shen YQ, et al. (2018) Combination of melatonin and rapamycin for head and neck cancer therapy: suppression of AKT/mTOR pathway activation, and activation of mitophagy and apoptosis via mitochondrial function regulation. J. Pineal Res. 64 (3). e12461. doi: 10.1111/jpi.12461.
59. Ye Q & She Q (2013) Integration of AKT and ERK signaling pathways in cancer: biological and therapeutic implications. J. Pharmacol. Clin. Toxicol. 1: 1009.
60. Liao B, Zhou H, Liang H, & Li C (2017) Regulation of ERK and AKT pathways by hepatitis B virus X protein via the Notch1 pathway in hepatocellular carcinoma. Int. J. Oncol. 51 (5): 1449-1459.
61. Li J, et al. (2016) pERK/pAkt phenotyping in circulating tumor cells as a biomarker for sorafenib efficacy in patients with advanced hepatocellular carcinoma. Oncotarget. 7 (3): 2646-2659.
62. Liu X, Tian S, Liu M, Jian L, & Zhao L (2016) Wogonin inhibits the proliferation and invasion, and induces the apoptosis of HepG2 and Bel7402 HCC cells through NFkappaB/Bcl-2, EGFR and EGFR downstream ERK/AKT signaling. Int. J. Mol. Med. 38 (4): 1250-1256.
63. Zhu P, et al. (2017) GPER/ERK&AKT/NF-kappaB pathway is involved in cadmium-induced proliferation, invasion and migration of GPER-positive thyroid cancer cells. Mol. Cell Endocrinol. 442: 68-80.
64. Liu QG, Li YJ, & Yao L (2018) Knockdown of AGR2 induces cell apoptosis and reduces chemotherapy resistance of pancreatic cancer cells with the involvement of ERK/AKT axis. Pancreatology 18 (6): 678-688.
65. Zhang B, Tao F, & Zhang H (2018) Metastasis-associated protein 2 promotes the metastasis of non-small cell lung carcinoma by regulating the ERK/AKT and VEGF signaling pathways. Mol. Med. Rep. 17 (4): 4899-4908.
66. Hoang TT, Tanrikulu IC, Vatland QA, Hoang TM, & Raines RT (2018) A human ribonuclease variant and ERK-pathway inhibitors exhibit highly synergistic toxicity for cancer cells. Mol. Cancer Ther. 17 (12): 2622-2632.
67. Jiao YN, et al. (2018) Marsdenia tenacissima extract induces apoptosis and suppresses autophagy through ERK activation in lung cancer cells. Cancer cell international. 18: 149. doi: 10.1186/s12935-018-0646-4.
68. Pereira SS, et al. (2018) MAPK/ERK pathway inhibition is a promising treatment target for adrenocortical tumors. J. Cell Biochem. 120 (1): 894-906
69. Ng HY, et al. (2018) Chemotherapeutic Treatments Increase PD-L1 Expression in Esophageal Squamous Cell Carcinoma through EGFR/ERK Activation. Transl. Ooncol. 11 (6): 1323-1333.
70. Lu YX, et al. (2016) Melatonin enhances sensitivity to fluorouracil in oesophageal squamous cell carcinoma through inhibition of Erk and Akt pathway. Cell Death Dis. 7 (10): e2432. doi: 10.1038/cddis.2016.330.
71. Jiang B, et al. (2004) Temporal control of NF-κB activation by ERK differentially regulates interleukin-1β-induced gene expression. J. Biol. Chem. 279 (2): 1323-1329.
72. Karin M, Cao Y, Greten FR, & Li Z-W (2002) NF-κB in cancer: from innocent bystander to major culprit. Nat. Rev. Cancer. 2 (4): 301.
73. Wang T, et al. (2005) Co-activation of ERK, NF-κB and GADD45β in response to ionizing radiation. J. Biol. Chem. 280 (13): 12593-12601.
74. Lu J-J, et al. (2016) Melatonin inhibits AP-2β/hTERT, NF-κB/COX-2 and Akt/ERK and activates caspase/Cyto C signaling to enhance the antitumor activity of berberine in lung cancer cells. Oncotarget. 7 (3): 2985-3001.
75. Liu L, et al. (2016) Inhibition of ERK1/2 signaling pathway is involved in melatonin's antiproliferative effect on human MG-63 osteosarcoma cells. Cell Physiol. Biochem. 39 (6): 2297-2307.
76. Shi Y, et al. (2004) Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 119 (7): 941-953.
77. Chen Y, et al. (2006) Crystal structure of human histone lysine-specific demethylase 1 (LSD1). Proc. Nati. Acad. Sci. 103 (38): 13956-13961.
78. Lee MG, Wynder C, Cooch N, & Shiekhattar R (2005) An essential role for CoREST in nucleosomal histone 3 lysine 4 demethylation. Nature 437 (7057): 432-435.
79. Cao C, et al. (2017) Functional interaction of histone deacetylase 5 (HDAC5) and lysine-specific demethylase 1 (LSD1) promotes breast cancer progression. Oncogene 36 (1): 133-145.
80. Yang Y, et al. (2018) LSD1 coordinates with the SIN3A/HDAC complex and maintains sensitivity to chemotherapy in breast cancer. J. Mol. cell Biol. 10 (4): 285-301.
81. Boulding T, et al. (2018) LSD1 activation promotes inducible EMT programs and modulates the tumour microenvironment in breast cancer. Sci. Rep. 8 (1): 73. doi: 10.1038/s41598-017-17913-x.
82. Chao A, et al. (2017) Lysine-specific demethylase 1 (LSD1) destabilizes p62 and inhibits autophagy in gynecologic malignancies. Oncotarget. 8 (43): 74434-74450.
83. Kumarasinghe IR & Woster PM (2018) Cyclic peptide inhibitors of lysine-specific demethylase 1 with improved potency identified by alanine scanning mutagenesis. Eur. J. Med. Chem. 148: 210-220.
84. Qin Y, et al. (2019) Inhibition of histone lysine-specific demethylase 1 elicits breast tumor immunity and enhances antitumor efficacy of immune checkpoint blockade. Oncogene 38: 390–405
85. Sarno F, et al. (2018) 3-Chloro-N'-(2-hydroxybenzylidene) benzohydrazide: An LSD1-Selective Inhibitor and Iron-Chelating Agent for Anticancer Therapy. Front Pharmacol. 9: 1006.
86. Shao G, et al. (2018) Inhibition of lysine-specific demethylase 1 prevents proliferation and mediates cisplatin sensitivity in ovarian cancer cells. Oncol. Lett. 15 (6): 9025-9032.
87. Xu S, et al. (2018) Optimization of 5-arylidene barbiturates as potent, selective, reversible LSD1 inhibitors for the treatment of acute promyelocytic leukemia. Bioorg. Med. Chem. 26 (17): 4871-4880.
88. Lu Z, et al. (2018) FLI-06 suppresses proliferation, induces apoptosis and cell cycle arrest by targeting LSD1 and Notch pathway in esophageal squamous cell carcinoma cells. Biomed. Pharmacother. 107: 1370-1376.
89. Yu Y, et al. (2013) High expression of lysine-specific demethylase 1 correlates with poor prognosis of patients with esophageal squamous cell carcinoma. Biochem. Biophys. Res. Commun. 437 (2): 192-198.
90. Hurt B, Schulick R, Edil B, El Kasmi KC, & Barnett C, Jr. (2017) Cancer-promoting mechanisms of tumor-associated neutrophils. Am. J. Surg. 214 (5): 938-944.
91. Manfroi B, et al. (2018) Tumor-associated neutrophils correlate with poor prognosis in diffuse large B-cell lymphoma patients. Blood cancer J. 8 (7): 66.
92. Fridlender ZG, et al. (2012) Transcriptomic analysis comparing tumor-associated neutrophils with granulocytic myeloid-derived suppressor cells and normal neutrophils. PLoS One 7 (2): e31524. doi: 10.1371/journal.pone.0031524.
93. Silva RNF, et al. (2018) Immunohistochemical analysis of neutrophils, interleukin-17, matrix metalloproteinase-9, and neoformed vessels in oral squamous cell carcinoma. J. Oral Pathol. Med. 47 (9): 856-863.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


