Melatonin is more effective on bone metabolism when given at early night than during the day in ovariectomized rats
Timing of melatonin administration on bone metabolism
Abstract
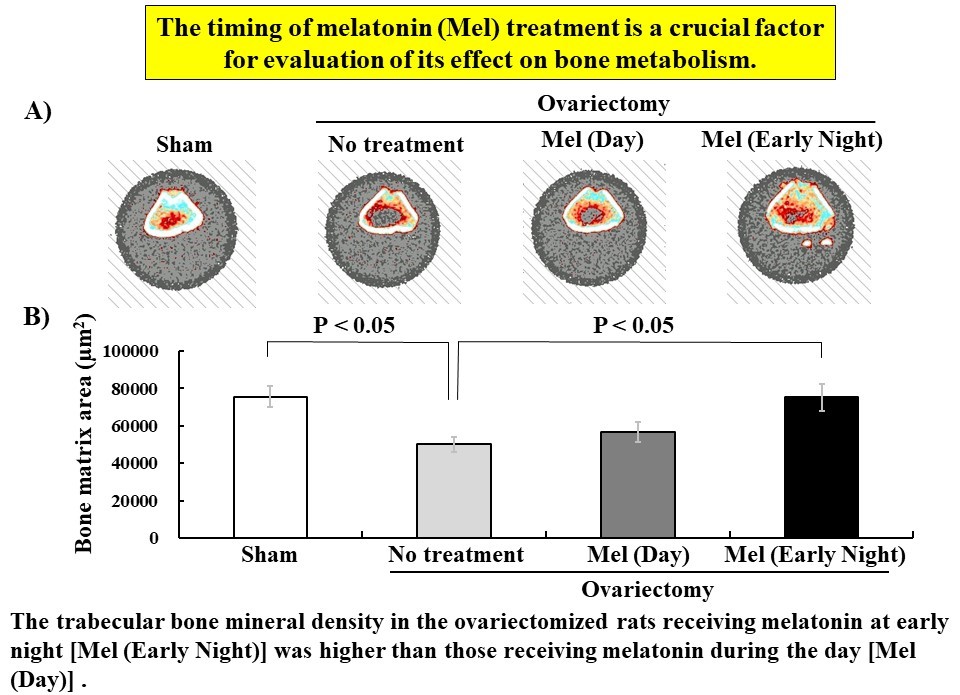
Melatonin has diverse effects, and has been reported to promote bone formation in addition to regulating the sleep–wake cycle. In the present study, we investigated the effects of melatonin on bone metabolism using ovariectomized (OVX) rats; a model of postmenopausal osteoporosis. Here, we focused on the differences in bone formation when melatonin was subcutaneous injected at day or early night. The OVX rats were injected with melatonin once daily (0.8 or 8 mg/head) between 11:00 to 14:00 or 18:00 to 19:30 for the day or early night, respectively, for six weeks. After completion of the injection, the femur and tibia in the OVX rats were dissected under general anesthesia and examined by quantitative computed tomography (pQCT) and histological analysis, respectively. Interestingly, the trabecular bone mineral density in the femur metaphysis of the OVX rats receiving 8 mg/head melatonin at early night was higher than those receiving melatonin during the day and they recovered to a similar level as the rats with sham treatment. In the diaphysis, the pQCT analysis results indicated that there was no significant difference in bone mineral density between the day and early night melatonin-injected OVX rats. Histological analysis of the secondary trabecular bone in the tibia of the OVX rats, revealed that the bone matrix area of the group receiving 8 mg/head melatonin at early night was higher compared with that of the day group and had a significant difference compared with OVX treatment rats. Taken together, the subcutaneous melatonin injection in OVX rats at early night was found to promote trabecular bone formation better than melatonin injection during the day. The timing of melatonin injection is a crucial factor when examining the influence of bone metabolism.
References
2. Cardinali DP, Srinivasan V, Brzezinski A, Brown GM (2012) Melatonin and its analogs in insomnia and depression. J. Pineal Res. 52: 365-375. https://doi.org/10.1111/j.1600-079X.2011.00962.x.
3. Wei S, et al. (2020) Efficacy and safety of melatonin for sleep onset insomnia in children and adolescents: a meta-analysis of randomized controlled trials. Sleep. Med. 68: 1-8. https://doi.org/10.1016/j.sleep.2019.02.017.
4. Hirayama J, et al. (2023) Physiological consequences of space flight, including abnormal bone metabolism, space radiation injury, and circadian clock dysregulation: implications of melatonin use and regulation as a countermeasure. J. Pineal Res. 74: e12834. https://doi.org/10.1111/jpi.12834.
5. Suzuki N, Hattori A (2002) Melatonin suppresses osteoclastic and osteoblastic activities in the scales of goldfish. J. Pineal Res. 33: 253-258. https://doi.org/10.1034/j.1600-079X.2002.02953.x.
6. Koyama H, Nakade O, Takada Y, Kaku T, Lau KHW (2002) Melatonin at pharmacologic doses increases bone mass by suppressing resorption through down-regulation of the RANKL-mediated osteoclast formation and activation. J. Bone Miner. Res. 17: 1219-1229. https://doi.org/10.1359/jbmr.2002.17.7.1219.
7. Suzuki N, Somei M, Seki A, Reiter RJ, Hattori A (2008) Novel bromomelatonin derivatives as potentially effective drugs to treat bone diseases. J. Pineal Res. 45: 229-234. https://doi.org/10.1111/j.1600-079X.2008.00623.x.
8. Maria S, et al. (2017) Melatonin-micronutrients Osteopenia Treatment Study (MOTS): a translational study assessing melatonin, strontium (citrate), vitamin D3 and vitamin K2 (MK7) on bone density, bone marker turnover and health related quality of life in postmenopausal osteopenic women following a one-year double-blind RCT and on osteoblast-osteoclast co-cultures. Aging 9: 256-285. https://doi.org/10.18632/aging.101158.
9. Maria S, et al. (2018) Biological effects of melatonin on osteoblast/osteoclast cocultures, bone, and quality of life: implications of a role for MT2 melatonin receptors, MEK1/2, and MEK5 in melatonin-mediated osteoblastogenesis. J. Pineal Res. 64: e12465. https://doi.org/10.1111/jpi.12465.
10. Ikegame M, et al. (2019) Melatonin is a potential drug for the prevention of bone loss during space flight. J. Pineal Res. 67: e12594. https://doi.org/10.1111/jpi.12594.
11. Wang X, et al. (2019) Melatonin prevents bone destruction in mice with retinoic acid–induced osteoporosis. Mol. Med. 25: 43. https://doi.org/10.1186/s10020-019-0107-0.
12. Igarashi-Migitaka J, et al. (2020) Oral administration of melatonin contained in drinking water increased bone strength in naturally aged mice. Acta Histochem. 122: 151596. https://doi.org/10.1016/j.acthis.2020.151596.
13. Bell NH, Johnson RH (1997) Bisphosphonates in the treatment of osteoporosis. Endocrine 6: 203-206. https://doi.org/10.1007/BF02738966.
14. Davis S, et al. (2016) A systematic review and economic evaluation of bisphosphonates for the prevention of fragility fractures. Health. Technol. Assess. 20: 1-406. https://doi.org/10.3310/hta20780.
15. McClung M, et al. (2013). Bisphosphonate therapy for osteoporosis: benefits, risks, and drug holiday. Am. J. Med. 126: 13-20. https://doi.org/10.1016/j.amjmed.2012.06.023.
16. Reyes C, Hitz M, Prieto-Alhambra D, Abrahamsen B (2016) Risks and benefits of bisphosphonate therapies. J. Cell Biochem. 117: 20-28. https://doi.org/10.1002/jcb.25266.
17. Reiter RJ (1993) The melatonin rhythm: both a clock and a calendar. Experientia 49: 654-664. https://doi.org/10.1007/BF01923947.
18. Allen AA, Pierce AT, Dauchy RT, Voros GB, Dobek GL (2022) Influence of light phase exposure to LED lighting on circadian levels of neuroendocrine hormones in Sprague-Dawley rats. J. Am. Assoc. Lab. Anim. Sci. 61: 333-343. http://doi.org/10.30802/AALAS-JAALAS-21-000123.
19. Ostrowska Z, Kos-Kudla B, Swietochowska E, Marek B, Kajdaniuk D, Górski J (2001) Assessment of the relationship between dynamic pattern of nighttime levels of melatonin and chosen biochemical markers of bone metabolism in a rat model of postmenopausal osteoporosis. Neuro. Endocrinol. Lett. 22: 129-136.
20. Ladizesky MG, Cutrera RA, Boggio V, Somoza J, Centrella JM, Mautalen C, Cardinali DP (2001) Effect of melatonin on bone metabolism in ovariectomized rats. Life Sci. 70: 557-565. http://doi.org/10.1016/S0024-3205(01)01431-X.
21. Iwamoto J, Seki A, Takeda T, Sato Y, Yamada H, Yeh JK (2006) Comparative effects of alendronate and alfacalcidol on cancellous and cortical bone mass and bone mechanical properties in ovariectomized rats. Exp. Anim. 55: 357-367. https://doi.org/10.1538/expanim.55.357.
22. Graham C, Cook MR, Gerkovich MM, Sastre A (2001) Examination of the melatonin hypothesis in women exposed at night to EMF or bright light. Environ. Health Perspect. 109: 501-507. http://doi.org/10.1289/ehp.01109501.
23. Feskanich D, Hankinson SE, Schernhammer ES (2009) Nightshift work and fracture risk: the nurses' health study. Osteoporos Int. 20: 537-542. http://doi.org/10.1007/s00198-008-0729-5.
24. Munmun F, Witt-Enderby PA (2021) Melatonin effects on bone: implications for use as a therapy for managing bone loss. J. Pineal Res. 71: e12749. http://doi.org/10.1111/jpi.12749.
25. Man GCW, et al. (2011) Abnormal melatonin receptor 1B expression in osteoblasts from girls with adolescent idiopathic scoliosis. J. Pineal Res. 50: 395-402. http://doi.org/10.1111/j.1600-079X.2011.00857.x.
26. Maria S, Witt-Enderby PA (2014) Melatonin effects on bone: potential use for the prevention and treatment for osteopenia, osteoporosis, and periodontal disease and for use in bone-grafting procedures. J. Pineal Res. 56: 115-125. http://doi.org/10.1111/jpi.12116.
27. Kim HJ, Kim HJ, Bae MK, Kim YD (2017) Suppression of osteoclastogenesis by melatonin: a melatonin receptor-independent action. Int. J. Mol. Sci. 18: 1142. http://doi.org/10.3390/ijms18061142.
28. Li T et al. (2019) Melatonin: another avenue for treating osteoporosis? J. Pineal Res. 66: e12548. http://doi.org/10.1111/jpi.12548.
29. Nakade O, Koyama H, Ariji H, Yajima A, Kaku T (1999) Melatonin stimulates proliferation and type I collagen synthesis in human bone cells in vitro. J. Pineal Res. 27: 106-110. http://doi.org/10.1111/j.1600-079x.1999.tb00603.x.
30. Uslu S, Uysal A, Oktem G, Yurtseven M, Tanyalçin T, Başdemir G (2007) Constructive effect of exogenous melatonin against osteoporosis after ovariectomy in rats. Anal. Quant. Cytol. Histol. 29: 317-325.
31. Witt-Enderby PA, et al. (2012) Effects on bone by the light/dark cycle and chronic treatment with melatonin and/or hormone replacement therapy in intact female mice. J. Pineal Res. 53: 374-384. http://doi.org/10.1111/j.1600-079X.2012.01007.x.
32. Zhou MS, Tao ZS (2022) Systemic administration with melatonin in the daytime has a better effect on promoting osseointegration of titanium rods in ovariectomized rats. Bone Joint Res. 11: 751-762. http://doi.org/10.1302/2046-3758.1111.BJR-2022-0017.R2.
33. Li TL, Liu HD, Ren MX, Zhou Z, Jiang WK, Yang M (2023) Daytime administration of melatonin has better protective effects on bone loss in ovariectomized rats. J. Orthop. Surg. Res. 18: 234. http://doi.org/10.1186/s13018-023-03695-8.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


