Melatonin attenuates cardiac injury caused by chromium-mediated oxidative stress in male Wistar rats: involvement of antioxidative mechanisms
Melatonin protects chromium-induced cardiac injuries
Abstract
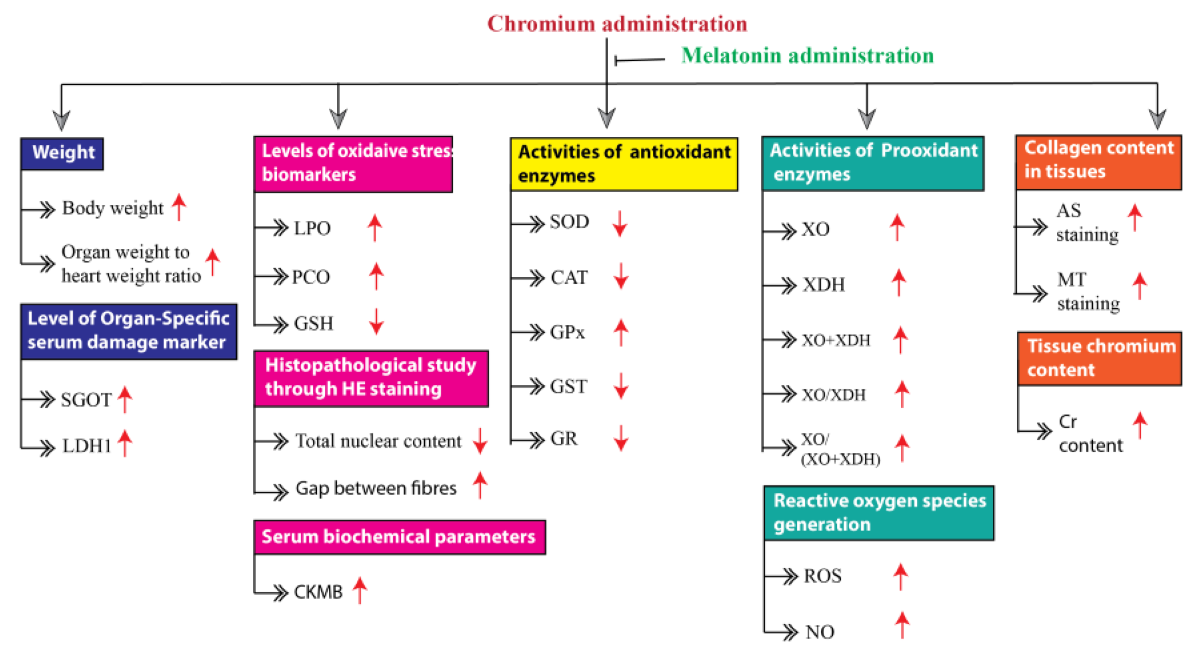
Cardiovascular disease (CVD) is a global health concern due to its high mortality. Heavy metals are the potential risk factor for CVD. Among other heavy metals, chromium (Cr) is considered a serious threat to human health due to its high oxidative capacity. In the current study, male Wistar rats were treated with Cr to induce cardiac tissue injuries, meanwhile, melatonin was given to test whether this treatment can protect against Cr-induced cardiac damage. The results showed that Cr markedly altered the heart weight, biomarkers of oxidative stress, activities of antioxidant and pro-oxidant enzymes, as well as tissue morphology. On contrary, melatonin treatment significantly suppressed all these alterations via its antioxidant activity. In addition, melatonin also significantly reduced tissue Cr concentration probably through its metal-chelating activity. The current study has demonstrated that melatonin is a promising antioxidant to protect the heart from Cr-induced oxidative damage, confirming that melatonin can be a future therapeutic agent for Cr-mediated toxicity in the heart or other organs.
References
2. Cosselman KE, Navas-Acien A, Kaufman JD (2015) Environmental factors in cardiovascular disease. Nat. Rev. Cardiol. 12 (11): 627–642. DOI: 10.1038/nrcardio.2015.152.
3. Ali H, Khan E, Ilahi I (2019) Environmental chemistry and ecotoxicology of hazardous heavy metals: environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019 : 1–14. DOI: 10.1155/2019/6730305.
4. Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Molecular, clinical and environmental toxicology. Luch A, editor. Molecular, Clinical and Environmental Toxicology. Basel: Springer Basel; 2012: 133–164 p. (Experientia Supplementum; vol. 101). DOI: 10.1007/978-3-7643-8340-4.
5. Zhang XH, Zhang X, Wang XC, Jin LF, Yang ZP, Jiang CX, Chen Q, Ren X Bin, Cao JZ, Wang Q, Zhu YM (2011) Chronic occupational exposure to hexavalent chromium causes DNA damage in electroplating workers. BMC Public Health. 11 (224): 1–8. DOI: 10.1186/1471-2458-11-224.
6. Becerra-Torres SL, Rodríguez-Vázquez ML, Medina-Ramírez IE, Jaramillo-Juárez F (2009) The flavonoid quercetin protects and prevents against potassium dichromate–induced systemic peroxidation of lipids and diminution in renal clearance of para-aminohippuric acid and inulin in the rat. Drug Chem. Toxicol. 32 (1): 88–91. DOI: 10.1080/01480540802449951.
7. Rajeev P, Rajput P, Singh DK, Singh AK, Gupta T (2018) Risk assessment of submicron PM-bound hexavalent chromium during wintertime. Hum. Ecol. Risk Assess. An Int. J. 24 (6): 1453–1463. DOI: 10.1080/10807039.2017.1414581.
8. Coyte RM, McKinley KL, Jiang S, Karr J, Dwyer GS, Keyworth AJ, Davis CC, Kondash AJ, Vengosh A (2020) Occurrence and distribution of hexavalent chromium in groundwater from North Carolina, USA. Sci. Total Environ. 711 (135135): 1–40. DOI: 10.1016/j.scitotenv.2019.135135.
9. Chromium Global Market Review 2022 and Forecast to 2031.
10. Mishra S, Bharagava RN (2016) Toxic and genotoxic effects of hexavalent chromium in environment and its bioremediation strategies. J. Environ. Sci. Heal. - Part C Environ. Carcinog. Ecotoxicol. Rev. 34 (1): 1–32. DOI: 10.1080/10590501.2015.1096883.
11. Sharma P, Singh SP, Parakh SK, Tong YW (2022) Health hazards of hexavalent chromium (Cr (VI)) and its microbial reduction. Bioengineered 13 (3): 4923–4938. DOI: 10.1080/21655979.2022.2037273.
12. von Burg R, Liu D (1993) Chromium and hexavalent chromium. J. Appl. Toxicol. 13 (3): 225–230. DOI: 10.1002/jat.2550130315.
13. Costa EJX, Shida CS, Biaggi MH, Ito AS, Lamy-Freund MT (1997) How melatonin interacts with lipid bilayers: A study by fluorescence and ESR spectroscopies. FEBS Lett. 416 (1): 103–106. DOI: 10.1016/S0014-5793(97)01178-2.
14. Costa M (1997) Toxicity and carcinogenicity of Cr(VI) in animal models and humans. Crit. Rev. Toxicol. 27 (5): 431–442. DOI: 10.3109/10408449709078442.
15. Shi X, Dalal NS (1990) On the hydroxyl radical formation in the reaction between hydrogen peroxide and biologically generated chromium(V) species. Arch. Biochem. Biophys. 277 (2): 342–350. DOI: 10.1016/0003-9861(90)90589-Q.
16. Sharma B, Singh S, Siddiqi NJ (2014) Biomedical implications of heavy metals induced imbalances in redox systems. Biomed Res. Int. 2014 (640754): 1–26. DOI: 10.1155/2014/640754.
17. Stohs SJ, Bagchi D (1995) Oxidative mechanisms in the toxicity of metal ions. Free Radic. Biol. Med. 18 (2): 321–336. DOI: 10.1016/0891-5849(94)00159-H.
18. Ghosh P, Dey T, Chattopadhyay A, Bandyopadhyay D (2021) An insight into the ameliorative effects of melatonin against chromium induced oxidative stress and DNA damage: a review. Melatonin Res. 4 (3): 377–407. DOI: 10.32794/MR112500101.
19. Nuran Ercal BSP, Hande Gurer-Orhan BSP, Nukhet Aykin-Burns BSP (2001) Toxic metals and oxidative stress part I: mechanisms involved in metal induced oxidative damage. Curr. Top. Med. Chem. 1 (6): 529–539. DOI: 10.2174/1568026013394831.
20. Valko M, Morris H, Cronin M (2005) Metals, toxicity and oxidative stress. Curr. Med. Chem. 12 (10): 1161–1208. DOI: 10.2174/0929867053764635.
21. Qi W, Reiter RJ, Tan DX, Garcia JJ, Manchester LC, Karbownik M, Calvo JR (2000) Chromium(III)-induced 8-hydroxydeoxyguanosine in DNA and its reduction by antioxidants: Comparative effects of melatonin, ascorbate, and vitamin E. Environ. Health Perspect. 108 (5): 399–403. DOI: 10.1289/ehp.00108399.
22. Xin Z, Zhang X, Hu W, Tan DX, Han M, Ji T, Jiang S, Yu Z, Reiter RJ, Yang Y (2019) The protective effects of melatonin on organisms against the environmental pollutants of heavy metal and non-mental toxins. Melatonin Res. 2 (4): 99–120. DOI: 10.32794/11250043.
23. Dominguez-Rodriguez A, Abreu-Gonzalez P, Sanchez-Sanchez JJ, Kaski JC, Reiter RJ (2010) Melatonin and circadian biology in human cardiovascular disease. J. Pineal Res. 49 (1): 1-10. DOI: 10.1111/j.1600-079X.2010.00773.x.
24. Reiter RJ, Mayo JC, Tan D-X, Sainz RM, Alatorre-Jimenez M, Qin L (2016) Melatonin as an antioxidant: under promises but over delivers. J. Pineal Res. 61 (3): 253–278. DOI: 10.1111/jpi.12360.
25. Rodriguez C, Mayo JC, Sainz RM, Antolín I, Herrera F, Martín V, Reiter RJ (2004) Regulation of antioxidant enzymes: A significant role for melatonin. J. Pineal Res. 36 (1): 1–9. DOI: 10.1046/j.1600-079X.2003.00092.x.
26. Tan D-X, Manchester LC, Hardeland R, Lopez-Burillo S, Mayo JC, Sainz RM, Reiter RJ (2003) Melatonin: a hormone, a tissue factor, an autocoid, a paracoid, and an antioxidant vitamin. J. Pineal Res. 34 (1): 75–78. DOI: 10.1034/j.1600-079X.2003.02111.x.
27. Acuña-Castroviejo D, Escames G, Venegas C, Díaz-Casado ME, Lima-Cabello E, López LC, Rosales-Corral S, Tan DX, Reiter RJ (2014) Extrapineal melatonin: Sources, regulation, and potential functions. Cell. Mol. Life Sci. 71 (16): 2997–3025. DOI: 10.1007/S00018-014-1579-2/FIGURES/4.
28. Cheng G, Ma T, Deng Z, Gutiérrez-Gamboa G, Ge Q, Xu P, Zhang Q, Zhang J, Meng J, Reiter RJ, Fang Y, Sun X (2021) Plant-derived melatonin from food: a gift of nature. Food Funct. 12 (7): 2829–2849. DOI: 10.1039/D0FO03213A.
29. Meng X, Li Y, Li S, Zhou Y, Gan R-Y, Xu D-P, Li H-B (2017) Dietary sources and bioactivities of melatonin. Nutrients 9 (4): 367. DOI: 10.3390/nu9040367.
30. A Hattori, H Migitaka, M Iigo, M Itoh, K Yamamoto, R Ohtani-Kaneko, M Hara, T Suzuki RJR (1985) Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem. Mol. Biol. Int. 35 (3): 627–634.
31. Kim E, Na KJ (1991) Nephrotoxicity of sodium dichromate depending on the route of administration. Arch. Toxicol. 65 (7): 537–541. DOI: 10.1007/BF01973713.
32. Mukherjee D, Roy SG, Bandyopadhyay A, Chattopadhyay A, Basu A, Mitra E, Ghosh AK, Reiter RJ, Bandyopadhyay D (2010) Melatonin protects against isoproterenol‐induced myocardial injury in the rat: antioxidative mechanisms. J. Pineal Res. 48 (3): 251–262. DOI: 10.1111/j.1600-079X.2010.00749.x.
33. Dey T, Ghosh A, Mishra S, Pal PK, Chattopadhyay A, Pattari SK, Bandyopadhyay D (2020) Attenuation of arsenic induced high fat diet exacerbated oxidative stress mediated hepatic and cardiac injuries in male Wistar rats by piperine involved antioxidative mechanisms. Food Chem. Toxicol. 142 (111477): 1–87. DOI: 10.1016/j.fct.2020.111477.
34. Strittmatter CF (1965) Studies on Avian Xanthine Dehydrogenases. Properties and Patterns of Appearance During Development. J. Biol. Chem. 240 (6): 2557–2564. DOI: 10.1016/S0021-9258(18)97361-8.
35. Varcoe JS (2001) Clinical biochemistry: techniques and instrumentation. World Scientific. WORLD SCIENTIFIC; 2001. DOI: 10.1142/4635.
36. Buege JA, Aust SD (1978) Microsomal lipid peroxidation. In: Journal of Physics: Conference Series. 1978. p. 302–310. DOI: 10.1016/S0076-6879(78)52032-6.
37. Bandyopadhyay D, Ghosh G, Bandyopadhyay A, Reiter RJ (2004) Melatonin protects against piroxicam-induced gastric ulceration. J. Pineal Res. 36 (3): 195–203. DOI: 10.1111/j.1600-079X.2004.00118.x.
38. Levine RL, Williams JA, Stadtman EP, Shacter E (1994) Carbonyl assays for determination of oxidatively modified proteins. In: Methods in Enzymology. Methods Enzymol; 1994. p. 346–357. DOI: 10.1016/S0076-6879(94)33040-9.
39. Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 25 (1): 192–205. DOI: 10.1016/0003-2697(68)90092-4.
40. Ewing JF, Janero DR (1995) Microplate superoxide dismutase assay employing a nonenzymatic superoxide generator. Anal. Biochem. 232 (2): 243–248. DOI: 10.1006/abio.1995.0014.
41. Beers RF, Sizer IW (1952) A sprectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 195 (1): 133–140. DOI: 10.1016/S0021-9258(19)50881-X.
42. Castro R, Piazzon MC, Noya M, Leiro JM, Lamas J (2008) Isolation and molecular cloning of a fish myeloperoxidase. Mol. Immunol. 45 (2): 428–437. DOI: 10.1016/j.molimm.2007.05.028.
43. Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-Transferases. J. Biol. Chem. 249 (22): 7130–7139. DOI: 10.1016/S0021-9258(19)42083-8.
44. Krohne-Ehrich G, Schirmer RH, Untucht-Grau R (1977) Glutathione reductase from human erythrocytes. isolation of the enzyme and sequence analysis of the redox-active peptide. Eur. J. Biochem. 80 (1): 65–71. DOI: 10.1111/j.1432-1033.1977.tb11856.x.
45. Greenlee L, Handler P (1964) Xanthine oxidase. J. Biol. Chem. 239 (4): 1090–1095. DOI: 10.1016/S0021-9258(18)91395-5.
46. Mitra E, Ghosh AK, Ghosh D, Mukherjee D, Chattopadhyay A, Dutta S, Pattari SK, Bandyopadhyay D (2012) Protective effect of aqueous Curry leaf (Murraya koenigii) extract against cadmium-induced oxidative stress in rat heart. Food Chem. Toxicol. 50 (5): 1340–1353. DOI: 10.1016/j.fct.2012.01.048.
47. Hilliard EP, Smith JD (1979) Minimum sample preparation for the determination of ten elements in pig faeces and feeds by atomic-absorption spectrophotometry and a spectrophotometric procedure for total phosphorus. Analyst 104 (1237): 313. DOI: 10.1039/an9790400313.
48. Capar SG, Tanner JT, Friedman MH, Boyer KW (1978) Multielement analysis of animal feed, animal wastes, and sewage sludge. Environ. Sci. Technol. 12 (7): 785–790. DOI: 10.1021/es60143a004.
49. Noronha Dutra AA, Steen EM, Woolf N (1984) The early changes induced by isoproterenol in the endocardium and adjacent myocardium. Am. J. Pathol. 114 (2): 231.
50. Mukherjee D, Ghosh AK, Bandyopadhyay A, Basu A, Datta S, Pattari SK, Reiter RJ, Bandyopadhyay D (2012) Melatonin protects against isoproterenol-induced alterations in cardiac mitochondrial energy-metabolizing enzymes, apoptotic proteins, and assists in complete recovery from myocardial injury in rats. J. Pineal Res. 53 (2): 166–179. DOI: 10.1111/j.1600-079X.2012.00984.x.
51. Bhogal RH, Curbishley SM, Weston CJ, Adams DH, Afford SC (2010) Reactive oxygen species mediate human hepatocyte injury during hypoxia/reoxygenation. Liver Transpl. 16 (11): 1303–1313. DOI: 10.1002/LT.22157.
52. Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 126 (1): 131–138. DOI: 10.1016/0003-2697(82)90118-X.
53. Suvarna K, Layton C, Bancroft J (2018) Bancroft’s theory and practice of histological techniques E-Book. 2018.
54. Junqueira LCU, Bignolas G, Brentani RR (1979) Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem. J. 11 (4): 447–455. DOI: 10.1007/BF01002772.
55. Sheehan DC, Hrapchak BB 1987. 2. ed. (1987) Theory and practice of histotechnology. Columbus Ohio: Battelle Press; 1987. 481 p.
56. Bancroft J, Gamble M (2008) Theory and practice of histological techniques.
57. Luna L (1968) Manual of histologic staining methods of the Armed Forces Institute of Pathology. McGrow-Hill, NY.
58. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193 (1): 265–275. DOI: 10.1016/s0021-9258(19)52451-6.
59. Yang A, Lo K, Zheng T, Yang J, Bai Y, Feng Y, Cheng N, Liu S (2020) Environmental heavy metals and cardiovascular diseases: Status and future direction. Chronic Dis. Transl. Med. 6 (4): 251–259. DOI: 10.1016/j.cdtm.2020.02.005.
60. Sahu BD, Koneru M, Bijargi SR, Kota A, Sistla R (2014) Chromium-induced nephrotoxicity and ameliorative effect of carvedilol in rats: Involvement of oxidative stress, apoptosis and inflammation. Chem. Biol. Interact. 223 (2014): 69–79. DOI: 10.1016/j.cbi.2014.09.009.
61. Ahmad MK, Syma S, Mahmood R (2011) Cr(VI) induces lipid peroxidation, protein oxidation and alters the activities of antioxidant enzymes in human erythrocytes. Biol. Trace Elem. Res. 144 (1–3): 426–435. DOI: 10.1007/s12011-011-9119-5.
62. Barhoma RAE (2018) The role of eugenol in the prevention of chromium-induced acute kidney injury in male albino rats. Alexandria J. Med. 54 (4): 711–715. DOI: 10.1016/j.ajme.2018.05.006.
63. Lopotych N, Panas N, Datsko T, Slobodian S (2020) Influence of heavy metals on hematologic parameters, body weight gain and organ weight in rats. Ukr. J. Ecol. 10 (1): 175–179. DOI: 10.15421/2020_28.
64. Honda K, Sahrul M, Hidaka H, Tatsukawa R (1983) Organ and tissue distribution of heavy metals, and their growth-related changes in antarctic fish, pagothenia borchgrevinki. Agric. Biol. Chem. 47 (11): 2521–2532. DOI: 10.1080/00021369.1983.10865986.
65. Soudani N, Troudi A, Bouaziz H, Ben Amara I, Boudawara T, Zeghal N (2011) Cardioprotective effects of selenium on chromium (VI)-induced toxicity in female rats. Ecotoxicol. Environ. Saf. 74 (3): 513–520. DOI: 10.1016/j.ecoenv.2010.06.009.
66. Kausar H, Singh B, Kaushik RK, Sinha P, Ghai R, Jain S (2017) Protective role of alpha-tocopherol in chromium induced toxicity in albino rat’s liver: a histopathological study. Ann. Int. Med. Dent. Res. 2 (5): 9–13. DOI: 10.21276/aimdr.2017.3.5.AT2.
67. Kopustinskiene DM, Bernatoniene J (2021) Molecular mechanisms of melatonin-mediated cell protection and signaling in health and disease. Pharmaceutics 13 (2): 129. DOI: 10.3390/pharmaceutics13020129.
68. Kocic G, Tomovic K, Kocic H, Sokolovic D, Djordjevic B, Stojanovic S, Arsic I, Smelcerovic A (2017) Antioxidative, membrane protective and antiapoptotic effects of melatonin, in silico study of physico-chemical profile and efficiency of nanoliposome delivery compared to betaine. RSC Adv. 7 (3): 1271–1281. DOI: 10.1039/C6RA24741E.
69. Holland SL, Avery S V. (2011) Chromate toxicity and the role of sulfur. Metallomics 3 (11): 1119. DOI: 10.1039/c1mt00059d.
70. Patlolla AK, Barnes C, Yedjou C, Velma VR, Tchounwou PB (2009) Oxidative stress, DNA damage, and antioxidant enzyme activity induced by hexavalent chromium in Sprague-Dawley rats. Environ. Toxicol. 24 (1): 66–73. DOI: 10.1002/tox.20395.
71. Shrivastava HY, Nair BU (2000) Protein degradation by peroxide catalyzed by chromium (III): role of coordinated ligand. Biochem. Biophys. Res. Commun. 270 (3): 749–754. DOI: 10.1006/bbrc.2000.2492.
72. Wang Y, Su H, Gu Y, Song X, Zhao J (2017) Carcinogenicity of chromium and chemoprevention: a brief update. Onco. Targets. Ther. 10 : 4065–4079. DOI: 10.2147/OTT.S139262.
73. Jozefczak M, Remans T, Vangronsveld J, Cuypers A (2012) Glutathione is a key player in metal-induced oxidative stress defenses. Int. J. Mol. Sci. 13 (3): 3145–3175. DOI: 10.3390/ijms13033145.
74. Soudani N, Ben Amara I, Sefi M, Boudawara T, Zeghal N (2011) Effects of selenium on chromium (VI)-induced hepatotoxicity in adult rats. Exp. Toxicol. Pathol. 63 (6): 541–548. DOI: 10.1016/j.etp.2010.04.005.
75. Wiegand HJ, Ottenwälder H, Bolt HM (1984) The reduction of chromium (VI) to chromium (III) by glutathione: An intracellular redox pathway in the metabolism of the carcinogen chromate. Toxicology 33 (3–4): 341–348. DOI: 10.1016/0300-483X(84)90050-7.
76. O’Brien T, Xu J, Patierno SR (2001) Effects of glutathione on chromium-induced DNA crosslinking and DNA polymerase arrest. Mol. Cell. Biochem. 222 (1–2): 173–182. DOI: 10.1023/A:1017918330073.
77. Menendez‐Pelaez A, Reiter RJ (1993) Distribution of melatonin in mammalian tissues: The relative importance of nuclear versus cytosolic localization. J. Pineal Res. 15 (2): 59–69. DOI: 10.1111/j.1600-079X.1993.tb00511.x.
78. Ceraulo L, Ferrugia M, Tesoriere L, Segreto S, Livrea MA, Turco Liveri V (1999) Interactions of melatonin with membrane models: Portioning of melatonin in AOT and lecithin reversed micelles. J. Pineal Res. 26 (2): 108–112. DOI: 10.1111/j.1600-079X.1999.tb00570.x.
79. Taysi S, Koc M, Büyükokuroǧlu ME, Altinkaynak K, Şahin YN (2003) Melatonin reduces lipid peroxidation and nitric oxide during irradiation-induced oxidative injury in the rat liver. J. Pineal Res. 34 (3): 173–177. DOI: 10.1034/j.1600-079X.2003.00024.x.
80. Manda K, Ueno M, Anzai K (2007) AFMK, a melatonin metabolite, attenuates X-ray-induced oxidative damage to DNA, proteins and lipids in mice. J. Pineal Res. 42 (4): 386–393. DOI: 10.1111/j.1600-079X.2007.00432.x.
81. Gulcin İ, Buyukokuroglu ME, Kufrevioglu OI (2003) Metal chelating and hydrogen peroxide scavenging effects of melatonin. J. Pineal Res. 34 (4): 278–281. DOI: 10.1034/j.1600-079X.2003.00042.x.
82. Mitra E, Bhattacharjee B, Pal PK, Ghosh AK, Mishra S, Chattopadhyay A, Bandyopadhyay D (2019) Melatonin protects against cadmium-induced oxidative damage in different tissues of rat: a mechanistic insight. Melatonin Res. 2 (2): 1–21. DOI: 10.32794/mr11250018.
83. Sugiyama M (1992) Role of physiological antioxidants in chromium (VI)- induced cellular injury. Free Radic. Biol. Med. 12 (5): 397–407. DOI: 10.1016/0891-5849(92)90089-y.
84. Chen L, Zhang J, Zhu Y, Zhang Y (2017) Interaction of chromium(III) or chromium(VI) with catalase and its effect on the structure and function of catalase: An in vitro study. Food Chem. 244 : 378–385. DOI: 10.1016/J.FOODCHEM.2017.10.062.
85. Baars AJ, Breimer DD (1980) The glutathione S-transferases. Their role in detoxification and toxification of xenobiotics. Ann. Biol. Clin. (Paris). 38 (1): 49–56.
86. Shi X, Dalal NS (1989) Chromium (V) and hydroxyl radical formation during the glutathione reductase-catalyzed reduction of chromium (VI). Biochem. Biophys. Res. Commun. 163 (1): 627–634. DOI: 10.1016/0006-291X(89)92183-9.
87. Banerjee S, Joshi N, Mukherjee R, Singh PK, Baxi D, Ramachandran A V. (2017) Melatonin protects against chromium (VI) induced hepatic oxidative stress and toxicity: Duration dependent study with realistic dosage. Interdiscip. Toxicol. 10 (1): 20–29. DOI: 10.1515/intox-2017-0003.
88. Cagnoli CM, Atabay C, Kharlamova E, Manev H (1995) Melatonin protects neurons from singlet oxygen‐induced apoptosis. J. Pineal Res. 18 (4): 222–226. DOI: 10.1111/j.1600-079X.1995.tb00163.x.
89. Mayo JC, Tan DX, Sainz RM, Lopez-Burillo S, Reiter RJ (2003) Oxidative damage to catalase induced by peroxyl radicals: Functional protection by melatonin and other antioxidants. Free Radic. Res. 37 (5): 543–553. DOI: 10.1080/1071576031000083206.
90. Marshall KA, Reiter RJ, Poeggeler B, Aruoma OI, Halliwell B (1996) Evaluation of the antioxidant activity of melatonin in vitro. Free Radic. Biol. Med. 21 (3): 307–315. DOI: 10.1016/0891-5849(96)00046-9.
91. Matuszak Z, Reszka KJ, Chignell CF (1997) Reaction of melatonin and related indoles with hydroxyl radicals: EPR and spin trapping investigations. Free Radic. Biol. Med. 23 (3): 367–372. DOI: 10.1016/S0891-5849(96)00614-4.
92. Zang LY, Cosma G, Gardner H, Vallyathan V (1998) Scavenging of reactive oxygen species by melatonin. Biochim. Biophys. Acta - Gen. Subj. 1425 (3): 469–477. DOI: 10.1016/S0304-4165(98)00099-3.
93. Reiter R, Acuna-Castriveijo D, Tan D-X, Burkhardt S (2006) Free radical-mediated molecular damage. Ann. N. Y. Acad. Sci. 939 (1): 200–215. DOI: 10.1111/j.1749-6632.2001.tb03627.x.
94. Mayo JC, Sainz RM, Antolín I, Herrera F, Martin V, Rodriguez C (2002) Melatonin regulation of antioxidant enzyme gene expression. Cell. Mol. Life Sci. 59 (10): 1706–1713. DOI: 10.1007/PL00012498.
95. Reiter RJ, Tan DX, Carmen O EG (2000) Actions of melatonin in the reduction of oxidative stress. J. Biomed. Sci. 7 (6): 444–458. DOI: 10.1007/BF02253360.
96. Chung HY, Baek BS, Song SH, Kim MS, Huh JI, Shim KH, Kim KW, Lee KH (1997) Xanthine dehydrogenase/xanthine oxldase and oxidative stress. J. Am. Aging Assoc. 20 (3): 127–140. DOI: 10.1007/s11357-997-0012-2.
97. Kerger BD, Finley BL, Corbett GE, Dodge DG, Paustenbach DJ (1997) Ingestion of chromium(vi) in drinking water by human volunteers: Absorption, distribution, and excretion of single and repeated doses. J. Toxicol. Environ. Health. 50 (1): 67–95. DOI: 10.1080/009841097160618.
98. Wilbur S, Abadin H, Fay M, Yu D, Tencza B, Ingerman L, Klotzbach J, James S (2012)Toxicological Profile for Chromium. Agency for Toxic Substances and Disease Registry (US)(September):1-9.www.atsdr.cdc.gov.
99. Ghosh P, Dey T, Majumder R, Datta M, Chattopadhyay A, Bandyopadhyay D (2023) Insights into the antioxidative mechanisms of melatonin in ameliorating chromium-induced oxidative stress-mediated hepatic and renal tissue injuries in male Wistar rats. Food Chem. Toxicol. 173 (113630): 113630. DOI: 10.1016/j.fct.2023.113630.
100. Cabaniss CD. Creatine Kinase. In: Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd edition. Boston: Butterworths; 1990. Chapter 32. Available from: https://www.ncbi.nlm.nih.gov/books/NBK352/?
101. Selker HP, Zalenski RJ, Antman EM, Aufderheide TP, Bernard SA, Bonow RO, Gibler WB, Hagen MD, Johnson P, Lau J, McNutt RA, Ornato J, Schwartz JS, Scott JD, Tunick PA, Weaver WD (1997) Creatine kinase. Ann. Emerg. Med. 29 (1): 59–63. DOI: 10.1016/S0196-0644(97)70308-1.
102. Pritchard J, Ackerman A, Kalyanaraman B (2000) Chromium (VI) increases endothelial cell expression of ICAM-1 and decreases nitric oxide activity. J. Environ. Pathol. Toxicol. Oncol. 19 (3): 251–260.
103. Porter R, Jáchymová M, Martásek P, Kalyanaraman B, Vásquez-Vivar J (2005) Reductive activation of Cr(VI) by nitric oxide synthase. Chem. Res. Toxicol. 18 (5): 834–843. DOI: 10.1021/tx049778e.
104. Aydogan S, Yerer MB, Goktas A (2006) Melatonin and nitric oxide. J. Endocrinol. Invest. 29 (3): 281–287. DOI: 10.1007/BF03345555.
105. Fabrizio RL, Gaia F, Eleonora F, Claudia R, Stefania C, Claudio L, Rita R (2013) Vascular endothelial cells and dysfunctions: role of melatonin. Front. Biosci. (Elite Ed). 5 (1): 119–129. DOI: 10.2741/E601.
106. Costa GM, Araujo SL, Xavier Júnior FAF, Morais GB de, Silveira JA de M, Viana D de A, Evangelista JSAM (2019) picrosirius red and masson’s trichrome staining techniques as tools for detection of collagen fibers in the skin of dogs with endocrine dermatopathologies. Ciência Anim. Bras. 20 (55398): 1–10. DOI: 10.1590/1089-6891v20e-55398.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


