The cardioprotective potential of melatonin on cardiac hypertrophy: A mechanistic overview
Melatonin protection in cardiac hypertrophy
Abstract
Cardiac hypertrophy (CH) is an increment of muscle mass to maintain the heart regular operations. A physiological cardiac hypertrophy due to exercise or other normal physiological process is characterized by normal contractile function and structural framework of heart tissue. In contrast, pathological hypertrophy occurs in response to increased pressure or volume overload from several cardiovascular diseases including hypertension, valvular diseases, cardiac infarction and heart failure. It is of major concern as it is one of the leading causes of death worldwide. Despite much progress in this field there is a scope for understanding of the molecular mechanisms of this condition. In this review, various types of cardiac hypertrophy and their intricate physio-pathological mechanisms have been discussed. In addition, the genetic mutations in sarcomere genes and oxidative stress are also closely linked to hypertrophic cardiomyopathy. Although several drugs against cardiac hypertrophy have been used, it appears that melatonin, due to its high bioavailability and low side effects, is a better candidate than the conventional medicine for treatment of hypertrophic cardiomyopathy. Melatonin, a hormone and a potent antioxidant, is secreted mainly from the pineal gland, but it is also synthesized from different peripheral tissues including the heart. This molecule can regulate a myriad of cellular functions. It can protect against cardiac hypertrophy via reducing oxidative stress, elevating Cu-Mn SOD via controlling several cell signalling pathways of Akt/mTOR, ROR-α and NLRP3 cascades. Melatonin also mitigates cardiac hypertrophy by suppressing pro-inflammatory cytokines including TNF-α and TGF-β and cardiac hypertrophy markers like β-MHC, ANP, BNP, LDH. This review focuses on the molecular mechanisms of cardiac hypertrophy and the defensive role of melatonin on it. We propose melatoninas a propitious adjunct for the treatment of cardiac hypertrophy.
References
2. Timmis A, Townsend N, Gale CP, Torbica A, Lettino M, Petersen SE, Mossialos EA, Maggioni AP, Kazakiewicz D, May HT, De Smedt D, Flather M, Zuhlke L, Beltrame JF, Huculeci R, Tavazzi L, Hindricks G, Bax J, Casadei B, Achenbach S, Wright L, Vardas P (2020) E Atlas Writing Group; ESC Atlas of Cardiology is a compendium of cardiovascular statistics compiled by the European Heart Agency, a department of the European Society of Cardiology.; Developed in collaboration with the national societies of the European Society of Cardiology member countries; European Society of Cardiology: Cardiovascular Disease Statistics 2019 (Executive Summary). Eur. Heart J. Qual. Care Clin. Outcomes. 6 (1): 7-9. doi: 10.1093/ehjqcco/qcz065.
3. Drazner MH, Rame JE, Marino EK, Gottdiener JS, Kitzman DW, Gardin JM, Manolio TA, Dries DL, Siscovick DS (2004) Increased left ventricular mass is a risk factor for the development of a depressed left ventricular ejection fraction within five years: the Cardiovascular Health Study. J. Am. Coll.Cardiol. 43 (12): 2207-2215. doi: 10.1016/j.jacc.2003.11.064.
4. Nakamura M, Sadoshima J (2018) Mechanisms of physiological and pathological cardiac hypertrophy. Nat. Rev. Cardiol. 15 (7): 387-407. doi: 10.1038/s41569-018-0007-y.
5. Frey N, Katus HA, Olson EN, Hill JA (2004) Hypertrophy of the heart: a new therapeutic target? Circulation 109 (13): 1580-1589. doi: 10.1161/01.CIR.0000120390. 68287.
6. Florescu C (2018) Genetic evaluation of hypertrophic cardiomyopathy current perspectives on cardiomyopathies. Circulation 7: 17-2167. doi: 10.5772/intechopen.79626.
7. Maron BJ, Gardin JM, Flack JM, Gidding SS, Kurosaki TT, Bild DE (1995) Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary artery risk development in (young) adults. Circulation 92 (4): 785-789. doi: 10.1161/01.cir.92.4.785.
8. Maron BJ, Casey SA, Hauser RG, Aeppli DM (2003) Clinical course of hypertrophic cardiomyopathy with survival to advanced age. J. Am. Coll.Cardiol. 42 (5): 882-888. doi: 10.1016/s0735-1097(03)00855-6.
9. Sasagawa S, Nishimura Y, Okabe S, Murakami S, Ashikawa Y, Yuge M, Kawaguchi K, Kawase R, Okamoto R, Ito M, Tanaka T (2016) Downregulation of GSTK1 is a common mechanism underlying hypertrophic cardiomyopathy. Front. Pharmacol. 7: 162. doi: 10.3389/fphar.2016.00162.
10. Cave A, Grieve D, Johar S, Zhang M, Shah AM (2005) NADPH oxidase-derived reactive oxygen species in cardiac pathophysiology. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 360 (1464): 2327-2334. doi: 10.1098/rstb.2005.1772.
11. Manchester LC, Coto-Montes A, Boga JA, Andersen LP, Zhou Z, Galano A, Vriend J, Tan DX, Reiter RJ (2015) Melatonin: an ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 59 (4): 403-419. doi: 10.1111/jpi.12267.
12. Cole LK, Mejia EM, Sparagna GC, Vandel M, Xiang B, Han X, Dedousis N, Kaufman BA, Dolinsky VW, Hatch GM (2020) Cardiolipin deficiency elevates susceptibility to a lipotoxic hypertrophic cardiomyopathy. J. Mol. Cell. Cardiol. 144: 24-34. doi: 10.1016/j.yjmcc.2020.05.001.
13. Graham D, Huynh NN, Hamilton CA, Beattie E, Smith RA, Cochemé HM, Murphy MP, Dominiczak AF (2009) Mitochondria-targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension 54 (2): 322-328. doi: 10.1161/HYPERTENSIONAHA.109.130351.
14. Green EM, Wakimoto H, Anderson RL, Evanchik MJ, Gorham JM, Harrison BC, Henze M, Kawas R, Oslob JD, Rodriguez HM, Song Y, Wan W, Leinwand LA, Spudich JA, McDowell RS, Seidman JG, Seidman CE (2016) A small-molecule inhibitor of sarcomere contractility suppresses hypertrophic cardiomyopathy in mice. Science 351 (6273): 617-621. doi: 10.1126/science. aad3456.
15. Ho CY, Lakdawala NK, Cirino AL, Lipshultz SE, Sparks E, Abbasi SA, Kwong RY, Antman EM, Semsarian C, González A, López B, Diez J, Orav EJ, Colan SD, Seidman CE. Diltiazem treatment for pre-clinical hypertrophic cardiomyopathy sarcomere mutation carriers: a pilot randomized trial to modify disease expression. JACC Heart Fail. 3 (2): 180-8. doi: 10.1016/j.jchf.2014.08.003
16. Okabe K, Matsushima S, Ikeda S, Ikeda M, Ishikita A, Tadokoro T, Enzan N, Yamamoto T, Sada M, Deguchi H, Shinohara K, Ide T, Tsutsui H (2020) DPP (Dipeptidyl Peptidase)-4 Inhibitor attenuates Ang II (Angiotensin II)-induced cardiac hypertrophy via GLP (Glucagon-Like Peptide)-1-dependent suppression of Nox (Nicotinamide Adenine Dinucleotide Phosphate Oxidase) 4-HDAC (Histone Deacetylase) 4 pathway. Hypertension. 75 (4): 991-1001. doi: 10.1161/HYPERTENSIONAHA.119.14400.
17. Na Takuathung M, Sakuludomkan W, Khatsri R, Dukaew N, Kraivisitkul N, Ahmadmusa B, Mahakkanukrauh C, Wangthaweesap K, Onin J, Srichai S, Buawangpong N, Koonrungsesomboon N (2022) Adverse effects of angiotensin-converting enzyme inhibitors in humans: a systematic review and meta-analysis of 378 randomized controlled trials. Int. J. Environ. Res. Public Health. 19 (14): 8373. doi: 10.3390/ijerph19148373.
18. Shaito A, Posadino AM, Younes N, Hasan H, Halabi S, Alhababi D, Al-Mohannadi A, Abdel-Rahman WM, Eid AH, Nasrallah GK, Pintus G (2020) Potential adverse effects of resveratrol: A literature review. Int. J. Mol. Sci. 21 (6): 2084. doi: 10.3390/ijms21062084.
19. Seabra ML, Bignotto M, Pinto LR Jr, Tufik S (2000) Randomized, double-blind clinical trial, controlled with placebo, of the toxicology of chronic melatonin treatment. J. Pineal Res. 29 (4): 193-200. doi: 10.1034/j.1600-0633.2002.290401. x.
20. Jahnke G, Marr M, Myers C, Wilson R, Travlos G, Price C (1999) Maternal and developmental toxicity evaluation of melatonin administered orally to pregnant Sprague-Dawley rats. Toxicol. Sci. 50 (2): 271-279. doi: 10.1093/toxsci/50.2.271.
21. Baker J, Kimpinski K (2018) Role of melatonin in blood pressure regulation: An adjunct anti-hypertensive agent. Clin. Exp. Pharmacol. Physiol. 45 (8): 755-766. doi: 10.1111/1440-1681.12942.
22. Cipolla-Neto J, Amaral FGD (2018) Melatonin as a hormone: New physiological and clinical insights. Endocr. Rev. 39 (6): 990-1028. doi: 10.1210/er.2018-00084.
23. Yu L, Fan C, Li Z, Zhang J, Xue X, Xu Y, Zhao G, Yang Y, Wang H (2017) Melatonin rescues cardiac thioredoxin system during ischemia-reperfusion injury in acute hyperglycemic state by restoring Notch1/Hes1/Akt signaling in a membrane receptor-dependent manner. J. Pineal Res. 62 (1). doi: 10.1111/jpi.12375.
24. Favero G, Rodella LF, Reiter RJ, Rezzani R (2014) Melatonin and its atheroprotective effects: a review. Mol. Cell. Endocrinol. 382 (2): 926-37. doi: 10.1016/j.mce.2013.11.016.
25. Simko F, Baka T, Paulis L, Reiter RJ (2016) Elevated heart rate and non-dipping heart rate as potential targets for melatonin: a review. J. Pineal Res. 61 (2): 127-137. doi: 10.1111/jpi.12348.
26. Mukherjee D, Roy SG, Bandyopadhyay A, Chattopadhyay A, Basu A, Mitra E, Ghosh AK, Reiter RJ, Bandyopadhyay D (2010) Melatonin protects against isoproterenol-induced myocardial injury in the rat: antioxidative mechanisms. J. Pineal Res. 48 (3): 251-262. doi: 10.1111/j.1600-079X.2010.00749. x.
27. Dominguez-Rodriguez A, Abreu-Gonzalez P, Reiter RJ (2014) The potential usefulness of serum melatonin level to predict heart failure in patients with hypertensive cardiomyopathy. Int. J. Cardiol. 174 (2): 415-7. doi: 10.1016/j.ijcard.2014.04.044.
28. Zhai M, Liu Z, Zhang B, Jing L, Li B, Li K, Chen X, Zhang M, Yu B, Ren K, Yang Y, Yi W, Yang J, Liu J, Yi D, Liang H, Jin Z, Reiter RJ, Duan W, Yu S (2017) Melatonin protects against the pathological cardiac hypertrophy induced by transverse aortic constriction through activating PGC-1β: In vivo and in vitro studies. J. Pineal Res. 63 (3). doi: 10.1111/jpi.12433.
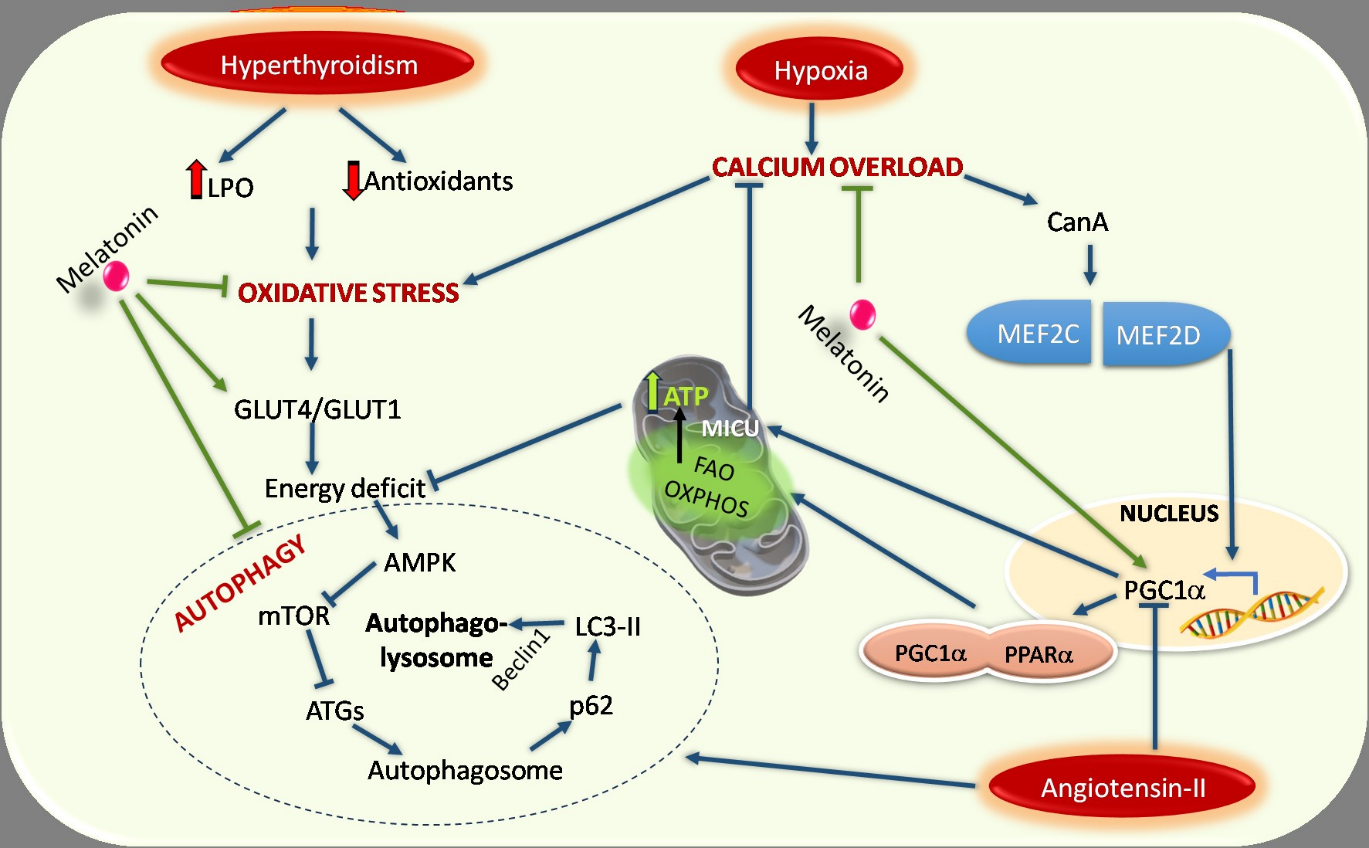
29. Ghosh G, De K, Maity S, Bandyopadhyay D, Bhattacharya S, Reiter RJ, Bandyopadhyay A (2007) Melatonin protects against oxidative damage and restores expression of GLUT4 gene in the hyperthyroid rat heart. J. Pineal Res. 42 (1): 71-82. doi: 10.1111/j.1600-079X.2006.00386. x.
30. Mohan M, Al-Talabany S, McKinnie A, Mordi IR, Singh JSS, Gandy SJ, Baig F, Hussain MS, Bhalraam U, Khan F, Choy AM, Matthew S, Houston JG, Struthers AD, George J, Lang CC (2019) A randomized controlled trial of metformin on left ventricular hypertrophy in patients with coronary artery disease without diabetes: the MET-REMODEL trial. Eur. Heart J. 40 (41): 3409-3417. doi: 10.1093/eurheartj/ehz203.
31. Martín Giménez VM, Inserra F, Tajer CD, Mariani J, Ferder L, Reiter RJ, Manucha W (2020) Lungs as target of COVID-19 infection: Protective common molecular mechanisms of vitamin D and melatonin as a new potential synergistic treatment. Life Sci. 254: 117808. doi: 10.1016/j.lfs.2020.117808.
32. Zhou Y, Hou Y, Shen J, Huang Y, Martin W, Cheng F (2020) Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2 Cell Discov. 6: 14. doi: 10.1038/s41421-020-0153-3.
33. Sandler H, & Dodge HT (1963). Left ventricular tension and stress in man. Cir. Res. 13 (2): 91-104.doi: 10.1161/01.res.13.2.91.
34. Hood WP Jr, Rackley CE, Rolett EL (1968) Wall stress in the normal and hypertrophied human left ventricle. Am. J. Cardiol. 22 (4): 550-558. doi: 10.1016/0002-9149(68)90161-6.
35. Grossman W, Jones D, McLaurin LP (1975) Wall stress and patterns of hypertrophy in the human left ventricle. J. Clin. Invest. 56 (1): 56-64. doi: 10.1172/JCI108079.
36. Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP (1990) Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N. Engl. J. Med. 322 (22): 1561-1566. doi: 10.1056/NEJM199005313222203.
37. Weber KT, Brilla CG, Janicki JS (1993) Myocardial fibrosis: functional significance and regulatory factors. Cardiovasc. Res. 27 (3): 341-348. doi: 10.1093/cvr/27.3.341.
38. Cohn JN, Bristow MR, Chien KR, Colucci WS, Frazier OH, Leinwand LA, Lorell BH, Moss AJ, Sonnenblick EH, Walsh RA, Mockrin SC, Reinlib L. (1997) Report of the National Heart, Lung, and Blood Institute Special Emphasis Panel on Heart Failure Research. Circulation 95 (4): 766-770. doi: 10.1161/01.cir.95.4.766.
39. Spinale FG, Oatmen KE, Sapp AA (2020) Myocardial Basis for Heart Failure.Heart Failure: A Companion to Braunwald’s Heart Dis. 62 (75): e7. doi:10.1016/b978-0-323-60987-6.00004-1
40. Wikman-Coffelt J, Parmley WW, Mason DT (1979) The cardiac hypertrophy process. Analyses of factors determining pathological vs. physiological development. Circ. Res. 45 (6): 697-707. doi: 10.1161/01.res.45.6.697.
41. Rupp H (1981) The adaptive changes in the isoenzyme pattern of myosin from hypertrophied rat myocardium as a result of pressure overload and physical training. Basic Res. Cardiol. 76 (1): 79-88. doi: 10.1007/BF01908164.
42. Pluim BM, Zwinderman AH, van der Laarse A, van der Wall EE (2000) The athlete's heart. A meta-analysis of cardiac structure and function. Circulation 101 (3): 336-344. doi: 10.1161/01.cir.101.3.336
43. Selby DE, Palmer BM, LeWinter MM, Meyer M (2011) Tachycardia-induced diastolic dysfunction and resting tone in myocardium from patients with a normal ejection fraction. J. Am. Coll. Cardiol. 58 (2): 147-54. doi: 10.1016/j.jacc.2010.10.069.
44. Katz AM (1990) Cardiomyopathy of overload. A major determinant of prognosis in congestive heart failure. N. Engl. J. Med. 322 (2): 100-110.doi:10.1056/NEJM199001113220206.
45. Sugden PH, Clerk A (1998) Cellularmechanisms of cardiac hypertrophy. J. Mol. Med. (Berl). 76 (11): 725-746. doi: 10.1007/s001090050275.
46. Sutton MG, Sharpe N (2000) Left ventricular remodelling after myocardial infarction: pathophysiology and therapy. Circulation 101 (25): 2981-8. doi: 10.1161/01.cir.101.25.2981.
47. RieggerAJ (1991) Left ventricular hypertrophy: favorable or unfavorable compensatory mechanisms? Herz. 1: 330-333. PMID: 1840298.
48. Stachera M, Przybyło P, Sznajder K, Gierlotka M (2021) Cardiac magnetic resonance in the assessment of hypertrophic cardiomyopathy phenotypes and stages - pictorial review. Pol. J. Radiol. 86: e672-e684. doi: 10.5114/pjr.2021.112310.
49. Maron BJ, Mathenge R, Casey SA, Poliac LC, Longe TF (1999) Clinical profile of hypertrophic cardiomyopathy identified de novo in rural communities. J. Am. Coll. Cardiol. 33 (6): 1590-1595. doi: 10.1016/s0735-1097(99)00039-x.
50. Hada Y, Sakamoto T, Amano K, Yamaguchi T, Takenaka K, Takahashi H, Takikawa R, Hasegawa I, Takahashi T, Suzuki J et al (1987) Prevalence of hypertrophic cardiomyopathy in a population of adult Japanese workers as detected by echocardiographic screening. Am. J. Cardiol. 59 (1): 183-184. doi: 10.1016/s0002-9149(87)80107-8.
51. Zou Y, Song L, Wang Z, Ma A, Liu T, Gu H, Lu S, Wu P, Zhang dagger Y, Shen dagger L, Cai Y, Zhen double dagger Y, Liu Y, Hui R (2004) Prevalence of idiopathic hypertrophic cardiomyopathy in China: a population-based echocardiographic analysis of 8080 adults. Am. J. Med. 116 (1): 14-18. doi: 10.1016/j.amjmed.2003.05.009.
52. Sachdev B, Takenaka T, Teraguchi H, Tei C, Lee P, McKenna WJ, Elliott PM (2002) Prevalence of Anderson-Fabry disease in male patients with late onset hypertrophic cardiomyopathy. Circulation 105 (12): 1407-1411.
53. Seo J, Kim M, Hong GR, Kim DS, Son JW, Cho IJ, Shim CY, Chang HJ, Ha JW, Chung N (2016) Fabry disease in patients with hypertrophic cardiomyopathy: a practical approach to diagnosis. J. Hum. Genet. 61 (9): 775-780. doi: 10.1038/jhg.2016.52.
54. Chimenti C, Pieroni M, Morgante E, Antuzzi D, Russo A, Russo MA, Maseri A, Frustaci A (2004) Prevalence of Fabry disease in female patients with late-onset hypertrophic cardiomyopathy. Circulation 110 (9): 1047-1153. doi: 10.1161/01.CIR.0000139847.74101.03.
55. Marian AJ, Braunwald E (2017) Hypertrophic Cardiomyopathy: Genetics, Pathogenesis, Clinical Manifestations, Diagnosis, and Therapy. Circ. Res. 121 (7): 749-770. doi: 10.1161/CIRCRESAHA.117.311059.
56. Schultheiss HP, Fairweather D, Caforio ALP, Escher F, Hershberger RE, Lipshultz SE, Liu PP, Matsumori A, Mazzanti A, McMurray J, Priori SG (2019) Dilated cardiomyopathy. Nat. Rev. Dis. Primers 5 (1): 32. doi: 10.1038/s41572-019-0084-1.
57. Sayin BY, Oto A (2022) Left Ventricular Hypertrophy: Etiology-Based Therapeutic Options. Cardiol. Ther. 11 (2): 203-230. doi: 10.1007/s40119-022-00260-y.
58. Jia G, Aroor AR, Hill MA, Sowers JR (2018) Role of Renin-Angiotensin-Aldosterone System Activation in Promoting Cardiovascular Fibrosis and Stiffness. Hypertension 72 (3): 537-548. doi: 10.1161/HYPERTENSIONAHA.118.11065.
59. Mozaffarian D, Caldwell JH (2001) Right ventricular involvement in hypertrophic cardiomyopathy: a case report and literature review. Clin. Cardiol. 24 (1): 2-8. doi: 10.1002/clc.4960240102.
60. Rohini A, Agrawal N, Koyani CN, Singh R (2010) Molecular targets and regulators of cardiac hypertrophy. Pharmacol. Res. 61 (4): 269-280. doi: 10.1016/j.phrs.2009.11.012.
61. Chien KR (2000) Genomic circuits and the integrative biology of cardiac diseases. Nature 407 (6801): 227-232. doi: 10.1038/35025196.
62. Sadoshima J, Takahashi T, Jahn L, Izumo S (1992) Rolesof mechano-sensitive ion channels, cytoskeleton, and contractile activity in stretch-induced immediate-early gene expression and hypertrophy of cardiac myocytes. Proc. Natl. Acad. Sci. U S A. 89 (20): 9905-9909. doi: 10.1073/pnas.89.20.9905.
63. Maillet M, van Berlo JH, Molkentin JD (2013) Molecular basis of physiological heart growth: fundamental concepts and new players. Nat. Rev. Mol. Cell Biol. 14 (1): 38-48. doi: 10.1038/nrm3495.
64. van Berlo JH, Maillet MJH, Maillet M, Molkentin JD (2013) Signaling effectors underlying pathologic growth and remodelling of the heart. J. Clin. Invest. 123 (1): 37-45. doi: 10.1172/JCI62839.
65. Bernardo BC, Weeks KL, Pretorius L, McMullen JR. (2010) Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol. Ther. 128 (1): 191-227. doi: 10.1016/j.pharmthera.2010.04.005.
66. Tham YK, Bernardo BC, Ooi JY, Weeks KL, McMullen JR (2015) Pathophysiology of cardiac hypertrophy and heart failure: signaling pathways and novel therapeutic targets. Arch. Toxicol. 89 (9): 1401-1438. doi: 10.1007/s00204-015-1477-x.
67. Shimizu I, Minamino T (2016) Physiological and pathological cardiac hypertrophy. J. Mol. Cell Cardiol. 97: 245-62. doi: 10.1016/j.yjmcc.2016.06.001.
68. Saltiel AR, Kahn CR (2001) Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414 (6865): 799-806. doi: 10.1038/414799a.
69. Boström P, Mann N, Wu J, Quintero PA, Plovie ER, Panáková D, Gupta RK, Xiao C, MacRae CA, Rosenzweig A, Spiegelman BM (2010) C/EBPβ controls exercise-induced cardiac growth and protects against pathological cardiac remodelling. Cell 143 (7): 1072-1083. doi: 10.1016/j.cell.2010.11.036.
70. Fernandes T, Baraúna VG, Negrão CE, Phillips MI, Oliveira EM (2015) Aerobic exercise training promotes physiological cardiac remodeling involving a set of microRNAs. Am. J.Physiol. Heart Circ. Physiol. 309 (4): H543-H552. doi: 10.1152/ajpheart.00899.2014.
71. Liu X, Xiao J, Zhu H, Wei X, Platt C, Damilano F, Xiao C, Bezzerides V, Boström P, Che L, Zhang C, Spiegelman BM, Rosenzweig A (2015) miR-222 is necessary for exercise-induced cardiac growth and protects against pathological cardiac remodelling. Cell Metab. 21 (4): 584-595. doi: 10.1016/j.cmet.2015.02.014.
72. Rose BA, Force T, Wang Y (2010) Mitogen-activated protein kinase signaling in the heart: angels versus demons in a heart-breaking tale. Physiol. Rev. 90 (4): 15071-1546. doi: 10.1152/physrev.00054.2009.
73. Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM, Jones F, Kimball TR, Molkentin JD (2004) Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ. Res. 94 (1): 110-118. doi: 10.1161/01.RES.0000109415.17511.18.
74. Sato PY, Chuprun JK, Schwartz M, Koch WJ (2015) The evolving impact of g protein-coupled receptor kinases in cardiac health and disease. Physiol. Rev. 95 (2): 377-404. doi: 10.1152/physrev.00015.2014.
75. Rigor DL, Bodyak N, Bae S, Choi JH, Zhang L, Ter-Ovanesyan D, He Z, McMullen JR, Shioi T, Izumo S, King GL, Kang PM (2009) Phosphoinositide 3-kinase Akt signaling pathway interacts with protein kinase Cbeta2 in the regulation of physiologic developmental hypertrophy and heart function. Am. J. Physiol. Heart Circ. Physiol. 296 (3): H566-H572. doi: 10.1152/ajpheart.00562.2008.
76. Chorghade S, Seimetz J, Emmons R, Yang J, Bresson SM, Lisio M, Parise G, Conrad NK, Kalsotra A (2017) Poly(A) tail length regulates PABPC1 expression to tune translation in the heart. Elife 6: e24139. doi: 10.7554/eLife.24139.
77. Saxton RA, Sabatini DM (2017) mTOR Signaling in Growth, Metabolism, and Disease. Cell 169 (2): 361-371. doi: 10.1016/j.cell.2017.03.035.
78. Sciarretta S, Forte M, Frati G, Sadoshima J (2018) New insights into the role of mTOR signaling in the cardiovascular system. Circ. Res. 122 (3): 489-505. doi: 10.1161/CIRCRESAHA.117.311147.
79. Nakamura M, Sadoshima J (2018) Mechanisms of physiological and pathological cardiac hypertrophy. Nat. Rev. Cardiol. 15 (7): 387-407. doi: 10.1038/s41569-018-0007-y.
80. Sciarretta S, Zhai P, Maejima Y, Del Re DP, Nagarajan N, Yee D, Liu T, Magnuson MA, Volpe M, Frati G, Li H, Sadoshima J (2015) mTORC2 regulates cardiac response to stress by inhibiting MST1. Cell Rep. 11 (1): 125-136. doi: 10.1016/j.celrep.2015.03.010.
81. Schumacher SM, Naga Prasad SV (2018) Tumor Necrosis Factor-α in Heart Failure: An Updated Review. Curr. Cardiol. Rep. 20 (11): 117. doi: 10.1007/s11886-018-1067-7.
82. Kuwahara F, Kai H, Tokuda K, Kai M, Takeshita A, Egashira K, Imaizumi T (2002) Transforming growth factor-beta function blocking prevents myocardial fibrosis and diastolic dysfunction in pressure-overloaded rats. Circulation 106 (1): 130-135. doi: 10.1161/01.cir.0000020689. 12472.e0.
83. Divakaran V, Adrogue J, Ishiyama M, Entman ML, Haudek S, Sivasubramanian N, Mann DL (2009) Adaptive and maladptive effects of SMAD3 signaling in the adult heart after hemodynamic pressure overloading. Circ. Heart Fail. 2 (6): 633-642. doi: 10.1161/CIRCHEARTFAILURE.108.823070.
84. Zhang D, Gaussin V, Taffet GE, Belaguli NS, Yamada M, Schwartz RJ, Michael LH, Overbeek PA, Schneider MD (2000) TAK1 is activated in the myocardium after pressure overload and is sufficient to provoke heart failure in transgenic mice. Nat. Med. 6 (5): 556-563. doi: 10.1038/75037.
85. Koitabashi N, Danner T, Zaiman AL, Pinto YM, Rowell J, Mankowski J, Zhang D, Nakamura T, Takimoto E, Kass DA (2011) Pivotal role of cardiomyocyte TGF-β signaling in the murine pathological response to sustained pressure overload. J. Clin. Invest. 121 (6): 2301-2312. doi: 10.1172/JCI44824.
86. Testa M, Yeh M, Lee P, Fanelli R, Loperfido F, Berman JW, LeJemtel TH (1996) Circulating levels of cytokines and their endogenous modulators in patients with mild to severe congestive heart failure due to coronary artery disease or hypertension. J. Am. Coll. Cardiol. 28 (4): 964-971. doi: 10.1016/s0735-1097(96)00268-9.
87. Meléndez GC, McLarty JL, Levick SP, Du Y, Janicki JS, Brower GL (2010) Interleukin 6 mediates myocardial fibrosis, concentric hypertrophy, and diastolic dysfunction in rats. Hypertension 56 (2): 225-231. doi: 10.1161/HYPERTENSIONAHA.109.148635.
88. Zhao L, Cheng G, Jin R, Afzal MR, Samanta A, Xuan YT, Girgis M, Elias HK, Zhu Y, Davani A, Yang Y, Chen X, Ye S, Wang OL, Chen L, Hauptman J, Vincent RJ, Dawn B (2016) Deletion of interleukin-6 attenuates pressure overload-induced left ventricular hypertrophy and dysfunction. Circ. Res. 118 (12): 1918-1929. doi: 10.1161/CIRCRESAHA.116.308688.
89. D'Cruz LG, Baboonian C, Phillimore HE, Taylor R, Elliott PM, Varnava A, Davison F, McKenna WJ, Carter ND (2000) Cytosine methylation confers instability on the cardiac troponin T gene in hypertrophic cardiomyopathy. J. Med. Genet. 37 (9): E18. doi: 10.1136/jmg.37.9.e18.
90. Papait R, Cattaneo P, Kunderfranco P, Greco C, Carullo P, Guffanti A, Viganò V, Stirparo GG, Latronico MV, Hasenfuss G, Chen J, Condorelli G (2013) Genome-wide analysis of histone marks identifying an epigenetic signature of promoters and enhancers underlying cardiac hypertrophy. Proc. Natl. Acad. Sci. USA. 110 (50): 20164-20169. doi: 10.1073/pnas.1315155110.
91. Eom GH, Nam YS, Oh JG, Choe N, Min HK, Yoo EK, Kang G, Nguyen VH, Min JJ, Kim JK, Lee IK, Bassel-Duby R, Olson EN, Park WJ, Kook H (2014) Regulation of acetylation of histone deacetylase 2 by p300/CBP-associated factor/histone deacetylase 5 in the development of cardiac hypertrophy. Circ. Res. 114 (7): 1133-1143. doi: 10.1161/CIRCRESAHA.114.303429.
92. Vega RB, Harrison BC, Meadows E, Roberts CR, Papst PJ, Olson EN, McKinsey TA (2004) Protein kinases C and D mediate agonist-dependent cardiac hypertrophy through nuclear export of histone deacetylase 5. Mol. Cell Biol. 24 (19): 8374-8375. doi: 10.1128/MCB.24.19.8374-8385.2004.
93. Popa-Fotea NM, Micheu MM, Bataila V, Scafa-Udriste A, Dorobantu L, Scarlatescu AI, Zamfir D, Stoian M, Onciul S, Dorobantu M (2019) Exploring the Continuum of Hypertrophic Cardiomyopathy-From DNA to Clinical Expression. Medicina (Kaunas). 55 (6): 299. doi: 10.3390/medicina55060299.
94. Chen C, Ponnusamy M, Liu C, Gao J, Wang K, Li P (2017) MicroRNA as a therapeutic target in cardiac remodeling. Biomed. Res. Int. 2017: 1278436. doi: 10.1155/2017/1278436.
95. Derda AA, Thum S, Lorenzen JM, Bavendiek U, Heineke J, Keyser B, Stuhrmann M, Givens RC, Kennel PJ, Schulze PC, Widder JD, Bauersachs J, Thum T (2015) Blood-based microRNA signatures differentiate various forms of cardiac hypertrophy. Int. J. Cardiol. 196: 115-122. doi: 10.1016/j.ijcard.2015.05.185.
96. Li Y, Wang K, Wei Y, Yao Q, Zhang Q, Qu H, Zhu G (2017) lncRNA-MIAT regulates cell biological behaviors in gastric cancer through a mechanism involving the miR-29a-3p/HDAC4 axis. Oncol. Rep. 38 (6): 3465-3472. doi: 10.3892/or.2017.6020.
97. Wolf CM (2019) Hypertrophic cardiomyopathy: genetics and clinical perspectives. Cardiovasc. Diagn. Ther. 9 (Suppl 2): S388-S415. doi: 10.21037/cdt.2019.02.01.
98. Gupta MK, Neelakantan TV, Sanghamitra M, Tyagi RK, Dinda A, Maulik S, Mukhopadhyay CK, Goswami SK (2006) An assessment of the role of reactive oxygen species and redox signaling in norepinephrine-induced apoptosis and hypertrophy of H9c2 cardiac myoblasts. Antioxid. Redox Signal. 8 (5-6): 1081-1093. doi: 10.1089/ars.2006.8.1081.
99. Lopes LR, Rahman MS, Elliott PM (2013) A systematic review and meta-analysis of genotype-phenotype associations in patients with hypertrophic cardiomyopathy caused by sarcomeric protein mutations. Heart 99 (24): 1800-1811. doi: 10.1136/heartjnl-2013-303939.
100. Ho CY, Charron P, Richard P, Girolami F, Van Spaendonck-Zwarts KY, Pinto Y (2015) Genetic advances in sarcomeric cardiomyopathies: state of the art. Cardiovasc Res. 105 (4): 397-3408. doi: 10.1093/cvr/cvv025.
101. Morita H, Rehm HL, Menesses A, McDonough B, Roberts AE, Kucherlapati R, Towbin JA, Seidman JG, Seidman CE (2008) Shared genetic causes of cardiac hypertrophy in children and adults. N. Engl. J. Med. 358 (18): 1899-1908. doi: 10.1056/NEJMoa075463.
102. Osio A, Tan L, Chen SN, Lombardi R, Nagueh SF, Shete S, Roberts R, Willerson JT, Marian AJ. (2007) Myozenin 2 is a novel gene for human hypertrophic cardiomyopathy. Circ. Res. 100 (6): 766-768. doi: 10.1161/01.RES.0000263008. 66799.aa.
103. Marian AJ. (2016) The Case of "missing causal genes" and the practice of medicine: A sherlock holmes approach of deductive reasoning. Circ. Res. 119 (1): 21-24. doi: 10.1161/CIRCRESAHA.116.308830.
104. Marian AJ (2014) Causality in genetics: the gradient of genetic effects and back to Koch's postulates of causality. Circ. Res. 114 (2): e18-21. doi: 10.1161/CIRCRESAHA.114.302904.
105. Hodatsu A, Konno T, Hayashi K, Funada A, Fujita T, Nagata Y, Fujino N, Kawashiri MA, Yamagishi M (2014) Compound heterozygosity deteriorates phenotypes of hypertrophic cardiomyopathy with founder MYBPC3 mutation: evidence from patients and zebrafish models. Am. J. Physiol. Heart Circ. Physiol. 307 (11): H1594-H604. doi: 10.1152/ajpheart.00637.2013.
106. Marian AJ, Yu QT, Mares A Jr, Hill R, Roberts R, Perryman MB (1992) Detection of a new mutation in the beta-myosin heavy chain gene in an individual with hypertrophic cardiomyopathy. J. Clin. Invest. 90 (6): 2156-2165. doi: 10.1172/JCI116101.
107. Watkins H, Seidman CE, Seidman JG, Feng HS, Sweeney HL (1996) Expression and functional assessment of a truncated cardiac troponin T that causes hypertrophic cardiomyopathy. Evidence for a dominant negative action. J. Clin. Invest. 98 (11): 2456-2461. doi: 10.1172/JCI119063.
108. Millat G, Bouvagnet P, Chevalier P, Dauphin C, Jouk PS, Da Costa A, Prieur F, Bresson JL, Faivre L, Eicher JC, Chassaing N, Crehalet H, Porcher R, Rodriguez-Lafrasse C, Rousson R. (2010) Prevalence and spectrum of mutations in a cohort of 192 unrelated patients with hypertrophic cardiomyopathy. Eur. J. Med. Genet. 53 (5): 261-267. doi: 10.1016/j.ejmg.2010.07.007.
109. Erdmann J, Daehmlow S, Wischke S, Senyuva M, Werner U, Raible J, Tanis N, Dyachenko S, Hummel M, Hetzer R, Regitz-Zagrosek V. (2003) Mutation spectrum in a large cohort of unrelated consecutive patients with hypertrophic cardiomyopathy. Clin. Genet. 64 (4): 339-349. doi: 10.1034/j.1399-0004.2003.00151. x.
110. Richard P, Charron P, Carrier L, Ledeuil C, Cheav T, Pichereau C, Benaiche A, Isnard R, Dubourg O, Burban M, Gueffet JP, Millaire A, Desnos M, Schwartz K, Hainque B, Komajda M (2003) Eurogene heart failure project. Hypertrophic cardiomyopathy: distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. Circulation 107 (17): 2227-2232. doi: 10.1161/01.CIR.0000066323.15244.54.
111. Matsumoto Y, Hayashi T, Inagaki N, Takahashi M, Hiroi S, Nakamura T, Arimura T, Nakamura K, Ashizawa N, Yasunami M, Ohe T, Yano K, Kimura A (2005) Functional analysis of titin/connectin N2-B mutations found in cardiomyopathy. J. Muscle Res. Cell Motil. 26 (6-8): 367-74. doi: 10.1007/s10974-005-9018-5.
112. Arimura T, Matsumoto Y, Okazaki O, Hayashi T, Takahashi M, Inagaki N, Hinohara K, Ashizawa N, Yano K, Kimura A (2007). Structural analysis of obscurin gene in hypertrophic cardiomyopathy. Biochem. Biophys. Res. Commun. 362 (2): 281-287. doi: 10.1016/j.bbrc.2007.07.183.
113. Arad M, Maron BJ, Gorham JM, Johnson WH Jr, Saul JP, Perez-Atayde AR, Spirito P, Wright GB, Kanter RJ, Seidman CE, Seidman JG (2005) Glycogen storage diseases presenting as hypertrophic cardiomyopathy. N. Engl. J. Med. 352 (4): 362-372. doi: 10.1056/NEJMoa033349.
114. Nakao S, Takenaka T, Maeda M, Kodama C, Tanaka A, Tahara M, Yoshida A, Kuriyama M, Hayashibe H, Sakuraba H, et al. (1995) An atypical variant of Fabry's disease in men with left ventricular hypertrophy. N. Engl. J. Med. 333 (5): 288-293. doi: 10.1056/NEJM199508033330504.
115. McConnell BK, Fatkin D, Semsarian C, Jones KA, Georgakopoulos D, Maguire CT, Healey MJ, Mudd JO, Moskowitz IP, Conner DA, Giewat M, Wakimoto H, Berul CI, Schoen FJ, Kass DA, Seidman CE, Seidman JG (2001) Comparison of two murine models of familial hypertrophic cardiomyopathy. Circ. Res. 88 (4): 383-389. doi: 10.1161/01.res.88.4.383.
116. Tyska MJ, Hayes E, Giewat M, Seidman CE, Seidman JG, Warshaw DM (2000) Single-molecule mechanics of R403Q cardiac myosin isolated from the mouse model of familial hypertrophic cardiomyopathy. Circ. Res. 86 (7): 737-744. doi: 10.1161/01.res.86.7.737.
117. Semsarian C, Ahmad I, Giewat M, Georgakopoulos D, Schmitt JP, McConnell BK, Reiken S, Mende U, Marks AR, Kass DA, Seidman CE, Seidman JG (2002) The L-type calcium channel inhibitor diltiazem prevents cardiomyopathy in a mouse model. J. Clin. Invest. 109 (8): 1013-1020. doi: 10.1172/JCI14677.
118. Popp MW, Maquat LE (2016) Leveraging rules of nonsense-mediated mRNA decay for genome engineering and personalized medicine. Cell 165 (6): 1319-1322. doi: 10.1016/j.cell.2016.05.053.
119. Siwaszek A, Ukleja M, Dziembowski A. Proteins involved in the degradation of cytoplasmic mRNA in the major eukaryotic model systems. RNA Biol. 11 (9): 1122-1136. doi: 10.4161/rna.34406.
120. Meurs KM, Kuan M (2011) Differential methylation of CpG sites in two isoforms of myosin binding protein C, an important hypertrophic cardiomyopathy gene. Environ. Mol. Mutagen. 52 (2): 161-164. doi: 10.1002/em.20596.
121. Chen JX, Zheng Y, West M, Tang MS (1998) Carcinogens preferentially bind at methylated CpG in the p53 mutational hot spots. Cancer Res. 58 (10): 2070-5.PMID: 9605744.
122. Glavaški M, Velicki L, Vučinić N (2023) Hypertrophic Cardiomyopathy: Genetic Foundations, Outcomes, Interconnections, and Their Modifiers. Medicina (Kaunas) 59 (8): 1424. doi: 10.3390/medicina59081424.
123. Maulik SK, Kumar S (2012) Oxidative stress and cardiac hypertrophy: a review. Toxicol. Mech. Meth. 22 (5): 359-366. doi: 10.3109/15376516.2012.666650.
124. Hauptmann N, Grimsby J, Shih JC, Cadenas E (1996) The metabolism of tyramine by monoamine oxidase A/B causes oxidative damage to mitochondrial DNA. Arch. Biochem. Biophys. 335 (2): 295-304. doi: 10.1006/abbi.1996.0510.
125. Di Meo S, Reed TT, Venditti P, Victor VM (2016) Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell Longev. 2016: 1245049. doi: 10.1155/2016/1245049. Jul 12.
126. Ray PD, Huang BW, Tsuji Y (2012) Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 24 (5): 981-990. doi: 10.1016/j.cellsig.2012.01.008.
127. Holmström KM, Finkel T (2014) Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell. Biol. 15 (6): 411-421. doi: 10.1038/nrm3801.
128. Quinlan CL, Perevoshchikova IV, Hey-Mogensen M, Orr AL, Brand MD (2013) Sites of reactive oxygen species generation by mitochondria oxidizing different substrates. Redox Biol. 1 (1): 304-312. doi: 10.1016/j.redox.2013.04.005.
129. Assem M, Teyssier JR, Benderitter M, Terrand J, Laubriet A, Javouhey A, David M, Rochette L (1997) Pattern of superoxide dismutase enzymatic activity and RNA changes in rat heart ventricles after myocardial infarction. Am. J. Pathol. 151 (2): 549-55.
130. Richardson AG, Schadt EE (2014) The role of macromolecular damage in aging and age-related disease. J. Gerontol. A. Biol. Sci. Med. Sci.69 (Suppl 1): S28-S32. doi: 10.1093/gerona/glu056.
131. Barja G (1999) Mitochondrial oxygen radical generation and leak: sites of production in states 4 and 3, organ specificity, and relation to aging and longevity. J. Bioenerg. Biomembr. 31 (4): 347-366. doi: 10.1023/a:1005427919188.
132. Ide T, Tsutsui H, Kinugawa S, Utsumi H, Kang D, Hattori N, Uchida K, Arimura Ki, Egashira K, Takeshita A (1999) Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circ. Res. 85 (4): 357-363. doi: 10.1161/01.res.85.4.357.
133. Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, Schumacker PT (2000) Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J. Biol. Chem. 275 (33): 25130-25138. doi: 10.1074/jbc.M001914200.
134. Lombardi M, Lazzeroni D, Pisano A, Girolami F, Alfieri O, La Canna G, d'Amati G, Olivotto I, Rimoldi OE, Foglieni C, Camici PG (2020) Mitochondrial energetics and Ca2+-activated atpase in obstructive hypertrophic cardiomyopathy. J. Clin. Med. 9 (6): 1799. doi: 10.3390/jcm9061799.
135. Ma T, Lin S, Wang B, Wang Q, Xia W, Zhang H, Cui Y, He C, Wu H, Sun F, Zhao Z, Gao P, Zhu Z, Liu D (2019) TRPC3 deficiency attenuates high salt-induced cardiac hypertrophy by alleviating cardiac mitochondrial dysfunction. Biochem. Biophys. Res. Commun. 519 (4): 674-681. doi: 10.1016/j.bbrc.2019.09.018.
136. Blasco N, Cámara Y, Núñez E, Beà A, Barés G, Forné C, Ruíz-Meana M, Girón C, Barba I, García-Arumí E, García-Dorado D, Vázquez J, Martí R, Llovera M, Sanchis D (2018) Cardiomyocyte hypertrophy induced by Endonuclease G deficiency requires reactive oxygen radicals’ accumulation and is inhibitable by the micropeptide humanin. Redox Biol. 16: 146-156. doi: 10.1016/j.redox.2018.02.021.
137. Münzel T, Gori T, Keaney JF Jr, Maack C, Daiber (2015) Pathophysiological role of oxidative stress in systolic and diastolic heart failure and its therapeutic implications. Eur. Heart J. 36 (38): 2555-2564. doi: 10.1093/eurheartj/ehv305.
138. Reddy S, Bernstein D (2015) Molecular Mechanisms of Right Ventricular Failure. Circulation 132 (18):1734-1742. doi: 10.1161/CIRCULATIONAHA.114.012975.
139. Kiyuna LA, Albuquerque RPE, Chen CH, Mochly-Rosen D, Ferreira JCB (2018) Targeting mitochondrial dysfunction and oxidative stress in heart failure: Challenges and opportunities. Free. Radic. Biol. Med. 129: 155-168. doi: 10.1016/j.freeradbiomed.2018.09.019.
140. Tang K, Zhao Y, Li H, Zhu M, Li W, Liu W, Zhu G, Xu D, Peng W, Xu YW (2017) Translocase of Inner Membrane 50 Functions as a Novel Protective Regulator of Pathological Cardiac Hypertrophy. J. Am. Heart Assoc. 6 (4): e004346. doi: 10.1161/JAHA.116.004346.
141. Babior BM, Curnutte JT, McMurrich BJ (1976) The particulate superoxide-forming system from human neutrophils. Properties of the system and further evidence supporting its participation in the respiratory burst. J. Clin. Invest. 58 (4): 989-996. doi: 10.1172/JCI108553.
142. Griendling KK, Sorescu D, Ushio-Fukai M (2000) NADPH oxidase: role in cardiovascular biology and disease. Circ. Res. 86 (5): 494-501. doi: 10.1161/01.res.86.5.494.
143. MacCarthy PA, Grieve DJ, Li JM, Dunster C, Kelly FJ, Shah AM (2001) Impaired endothelial regulation of ventricular relaxation in cardiac hypertrophy: role of reactive oxygen species and NADPH oxidase. Circulation 104 (24): 2967-2974. doi: 10.1161/hc4901.100382.
144. Grieve DJ, Byrne JA, Siva A, Layland J, Johar S, Cave AC, Shah AM (2006) Involvement of the nicotinamide adenosine dinucleotide phosphate oxidase isoform Nox2 in cardiac contractile dysfunction occurring in response to pressure overload. J. Am. Coll. Cardiol. 47 (4): 817-826. doi: 10.1016/j.jacc.2005.09.051.
145. Harvey AP, Robinson E, Edgar KS, McMullan R, O'Neill KM, Alderdice M, Amirkhah R, Dunne PD, McDermott BJ, Grieve DJ (2020) Downregulation of PPARα during experimental left ventricular hypertrophy is critically dependent on Nox2 NADPH oxidase signalling. Int. J. Mol. Sci. 21 (12): 4406. doi: 10.3390/ijms21124406.
146. Kar D, Bandyopadhyay A (2018) Targeting Peroxisome Proliferator Activated Receptor α (PPAR α) for the Prevention of Mitochondrial Impairment and Hypertrophy in Cardiomyocytes. Cell. Physiol. Biochem. 49 (1): 245-259. doi: 10.1159/000492875.
147. Galán M, Varona S, Guadall A, Orriols M, Navas M, Aguiló S, de Diego A, Navarro MA, García-Dorado D, Rodríguez-Sinovas A, Martínez-González J, Rodriguez C (2017) Lysyl oxidase overexpression accelerates cardiac remodeling and aggravates angiotensin II-induced hypertrophy. FASEB J. 31 (9): 3787-3799. doi: 10.1096/fj.201601157RR.
148. Bhatti SN, Li JM (2020) Nox2 dependent redox-regulation of Akt and ERK1/2 to promote left ventricular hypertrophy in dietary obesity of mice. Biochem. Biophys. Res. Commun. 528 (3): 506-513. doi: 10.1016/j.bbrc.2020.05.162.
149. Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z (2007) Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 26 (7): 1749-60. doi: 10.1038/sj.emboj.7601623.
150. Ramachandra CJA, Cong S, Chan X, Yap EP, Yu F, Hausenloy DJ (2021) Oxidative stress in cardiac hypertrophy: From molecular mechanisms to novel therapeutic targets. Free Radic. Biol. Med. 166: 297-312. doi: 10.1016/j.freeradbiomed.2021.02.040.
151. Wang SY, Ni X, Hu KQ, Meng FL, Li M, Ma XL, Meng TT, Wu HH, Ge D, Zhao J, Li Y, Su GH2020) Cilostazol alleviate nicotine induced cardiomyocytes hypertrophy through modulation of autophagy by CTSB/ROS/p38MAPK/JNK feedback loop. Int. J. Biol. Sci. 16 (11): 2001-2013. doi: 10.7150/ijbs.43825.
152. Xie YP, Lai S, Lin QY, Xie X, Liao JW, Wang HX, Tian C, Li HH (2018) CDC20 regulates cardiac hypertrophy via targeting LC3-dependent autophagy. Theranostics 8 (21): 5995-6007. doi: 10.7150/thno.27706.
153. Ji YX, Zhang P, Zhang XJ, Zhao YC, Deng KQ, Jiang X, Wang PX, Huang Z, Li H (2016) The ubiquitin E3 ligase TRAF6 exacerbates pathological cardiac hypertrophy via TAK1-dependent signalling. Nat. Commun. 7: 11267. doi: 10.1038/ncomms11267.
154. Yang LL, Xiao WC, Li H, Hao ZY, Liu GZ, Zhang DH, Wu LM, Wang Z, Zhang YQ, Huang Z, Zhang YZ (2022) E3 ubiquitin ligase RNF5 attenuates pathological cardiac hypertrophy through STING. Cell Death Dis. 13 (10): 889. doi: 10.1038/s41419-022-05231-8.
155. Xie Y, Gao Y, Gao R, Yang W, Dong Z, Moses RE, Sun A, Li X, Ge J (2020) The proteasome activator REGγ accelerates cardiac hypertrophy by declining PP2Acα-SOD2 pathway. Cell Death Differ. 27 (10): 2952-2972. doi: 10.1038/s41418-020-0554-8.
156. Morin D, Long R, Panel M, Laure L, Taranu A, Gueguen C, Pons S, Leoni V, Caccia C, Vatner SF, Vatner DE, Qiu H, Depre C, Berdeaux A, Ghaleh B (2019) Hsp22 overexpression induces myocardial hypertrophy, senescence and reduced life span through enhanced oxidative stress. Free Radic. Biol. Med. 137: 194-200. doi: 10.1016/j.freeradbiomed.2019.04.035.
157. Dewachter L, Dewachter C (2018) Inflammation in right ventricular failure: Does it matter? Front. Physiol. 9: 1056. doi: 10.3389/fphys.2018.01056.
158. Yang Y, Lv J, Jiang S, Ma Z, Wang D, Hu W, Deng C, Fan C, Di S, Sun Y, Yi W (2016) The emerging role of Toll-like receptor 4 in myocardial inflammation. Cell Death Dis. 7 (5): e2234. doi: 10.1038/cddis.2016.140.
159. Mian MOR, He Y, Bertagnolli M, Mai-Vo TA, Fernandes RO, Boudreau F, Cloutier A, Luu TM, Nuyt AM (2019) TLR (Toll-Like Receptor) 4 antagonism prevents left ventricular hypertrophy and dysfunction caused by neonatal hyperoxia exposure in rats. Hypertension 74 (4): 843-853. doi: 10.1161/HYPERTENSIONAHA.119.13022.
160. Chen D, Li Z, Bao P, Chen M, Zhang M, Yan F, Xu Y, Ji C, Hu X, Sanchis D, Zhang Y, Ye J (2019) Nrf2 deficiency aggravates Angiotensin II-induced cardiac injury by increasing hypertrophy and enhancing IL-6/STAT3-dependent inflammation. Biochem. Biophys. Acta. Mol. Basis Dis. 1865 (6): 1253-1264. doi: 10.1016/j.bbadis.2019.01.020.
161. Ma ZG, Yuan YP, Zhang X, Xu SC, Kong CY, Song P, Li N, Tang QZ (2019) C1q-tumour necrosis factor-related protein-3 exacerbates cardiac hypertrophy in mice. Cardiovasc. Res.115 (6): 1067-1077. doi: 10.1093/cvr/cvy279.
162. Yu Q, Kou W, Xu X, Zhou S, Luan P, Xu X, Li H, Zhuang J, Wang J, Zhao Y, Xu Y, Peng W (2019) FNDC5/Irisin inhibits pathological cardiac hypertrophy. Clin. Sci. (Lond). 133 (5): 611-627. doi: 10.1042/CS20190016.
163. Palandri C, Santini L, Argirò A, Margara F, Doste R, Bueno-Orovio A, Olivotto I, Coppini R (2022) Pharmacological management of hypertrophic cardiomyopathy: from bench to bedside. Drugs. 2 (8): 889-912. doi: 10.1007/s40265-022-01728-w.
164. Cohen LS, Braunwald E (1967) Amelioration of angina pectoris in idiopathic hypertrophic subaortic stenosis with beta-adrenergic blockade. Circulation 35 (5): 847-851. doi: 10.1161/01.cir.35.5.847.
165. Gilligan DM, Chan WL, Joshi J, Clarke P, Fletcher A, Krikler S, Oakley CM (1993) A double-blind, placebo-controlled crossover trial of nadolol and verapamil in mild and moderately symptomatic hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 21 (7):1672-9. doi: 10.1016/0735-1097(93)90386-f.
166. Khatua TN, Borkar RM, Mohammed SA, Dinda AK, Srinivas R, Banerjee SK (2017) Novel Sulfur Metabolites of Garlic Attenuate Cardiac Hypertrophy and Remodeling through Induction of Na+/K+-ATPase Expression. Front. Pharmacol. 8: 18. doi: 10.3389/fphar.2017.00018.
167. Hasan P, Saotome M, Ikoma T, Iguchi K, Kawasaki H, Iwashita T, Hayashi H, Maekawa Y (2018) Mitochondrial fission protein, dynamin-related protein 1, contributes to the promotion of hypertensive cardiac hypertrophy and fibrosis in Dahl-salt sensitive rats. J. Mol. Cell Cardiol. 121: 103-106. doi: 10.1016/j.yjmcc.2018.07.004.
168. Xie F, Wu D, Huang SF, Cao JG, Li HN, He L, Liu MQ, Li LF, Chen LX (2017) The endoplasmic reticulum stress-autophagy pathway is involved in apelin-13-induced cardiomyocyte hypertrophy in vitro. Acta. Pharmacol. Sin. 38 (12): 1589-1600. doi: 10.1038/aps.2017.97.
169. Zhu W, Wu RD, Lv YG, Liu YM, Huang H, Xu JQ (2020) BRD4 blockage alleviates pathological cardiac hypertrophy through the suppression of fibrosis and inflammation via reducing ROS generation. Biomed. Pharmacother. 121: 109368. doi: 10.1016/j.biopha.2019.109368.
170. Liu L, Zhang D, Li Y (2020) LncRNAs in cardiac hypertrophy: From basic science to clinical application. J. Cell Mol. Med. 24 (20): 11638-11645. doi: 10.1111/jcmm.15819.
171. Roma-Rodrigues C, Raposo LR, Fernandes AR (2015) MicroRNAs based therapy of hypertrophic cardiomyopathy: The road travelled so far. Biomed. Res. Int. 2015: 983290. doi: 10.1155/2015/983290.
172. Amin R, Muthuramu I, Aboumsallem JP, Mishra M, Jacobs F, De Geest B (2017) Selective HDL-raising human Apo A-I gene therapy counteracts cardiac hypertrophy, reduces myocardial fibrosis, and improves cardiac function in mice with chronic pressure overload. Int. J. Mol. Sci. 18 (9): 2012. doi: 10.3390/ijms18092012.
173. Behrens-Gawlik V, Mearini G, Gedicke-Hornung C, Richard P, Carrier L (2014) MYBPC3 in hypertrophic cardiomyopathy: from mutation identification to RNA-based correction. Pflugers. Arch. 466 (2): 215-223. doi: 10.1007/s00424-013-1409-7.
174. Carrier L, Mearini G, Stathopoulou K, Cuello F (2015) Cardiac myosin-binding protein C (MYBPC3) in cardiac pathophysiology. Gene 573 (2): 188-197. doi: 10.1016/j.gene.2015.09.008.
175. Ishikawa K, Fish KM, Tilemann L, Rapti K, Aguero J, Santos-Gallego CG, Lee A, Karakikes I, Xie C, Akar FG, Shimada YJ, Gwathmey JK, Asokan A, McPhee S, Samulski J, Samulski RJ, Sigg DC, Weber T, Kranias EG, Hajjar RJ (2014) Cardiac I-1c overexpression with reengineered AAV improves cardiac function in swine ischemic heart failure. Mol. Ther. 22 (12): 2038-2045. doi: 10.1038/mt.2014.127.
176. Jessup M, Greenberg B, Mancini D, Cappola T, Pauly DF, Jaski B, Yaroshinsky A, Zsebo KM, Dittrich H, Hajjar RJ (2011) Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID) Investigators: a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-ATPase in patients with advanced heart failure. Circulation 124 (3): 304-313. doi: 10.1161/CIRCULATIONAHA.111.022889.
177. Greenberg B, Butler J, Felker GM, Ponikowski P, Voors AA, Desai AS, Barnard D, Bouchard A, Jaski B, Lyon AR, Pogoda JM, Rudy JJ, Zsebo KM (2016) Calcium upregulation by percutaneous administration of gene therapy in patients with cardiac disease (CUPID 2): a randomised, multinational, double-blind, placebo-controlled, phase 2b trial. Lancet 387 (10024): 1178-1186. doi: 10.1016/S0140-6736(16)00082-9.
178. Wu YH, Swaab DF (2005) The human pineal gland and melatonin in aging and Alzheimer's disease. J. Pineal Res. 38 (3):145-152. doi: 10.1111/j.1600-079X.2004.00196. x.
179. Jiki Z, Lecour S, Nduhirabandi F (2018) Cardiovascular Benefits of Dietary Melatonin: A Myth or a Reality? Front. Physiol. 9: 528. doi: 10.3389/fphys.2018.00528.
180. Roohbakhsh A, Shamsizadeh A, Hayes AW, Reiter RJ, Karimi G (2018) Melatonin as an endogenous regulator of diseases: The role of autophagy. Pharmacol. Res. 133: 265-276. doi: 10.1016/j.phrs.2018.01.022.
181. Lochner A, Marais E, Huisamen B (2018) Melatonin and cardioprotection against ischaemia/reperfusion injury: What's new? A review. J. Pineal Res. 65 (1): e12490. doi: 10.1111/jpi.12490.
182. Szárszoi O, Asemu G, Vanecek J, Ost'ádal B, Kolár F (2001) Effects of melatonin on ischemia and reperfusion injury of the rat heart. Cardiovasc. Drugs Ther. 15 (3): 251-257. doi: 10.1023/a:1011920407691.
183. Dobsak P, Siegelova J, Eicher JC, Jancik J, Svacinova H, Vasku J, Kuchtickova S, Horky M, Wolf JE (2003) Melatonin protects against ischemia-reperfusion injury and inhibits apoptosis in isolated working rat heart. Pathophysiology 9 (3): 179-187. doi: 10.1016/s0928-4680(02)00080-9
184. Diez ER, Prados LV, Carrión A, Ponce ZA, Miatello RM (2009) A novel electrophysiologic effect of melatonin on ischemia/reperfusion-induced arrhythmias in isolated rat hearts. J. Pineal Res. 46 (2): 155-160. doi: 10.1111/j.1600-079X.2008.00643. x.
185. Han D, Huang W, Li X, Gao L, Su T, Li X, Ma S, Liu T, Li C, Chen J, Gao E, Cao F. (2016) Melatonin facilitates adipose-derived mesenchymal stem cells to repair the murine infarcted heart via the SIRT1 signaling pathway. J. Pineal Res. 60 (2): 178-192. doi: 10.1111/jpi.12299.
186. Nduhirabandi F, Lamont K, Albertyn Z, Opie LH, Lecour S (2016) Role of toll-like receptor 4 in melatonin-induced cardioprotection. J. Pineal Res. 60 (1): 39-47. doi: 10.1111/jpi.12286.
187. He B, Zhao Y, Xu L, Gao L, Su Y, Lin N, Pu J (2016) The nuclear melatonin receptor RORα is a novel endogenous defender against myocardial ischemia/reperfusion injury. J. Pineal Res. 60 (3): 313-26. doi: 10.1111/jpi.12312.
188. Paulis L, Simko F, Laudon M (2012) Cardiovascular effects of melatonin receptor agonists. Expert Opin. Investig. Drugs. 21 (11): 1661-1678. doi: 10.1517/13543784.2012.714771.
189. Genade S, Genis A, Ytrehus K, Huisamen B, Lochner A (2008) Melatonin receptor-mediated protection against myocardial ischaemia/reperfusion injury: role of its anti-adrenergic actions. J. Pineal Res. 45 (4): 449-458. doi: 10.1111/j.1600-079X.2008.00615. x.
190. Lochner A, Huisamen B, Nduhirabandi F (2013) Cardioprotective effect of melatonin against ischaemia/reperfusion damage. Front. Biosci. (Elite Ed). 5 (1): 305. doi: 10.2741/e617.
191. Tobeiha M, Jafari A, Fadaei S, Mirazimi SMA, Dashti F, Amiri A, Khan H, Asemi Z, Reiter RJ, Hamblin MR, Mirzaei H (2022) Evidence for the benefits of melatonin in cardiovascular disease. Front. Cardiovasc. Med. 9: 888319. doi: 10.3389/fcvm.2022.888319.
192. Jockers R, Delagrange P, Dubocovich ML, Markus RP, Renault N, Tosini G, Cecon E, Zlotos DP (2016) Update on melatonin receptors: IUPHAR Review 20. Br. J. Pharmacol. 173 (18): 2702-2725. doi: 10.1111/bph.13536.
193. Ekmekcioglu C, Thalhammer T, Humpeler S, Mehrabi MR, Glogar HD, Hölzenbein T, Markovic O, Leibetseder VJ, Strauss-Blasche G, Marktl W (2003)The melatonin receptor subtype MT2 is present in the human cardiovascular system. J. Pineal Res. 35 (1): 40-44. doi: 10.1034/j.1600-079x.2003.00051. x.
194. Pang CS, Xi SC, Brown GM, Pang SF, Shiu SY (2002)2[125I] Iodomelatonin binding and interaction with beta-adrenergic signaling in chick heart/coronary artery physiology. J Pineal Res. 32 (4): 243-252. doi: 10.1034/j.1600-079x.2002.01860. x.
195. Zhao D, Yu Y, Shen Y, Liu Q, Zhao Z, Sharma R, Reiter RJ (2019) Melatonin synthesis and function: Evolutionary history in animals and plants. Front. Endocrinol. (Lausanne) 10: 249. doi: 10.3389/fendo.2019.00249.
196. Mukherjee D, Ghosh AK, Dutta M, Mitra E, Mallick S, Saha B, Reiter RJ, Bandyopadhyay D. (2015) Mechanisms of isoproterenol-induced cardiac mitochondrial damage: protective actions of melatonin. J. Pineal Res. 58 (3): 275-290. doi: 10.1111/jpi.12213.
197. Liu J, Clough SJ, Hutchinson AJ, Adamah-Biassi EB, Popovska-Gorevski M, Dubocovich (2016) ML. MT1 and MT2 melatonin receptors: A therapeutic perspective. Annu. Rev. Pharmacol. Toxicol. 56: 361-383. doi: 10.1146/annurev-pharmtox-010814-124742.
198. Lochner A, Genade S, Davids A, Ytrehus K, Moolman JA (2006) Short- and long-term effects of melatonin on myocardial post-ischemic recovery. J. Pineal Res. 40 (1): 56-63. doi: 10.1111/j.1600-079X.2005.00280.x.
199. Lamont K, Nduhirabandi F, Adam T, Thomas DP, Opie LH, Lecour S (2015) Role of melatonin, melatonin receptors and STAT3 in the cardioprotective effect of chronic and moderate consumption of red wine. Biochem. Biophys. Res. Commun. 465 (4): 719-724. doi: 10.1016/j.bbrc.2015.08.064.
200. Nogueiras R, Habegger KM, Chaudhary N, Finan B, Banks AS, Dietrich MO, Horvath TL, Sinclair DA, Pfluger PT, Tschöp MH (2012) Sirtuin 1 and sirtuin 3: physiological modulators of metabolism. Physiol. Rev. 92 (3): 1479-1514. doi: 10.1152/physrev.00022.2011.
201. Yu L, Sun Y, Cheng L, Jin Z, Yang Y, Zhai M, Pei H, Wang X, Zhang H, Meng Q, Zhang Y, Yu S, Duan W (2014) Melatonin receptor-mediated protection against myocardial ischemia/reperfusion injury: role of SIRT1. J. Pineal Res. 57 (2): 228-238. doi: 10.1111/jpi.12161.
202. Feng J, Chen X, Liu R, Cao C, Zhang W, Zhao Y, Nie S (2018) Melatonin protects against myocardial ischemia-reperfusion injury by elevating Sirtuin3 expression and manganese superoxide dismutase activity. Free Radic. Res. 52 (8): 840-849. doi: 10.1080/10715762.2018.1461215.
203. Kurutas EB (2016) The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr. J. 15 (1): 71. doi: 10.1186/s12937-016-0186-5.
204. Huo X, Wang C, Yu Z, Peng Y, Wang S, Feng S, Zhang S, Tian X, Sun C, Liu K, Deng S, Ma X (2017) Human transporters, PEPT1/2, facilitate melatonin transportation into mitochondria of cancer cells: An implication of the therapeutic potential. J. Pineal Res. 62 (4). doi: 10.1111/jpi.12390.
205. Hevia D, González-Menéndez P, Quiros-González I, Miar A, Rodríguez-García A, Tan DX, Reiter RJ, Mayo JC, Sainz RM (2015) Melatonin uptake through glucose transporters: a new target for melatonin inhibition of cancer. J. Pineal Res. 58 (2): 234-250. doi: 10.1111/jpi.12210.
206. Del Re DP (2016) Hippo Signaling in the Heart-Non-Canonical Pathways Impact Growth, Survival and Function. Circ. J. 80 (7): 1504-1510. doi: 10.1253/circj. CJ-16-0426.
207. Higgins AY, O'Halloran TD, Chang JD (2015) Chemotherapy-induced cardiomyopathy. Heart Fail. Rev. 20 (6): 721-730. doi: 10.1007/s10741-015-9502-y.
208. Zhang YW, Shi J, Li YJ, Wei L (2009) Cardiomyocyte death in doxorubicin-induced cardiotoxicity. Arch. Immunol. Ther. Exp (Warsz). 57 (6): 435-445. doi: 10.1007/s00005-009-0051-8.
209. Zhou H, Li D, Zhu P, Ma Q, Toan S, Wang J, Hu S, Chen Y, Zhang Y (2018) Inhibitory effect of melatonin on necroptosis via repressing the Ripk3-PGAM5-CypD-mPTP pathway attenuates cardiac microvascular ischemia-reperfusion injury. J. Pineal Res. 65 (3): e12503. doi: 10.1111/jpi.1250329770487.
210. L'Heureux M, Sternberg M, Brath L, Turlington J, Kashiouris MG (2020) Sepsis-induced cardiomyopathy: a comprehensive review. Curr. Cardiol. Rep. 22 (5): 35. doi: 10.1007/s11886-020-01277-2.
211. Zhang J, Wang L, Xie W, Hu S, Zhou H, Zhu P, Zhu H (2020) Melatonin attenuates ER stress and mitochondrial damage in septic cardiomyopathy: A new mechanism involving BAP31 upregulation and MAPK-ERK pathway. J. Cell Physiol. 235 (3): 2847-2856. doi: 10.1002/jcp.29190.
212. Yang JB, Kang YM, Zhang C, Yu XJ, Chen WS (2019) Infusion of melatonin into the paraventricular nucleus ameliorates myocardial ischemia-reperfusion injury by regulating oxidative stress and inflammatory cytokines. J. Cardiovasc. Pharmacol. 74 (4): 336-347. doi: 10.1097/FJC.0000000000000711.
213. Zhou H, Zhang Y, Hu S, Shi C, Zhu P, Ma Q, Jin Q, Cao F, Tian F, Chen Y (2017) Melatonin protects cardiac microvasculature against ischemia/reperfusion injury via suppression of mitochondrial fission-VDAC1-HK2-mPTP-mitophagy axis. J. Pineal Res. 63 (1): e12413. doi: 10.1111/jpi.12413.
214. Cai B, Ma W, Bi C, Yang F, Zhang L, Han Z, Huang Q, Ding F, Li Y, Yan G, Pan Z, Yang B, Lu Y (2016) Long noncoding RNA H19 mediates melatonin inhibition of premature senescence of c-kit(+) cardiac progenitor cells by promoting miR-675. J. Pineal Res. 1 (1): 82-95. doi: 10.1111/jpi.12331.
215. Yang G, Song M, Hoang DH, Tran QH, Choe W, Kang I, Kim SS, Ha J (2020) Melatonin prevents doxorubicin-induced cardiotoxicity through suppression of AMPKα2-dependent mitochondrial damage. Exp. Mol. Med. 52 (12): 2055-2068. doi: 10.1038/s12276-020-00541-3.
216. Andersen LP, Gögenur I, Rosenberg J, Reiter RJ. (2016) The Safety of Melatonin in Humans. Clin. Drug Investig. 36 (3): 169-175. doi: 10.1007/s40261-015-0368-5.
217. Feldman AM, Weinberg EO, Ray PE, Lorell BH (1993) Selective changes in cardiac gene expression during compensated hypertrophy and the transition to cardiac decompensation in rats with chronic aortic banding. Circ. Res. 73 (1): 184-192. doi: 10.1161/01.res.73.1.184.
218. Pandya K, Pulli B, Bultman S, Smithies O (2010) Reversible epigenetic modifications of the two cardiac myosin heavy chain genes during changes in expression. Gene Expr. 15 (2): 51-59. doi: 10.3727/105221611x12973615737505.
219. Yeung HM, Hung MW, Fung ML (2008) Melatonin ameliorates calcium homeostasis in myocardial and ischemia-reperfusion injury in chronically hypoxic rats. J. Pineal Res. 45 (4): 373-382. doi: 10.1111/j.1600-079X.2008. 00601.x
220. Finck BN, Kelly DP. (2007) Peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1) regulatory cascade in cardiac physiology and disease. Circulation 115 (19): 2540-2548. doi: 10.1161/CIRCULATIONAHA.107.670588.
221. Mallilankaraman K, Doonan P, Cárdenas C, Chandramoorthy HC, Müller M, Miller R, Hoffman NE, Gandhirajan RK, Molgó J, Birnbaum MJ, Rothberg BS, Mak DO, Foskett JK, Madesh M (2012) MICU1 is an essential gatekeeper for MCU-mediated mitochondrial Ca (2+) uptake that regulates cell survival. Cell 151 (3): 630-644. doi: 10.1016/j.cell.2012.10.011.
222. Yang Y, Du J, Xu R, Shen Y, Yang D, Li D, Hu H, Pei H, Yang Y (2020) Melatonin alleviates angiotensin-II-induced cardiac hypertrophy via activating MICU1 pathway. Aging (Albany NY). 13 (1): 493-515. doi: 10.18632/aging.202159.
223. Maarman G, Blackhurst D, Thienemann F, Blauwet L, Butrous G, Davies N, Sliwa K, Lecour S (2015) Melatonin as a preventive and curative therapy against pulmonary hypertension. J. Pineal Res. 59 (3): 343-353. doi: 10.1111/jpi.12263.
224. Levine B, Kroemer G. (2019) Biological functions of autophagy genes: A disease perspective. Cell. 176 (1-2): 11-42. doi: 10.1016/j.cell.2018.09.048.
225. Weng LQ, Zhang WB, Ye Y, Yin PP, Yuan J, Wang XX, Kang L, Jiang SS, You JY, Wu J, Gong H, Ge JB, Zou YZ (2014) Aliskiren ameliorates pressure overload-induced heart hypertrophy and fibrosis in mice. Acta.Pharmacol. Sin. 2014 35 (8): 1005-1014. doi: 10.1038/aps.2014.45.
226. Xu CN, Kong LH, Ding P, Liu Y, Fan ZG, Gao EH, Yang J, Yang LF (2020) Melatonin ameliorates pressure overload-induced cardiac hypertrophy by attenuating Atg5-dependent autophagy and activating the Akt/mTOR pathway. Biochem. Biophys. Acta. Mol. Basis. Dis. 1866 (10): 165848. doi: 10.1016/j.bbadis.2020.165848.
227. Girotti L, Lago M, Ianovsky O, Elizari MV, Dini A, Pérez Lloret S, Albornoz LE, Cardinali DP (2003) Low urinary 6-sulfatoxymelatonin levels in patients with severe congestive heart failure. Endocrine 22 (3): 245-8. doi: 10.1385/ENDO:22:3:245.
228. Song YJ, Zhong CB, Wu W (2020) Cardioprotective effects of melatonin: Focusing on its roles against diabetic cardiomyopathy. Biomed. Pharmacother. 128: 110260. doi: 10.1016/j.biopha.2020.110260.
229. Zhao X, Wang X, Wang J, Yuan J, Zhang J, Zhu X, Lei C, Yang Q, Wang B, Cao F, Liu L (2020) A Peptide-functionalized magnetic nanoplatform-loaded melatonin for targeted amelioration of fibrosis in pressure overload-induced cardiac hypertrophy. Int. J. Nanomedicine. 15: 1321-1333. doi: 10.2147/IJN.S235518.
230. Jiang J, Liang S, Zhang J, Du Z, Xu Q, Duan J, Sun Z (2021) Melatonin ameliorates PM2.5 -induced cardiac perivascular fibrosis through regulating mitochondrial redox homeostasis. J. Pineal. Res. 70 (1): e12686. doi: 10.1111/jpi.12686.
231. Lu Q, Yi X, Cheng X, Sun X, Yang X (2015) Melatonin protects against myocardial hypertrophy induced by lipopolysaccharide. In Vitro Cell. Dev. Biol. Anim. 51 (4):353-360. doi: 10.1007/s11626-014-9844-0.
232. Xu L, Su Y, Zhao Y, Sheng X, Tong R, Ying X, Gao L, Ji Q, Gao Y, Yan Y, Yuan A, Wu F, Lan F, Pu J (2019) Melatonin differentially regulates pathological and physiological cardiac hypertrophy: Crucial role of circadian nuclear receptor RORα signaling. J. Pineal. Res. 67 (2): e12579. doi: 10.1111/jpi.12579.
233. Favero G, Franco C, Stacchiotti A, Rodella LF, Rezzani R (2020) Sirtuin1 Role in the Melatonin Protective Effects Against Obesity-Related Heart Injury. Front. Physiol. 11: 103. doi: 10.3389/fphys.2020.00103.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


