Antioxidant activity of pineal methoxyindoles on hepatocyte plasmatic membrane
Antioxidant activity of methoxyindoles on cell membrane.
Abstract
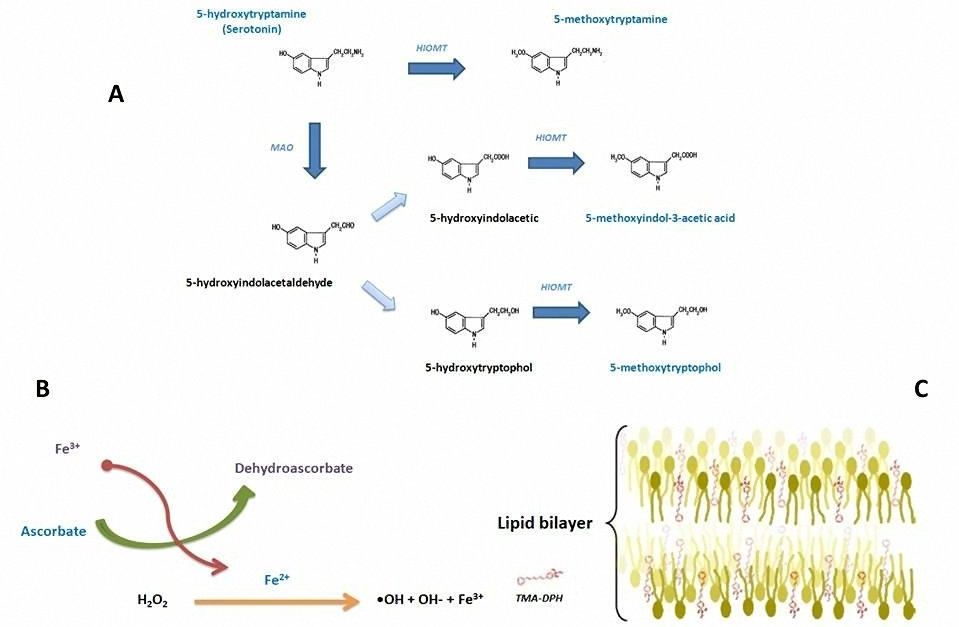
Antioxidant effect of several pineal derived molecules has been well documented. Here, the protective effects of 5-methoxytryptophol (5-MTOH) and 5-methoxyindol-3-acetic acid (5-MIAA) on hepatic cell membrane lipid peroxidation and cell membrane rigidity induced by FeCl3 plus ascorbic acid have been systemically investigated. The membrane fluidity was evaluated by fluorescence spectroscopy, malondialdehyde (MDA) and 4-hydroxyalkenals (4-HDA) concentrations and carbonyl groups of protein were measured as the parameters of lipid and protein damage, respectively. Results showed that oxidative stress increased membrane rigidity, MDA and 4-HDA concentrations as well as carbonyl content in a concentration-dependent manner. 5-MTOH, but not 5-MIAA, significantly attenuated these oxidative indecies. In absence of oxidative stress, none of these methoxyindoleamines modified the content of MDA, 4-HDA or carbonylation. However 5-MIAA at its highest concentration slightly modified membrane fluidity. The results suggest that structural modification of C3 in the methoxyindoleamine, that is, the carboxyl group replaced by hydroxyl group in this site could improve the ability of 5-methoxyindoleamine derivatives to preserve membrane fluidity of cells which are under oxidative stress.
References
2. Zawilska JB, Sadowska M. (2002) Prolonged treatment with glucocorticoid dexa-methasone suppresses melatonin production by the chick pineal gland and retina. Pol. J. Pharmacol. 54: 61-66.
3. Mhatre MC, van Jaarsveld AS, Reiter RJ. (1988) Melatonin in the lacrimal gland: first demonstration and experimental manipulation. Biochem. Biophys. Res. Com-mun. 153: 1186-1192.
4. Ananth C, Gopalakrishnakone P, Kaur C. (2003) Protective role of melatonin in domoic acid-induced neuronal damage in the hippocampus of adult rats. Hippo-campus 13: 375-387.
5. Söderquist F, Hellström PM, Cunningham JL. (2015) Human gastroenteropancreat-ic expression of melatonin and its receptors MT1 and MT2. PLoS One 10 (3): e0120195. doi: 10.1371/journal.pone.0120195.
6. Reiter RJ, Rosales-Corral SA, Manchester LC, et al. (2014) Melatonin in the bili-ary tract and liver: health implications. Curr. Pharm. Des. 20: 4788-4801
7. Tan DX, Manchester LC, Reiter RJ, et al. (1999) High physiological levels of mel-atonin in the bile of mammals. Life Sci. 65: 2523-2529.
8. Fernández BE, Díaz E, Fernández C, et al. (2013) Ovarian aging: melatonin regula-tion of the cytometric and endocrine evolutive pattern. Curr. Aging Sci. 6: 1-7.
9. Guerrero JM, Reiter RJ. (2002) Melatonin-immune system relationships. Curr. Top Med. Chem. 2: 167-179.
10. Poeggeler B, Thuermann S, Dose A, et al. (2002) Melatonin's unique radical scav-enging properties - roles of its functional substituents as revealed by a comparison with its structural analogs. J. Pineal Res. 33: 20-30.
11. Parada E, Buendia I, León R, et al. (2014) Neuroprotective effect of melatonin against ischemia is partially mediated by alpha-7 nicotinic receptor modulation and HO-1 overexpression. J. Pineal Res. 56: 204-212. doi: 10.1111/jpi.12113.
12. Esteban-Zubero E, Alatorre-Jiménez MA, López-Pingarrón L, et al. (2016) Mela-tonin's role in preventing toxin-related and sepsis-mediated hepatic damage: A re-view. Pharmacol. Res. 105: 108-120. doi: 10.1016/j.phrs.2016.01.018.
13. García JJ, Reiter RJ, Guerrero JM, et al. (1997) Melatonin prevents changes in mi-crosomal membrane fluidity during induced lipid peroxidation. FEBS Lett. 408: 297-300.
14. Reiter RJ, Tan DX, Galano A. (2014) Melatonin reduces lipid peroxidation and membrane viscosity. Front Physiol. 5: 377. doi: 10.3389/fphys.2014.00377
15. Galano A, Medina ME, Tan DX, et al. (2015) Melatonin and its metabolites as copper chelating agents and their role in inhibiting oxidative stress: a physico-chemical analysis. J. Pineal Res. 58: 107-116. doi: 10.1111/jpi.12196.
16. Gavazza M, Catala A. (2003) Melatonin preserves arachidonic and docosapentae-noic acids during ascorbate-Fe2+ peroxidation of rat testis microsomes and mito-chondria. Int. J. Biochem. Cell Biol. 35: 359-366.
17. Guajardo MH, Terrasa AM, Catala A. (2003) Protective effect of indoleamines on in vitro ascorbate-Fe2+ dependent lipid peroxidation of rod outer segment mem-branes of bovine retina. J. Pineal Res. 35: 276-282.
18. Millán-Plano S, Piedrafita E, Miana-Mena FJ, et al. (2010) Melatonin and struc-turally-related compounds protect synaptosomal membranes from free radical damage. Int. J. Mol. Sci. 11: 312-328.
19. García JJ, Reiter RJ, Cabrera JJ, et al. (2000) 5-methoxytryptophol preserves he-patic microsomal membrane fluidity during oxidative stress. J. Cell Biochem. 76: 651-657.
20. Yu BP, Suescun EA, Yang SY. (1992) Effect of age-related lipid peroxidation on membrane fluidity and phospholipase A2: modulation by dietary restriction. Mech. Ageing Dev. 65: 17-33.
21. Lerner AB, Case JD, Takahashi Y, et al. (1958) Isolation of melatonin, the pineal gland factor that lightens melanocytes. J. Am. Chem. Soc. 80: 2587-2587.
22. McIsaac WM, Farrell G, Taborsky RG, et al. (1965) Indole Compounds: Isolation from Pineal Tissue. Science 148: 102-103.
23. Ceinos RM, Rábade S, Soengas JL, et al. (2005) Indoleamines and 5-methoxyindoles in trout pineal organ in vivo: daily changes and influence of pho-toperiod. Gen. Comp. Endocrinol. 144: 67-77.
24. Míguez JM, Recio J, Sánchez-Barceló E, et al. (1998) Changes with age in daytime and nighttime contents of melatonin, indoleamines, and catecholamines in the pin-eal gland: a comparative study in rat and Syrian hamster. J. Pineal Res. 25: 106-115.
25. Míguez JM, Recio J, Vivien-Roels B, et al. (1996) Diurnal changes in the content of indoleamines, catecholamines, and methoxyindoles in the pineal gland of the Djungarian hamster (Phodopus sungorus): effect of photoperiod. J. Pineal Res. 21: 7-14.
26. Skene DJ, Smith I, Arendt J. (1986) Radioimmunoassay of pineal 5-methoxytryptophol in different species: comparison with pineal melatonin content. J. Endocrinol. 110: 177-184.
27. Zawilska JB, Vivien-Roels B, Skene DJ, et al. (2000) Phase-shifting effects of light on the circadian rhythms of 5-methoxytryptophol and melatonin in the chick pineal gland. J. Pineal Res. 29: 1-7.
28. Zawilska JB, Skene DJ, Nowak JZ. (1998) 5-Methoxytryptophol rhythms in the chick pineal gland: effect of environmental lighting conditions. Neurosci. Lett. 251: 33-36.
29. Hofman MA, Skene DJ, Swaab DF. (1995) Effect of photoperiod on the diurnal melatonin and 5-methoxytryptophol rhythms in the human pineal gland. Brain Res. 671: 254-260.
30. Blanc A, Vivien-Roels B, Pévet P, et al. (2003) Melatonin and 5-methoxytryptophol (5-ML) in nervous and/or neurosensory structures of a gastro-pod mollusc (Helix aspersa maxima): synthesis and diurnal rhythms. Gen. Comp. Endocrinol. 131: 168-175.
31. Vivien-Roels B, Pevet P, Masson-Pevet M, et al. (1992) Seasonal variations in the daily rhythm of pineal gland and/or circulating melatonin and 5-methoxytryptophol concentrations in the European hamster, Cricetus cricetus. Gen. Comp. Endocrinol. 86: 239-247.
32. Mattam U, Jagota A. (2015) Daily rhythms of serotonin metabolism and the ex-pression of clock genes in suprachiasmatic nucleus of rotenone-induced Parkin-son's disease male Wistar rat model and effect of melatonin administration. Bi-ogerontology 16: 109-123. doi: 10.1007/s10522-014-9541-0.
33. Molina-Carballo A, Muñoz-Hoyos A, Martin-García JA, et al. (1996) 5-Methoxytryptophol and melatonin in children: differences due to age and sex. J. Pineal Res. 21: 73-79.
34. Malpaux B, Thiéry JC, Chemineau P. (1999) Melatonin and the seasonal control of reproduction. Reprod. Nutr. Dev. 39: 355-366.
35. Wang H. Ng TB. (2000) Hypotensive activity of the pineal indoleamine hormones melatonin, 5- methoxytryptophol and 5-methoxytryptamine. Pharmacol. Toxicol. 86: 125-128.
36. Conti V, Corbi G, Simeon V, et al. (2015) Aging-related changes in oxidative stress response of human endothelial cells. Aging Clin. Exp. Res. 27: 547-553. doi: 10.1007/s40520-015-0357-9.
37. Gebicki JM. (2016) Oxidative stress, free radicals and protein peroxides. Arch. Biochem. Biophys. 595: 33-39. doi: 10.1016/j.abb.2015.10.021.
38. Fenton HJH. (1894) Oxidation of tartaric acid in presence of iron. J. Chem. Soc. 65: 899-910.
39. Fairweather-Tait SJ, Wawer AA, Gillings R, et al. (2014) Iron status in the elderly. Mech. Ageing Dev. 136-137: 22-28. doi: 10.1016/j.mad.2013.11.005.
40. Sahu SC, Washington MC. (1992) Effect of ascorbic acid and curcumin on querce-tin-induced nuclear DNA damage, lipid peroxidation and protein degradation. Can-cer Lett. 63: 237-241.
41. Abe K, Takai N, Fukumoto K, et al. (2014) In vivo imaging of reactive oxygen species in mouse brain by using [3H]-hydromethidine as a potential radical trap-ping radiotracer. J. Cereb. Blood Flow Metab. 34: 1907-1913. doi: 10.1038/jcbfm.2014.160.
42. Cadenas E, Simic MG, Sies H. (1989) Antioxidant activity of 5-hydroxytryptophan, 5-hydroxyindole, and DOPA against microsomal lipid peroxi-dation and its dependence on vitamin E. Free Radic. Res. Commun. 6: 11-17.
43. Millán-Plano S, García JJ, Martínez-Ballarín E, et al. (2003) Melatonin and pino-line prevent aluminium-induced lipid peroxidation in rat synaptosomes. J. Trace Elem. Med. Biol. 17: 39-44.
44. Wong SF, Halliwell B, Richmond R, et al. (1981) The role of superoxide and hy-droxyl radicals in the degradation of hyaluronic acid induced by metal ions and by ascorbic acid. J. Inorg. Biochem. 14: 127-134.
45. Lysek N, Kinscherf R, Claus R, et al. (2003) L-5-Hydroxytryptophan: antioxidant and anti-apoptotic principle of theintertidal sponge Hymeniacidon heliophila Z. Naturforsch [C] 58: 568-572.
46. Rice-Evans C, Burdon R. (1993) Free radical-lipid interactions and their pathologi-calconsequences. Prog. Lipid Res. 32: 71-110.
47. Bradford MM. (1976) A rapid and sensitive method for the quantitation of mi-crogram quantities ofprotein utilizing the principle of protein-dye binding. Anal Biochem. 72: 248-254.
48. Janero DR. (1990) Malondialdehyde and thiobarbituric acid-reactivity as diagnos-tic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Biol. Med. 9: 515-540.
49. Valenzuela A. (1991) The biological significance of malondialdehyde determina-tion in the assessment of tissue oxidative stress. Life Sci. 48: 301-309.
50. Levine RL, Garland D, Oliver CN, et al. (1990) Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 186: 464-478.
51. Levine RL, Williams JA, Stadtman ER, et al. (1994) Carbonyl assays for determi-nation of oxidatively modified proteins. Methods Enzymol. 233: 346-357.
52. Dean RT, Fu S, Stocker R, et al. (1997) Biochemistry and pathology of radical-mediated protein oxidation. Biochem. J. 324 (Pt 1): 1-18.
53. Millán-Plano S, García JJ, Martínez-Ballarín E, et al. (2002) Strontium effects on lipid peroxidation due to FeCl 3 and ascorbicacid in rat synaptosomes, in Metal Ions in Biology and Medicine, Khassanova L, et al., Editors. John Libbey Eurotext: París. 502-505.
54. Ghosh C, Dick RM, Ali SF. (1993) Iron/ascorbate-induced lipid peroxidation changesmembrane fluidity and muscarinic cholinergic receptor binding in rat frontal cortex. Neurochem. Int. 23: 479-484.
55. Aleardi AM, Benard G, Augereau O, et al. (2005) Gradual alteration of mitochon-drial structure and function by beta-amyloids: importance of membrane viscosity changes, energy deprivation, reactive oxygen species production, and cytochrome c release. J. Bioenerg. Biomembr. 37: 207-225.
56. Maturu P, Vaddi DR, Pannuru P, et al. (2010) Alterations in erythrocyte membrane fluidity and Na+/K+ -ATPase activity in chronic alcoholics. Mol. Cell Biochem. 339: 35-42. doi: 10.1007/s11010-009-0367-z.
57. Tai WY, Yang YC, Lin HJ, et al. (2010) Interplay between structure and fluidity of model lipid membranes under oxidative attack. J. Phys. Chem. B. 114: 15642-15649. doi: 10.1021/jp1014719.
58. Calhoon EA, Ro J, Williams JB. (2015) Perspectives on the membrane fatty acid unsaturation/pacemaker hypotheses of metabolism and aging. Chem. Phys. Lipids. 191: 48-60. doi: 10.1016/j.chemphyslip.2015.08.008.
59. Dmitriev LF. (2001) Activity of key enzymes in microsomal and mitochondrial membranes depends on the redox reactions involving lipid radicals. Membr. Cell Biol. 14: 649-662.
60. Mayo JC, Tan DX, Sainz RM, et al. (2003) Protection against oxidative protein damage induced by metal-catalyzedreaction or alkylperoxyl radicals: comparative effects of melatonin and other antioxidants. Biochim. Biophys. Acta. 1620: 139-150.
61. Reiter RJ, Paredes SD, Korkmaz A et al. (2008) Melatonin in relation to the "strong" and "weak" versions of the free radicaltheory of aging. Adv. Med. Sci. 53: 119-129. doi: 10.2478/v10039-008-0032-x.
62. Wang HX, Liu F, Ng TB. (2001) Examination of pineal indoles and 6-methoxy-2-benzoxazolinone for antioxidant and antimicrobial effects. Comp. Biochem. Phys-iol. C. Toxicol. Pharmacol. 130: 379-388.
63. Ng TB, Liu F, Zhao L. (2000) Antioxidative and free radical scavenging activities of pinealindoles. J. Neural Transm. (Vienna) 107: 1243-1251.
64. Wang P, Kang J, Zheng R, et al. (1996) Scavenging effects of phenylpropanoid glycosides from Pedicularis on superoxide anion and hydroxyl radical by the spin trapping method (95) 02255-4. Biochem. Pharmacol. 51: 687-691.
65. Poeggeler B, Reiter RJ, Hardeland R, et al. (1996) Melatonin and structurally-related, endogenous indoles act as potent electron donors and radical scavengers in vitro. Redox Rep. 2: 179-184.
66. Burczynski JM, Southard SJ, Hayes JR, et al. (2001) Changes in mitochondrial and microsomal lipid peroxidation and fatty acid profiles in adrenal glands, testes, and livers from alpha-tocopherol-deficient rats. Free Radic. Biol. Med. 30: 1029-1035.
67. Kazanci N, Severcan F. (2007) Concentration dependent different action of tamoxi-fen on membrane fluidity. Biosci. Rep. 27: 247-255.
68. Ortega-Gutiérrez S, García JJ, Martínez-Ballarín E, et al. (2002) Melatonin im-proves deferoxamine antioxidant activity in protectingagainst lipid peroxidation caused by hydrogen peroxide in rat brain homogenates. Neurosci. Lett. 323: 55-59.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


