Why are aging and stress associated with dementia, cancer, and other diverse medical conditions? Role of pineal melatonin interactions with the HPA axis in mitochondrial regulation via BAG-1
Melatonin, cortisol, BAG-1 and mitochondria
Abstract
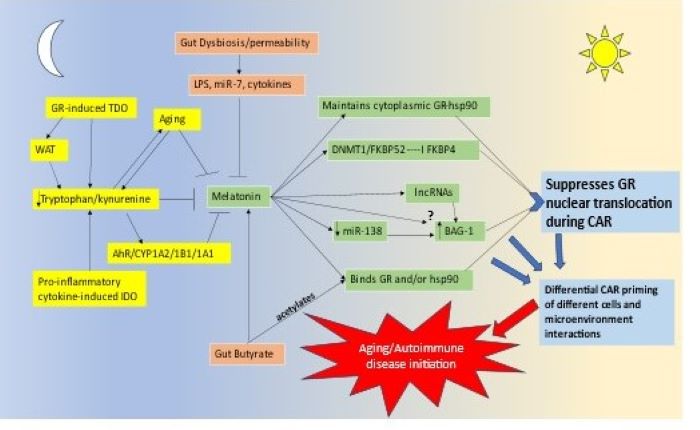
Pineal melatonin and the cortisol awakening response (CAR) are integral aspects of the circadian rhythm. Pineal melatonin release during sleep is proposed to optimize mitochondrial function and dampen any residual oxidant and inflammatory activity. Little is known about CAR, which is generally thought to prepare the body for the coming day, primarily through the activation of the glucocorticoid receptor (GR). Melatonin, like the gut microbiome-derived butyrate, suppresses GR nuclear translocation, indicating that pineal melatonin and night-time butyrate may interact to modulate CAR effects via the GR, including CAR priming of immune and glia cells that underpin the pathogenesis of most medical conditions. Cutting edge research shows that the GR can be chaperoned by bcl2-associated athanogene (BAG)-1 to mitochondria, where GR can have significant and diverse impacts on mitochondrial function. A number of lines of evidence indicate that melatonin indirectly increases BAG-1, including via epigenetic mechanisms, such as derepressing miR-138 inhibition of BAG-1. The dramatic decrease in pineal melatonin production over aging will therefore have significant impacts on GR nuclear translocation, but also possibly the levels of BAG-1 mediated mitochondrial translocation of the GR. This may have dramatic consequences for how CAR ‘prepares the body for the coming day’, via the differential consequence of GR location in the cytoplasm, nucleus or mitochondria, with differential effects in different cell types. The interactions of melatonin/butyrate/BAG-1/GR are especially important in the hypothalamus, where a maintained heightened melatonin concentration occurs over the night due to the direct release of pineal melatonin, via the pineal recess, into the third ventricle. The interaction of melatonin/butyrate/BAG-1/GR will have differential effects in different cell types, thereby altering the intercellular homeostatic interactions in a given microenvironment that will contribute to the pathogenesis of many aging-associated conditions, including neurodegenerative conditions and cancer. This reframes the nature of the circadian rhythm as well as how stress-associated hypothalamus-pituitary-adrenal (HPA) axis may modulate both the pathogenesis and course of diverse medical presentations. This has a number of research and treatment implications across a host of current medical conditions.
References
2. Cheng WY, Ho YS, Chang RC (2022) Linking circadian rhythms to microbiome-gut-brain axis in aging-associated neurodegenerative diseases. Ageing Res. Rev. 78: 101620. https://doi.org/10.1016/j.arr.2022.101620.
3. Maldonado E, Morales-Pison S, Urbina F, Solari A (2023) Aging hallmarks and the role of oxidative stress. Antioxidants (Basel). 12 (3): 651. https://doi.org/10.3390/antiox12030651.
4. James KA, Stromin JI, Steenkamp N, Combrinck MI (2023) Understanding the relationships between physiological and psychosocial stress, cortisol and cognition. Front. Endocrinol. (Lausanne). 14: 1085950. https://doi.org/10.3389/fendo.2023.1085950.
5. Simons RL, Ong ML, Beach SRH, Lei MK, Philibert R, Mielke MM (2023) Direct and indirect effects of socioeconomic status and discrimination on subjective cognitive decline: A longitudinal study of African American women. J. Gerontol. B. Psychol. Sci. Soc. Sci. 78 (5): 799–808. https://doi.org/10.1093/geronb/gbad029.
6. Petrican R, Fornito, A (2023) Adolescent neurodevelopment and psychopathology: The interplay between adversity exposure and genetic risk for accelerated brain ageing. Dev. Cogn. Neurosci. 60: 101229. https://doi.org/10.1016/j.dcn.2023.101229.
7. Reed RG, Presnell S.R, Al-Attar, A, Lutz, C. T, Segerstrom, SC (2023) Life stressors and immune aging: Protective effects of cognitive reappraisal. Brain. Behav. Immun. 110: 212–221. https://doi.org/10.1016/j.bbi.2023.02.018.
8. Bürgin D, Varghese N, Eckert A, Clemens V, Unternährer E, Boonmann C, O'Donovan A, Schmid M (2022) Higher hair cortisol concentrations associated with shorter leukocyte telomere length in high-risk young adults. Sc. Rep. 12 (1): 11730. https://doi.org/10.1038/s41598-022-14905-4.
9. De Gaetano A, Gibellini L, Zanini G, Nasi M, Cossarizza A, Pinti M (2021) Mitophagy and oxidative stress: The role of aging. Antioxidants (Basel) 10 (5): 794. https://doi.org/10.3390/antiox10050794.
10. Anderson G, Almulla AF, Reiter RJ, Maes M (2023) Redefining autoimmune disorders' pathoetiology: Implications for mood and psychotic disorders' association with neurodegenerative and classical autoimmune disorders. Cells 12 (9): 1237. https://doi.org/10.3390/cells12091237.
11. Anderson G. (2023) Type I diabetes pathoetiology and pathophysiology: Roles of the gut microbiome, pancreatic cellular interactions, and the 'bystander' activation of memory CD8+ T cells. Int. J. Mol. Sci. 24 (4): 3300. https://doi.org/10.3390/ijms24043300.
12. Anderson G. (2020) Tumour microenvironment: roles of the aryl hydrocarbon receptor, O-GlcNAcylation, acetyl-CoA and melatonergic pathway in regulating dynamic metabolic interactions across cell types- Tumour microenvironment and metabolism. Int. J. Mol. Sci. 22 (1): 141. https://doi.org/10.3390/ijms22010141.
13. Cheng A, Hou Y,Mattson MP (2010) Mitochondria and neuroplasticity. ASN. neuro. 2 (5): e00045. https://doi.org/10.1042/AN20100019.
14. Silva AM, Ribeiro CT, Bernardino RL, Jarak I, Carvalho RA, Pereira-Sampaio MA, de Souza DB, Alves MG, Oliveira PF (2022) Stress hormone corticosterone controls metabolic mitochondrial performance and inflammatory signaling of in vitro cultured Sertoli cells. Biomedicines 10 (9): 2331. https://doi.org/10.3390/biomedicines10092331.
15. Hou Y, Xie J, Wang S, Li D, Wang L, Wang H, Ni X, Leng S, Li G, Hou M, Peng J (2022) Glucocorticoid receptor modulates myeloid-derived suppressor cell function via mitochondrial metabolism in immune thrombocytopenia. Cell. Mol. Immunol. 19 (7): 764–776. https://doi.org/10.1038/s41423-022-00859-0.
16. Yuan J, Gao YS, Liu DL, Pang Tai AC, Zhou H, Papadimitriou JM, Zhang CQ, Zheng MH, Gao JJ (2022) PINK1-mediated mitophagy contributes to glucocorticoid-induced cathepsin K production in osteocytes. J. Orthop. Translat. 38: 229–240. https://doi.org/10.1016/j.jot.2022.11.003.
17. Choi GE, Lee HJ, Chae CW, Cho JH, Jung YH, Kim JS, Kim SY, Lim JR, Han HJ (2021) BNIP3L/NIX-mediated mitophagy protects against glucocorticoid-induced synapse defects. Nat. Commun. 12(1):487. https://doi.org/10.1038/s41467-020-20679-y.
18. Tesic V, Ciric J, Jovanovic Macura I, Zogovic N, Milanovic D, Kanazir S, Perovic M (2021) Corticosterone and glucocorticoid receptor in the cortex of rats during aging-The effects of long-term food restriction. Nutrients. 1 (12): 4526. https://doi.org/10.3390/nu13124526.
19. Verma AK, Singh S, Rizvi SI (2023) Aging, circadian disruption and neurodegeneration: Interesting interplay. Exp. Gerontol. 172: 112076. https://doi.org/10.1016/j.exger.2022.112076.
20. Ahmad F, Sachdeva P, Sarkar J, Izhaar R (2022) Circadian dysfunction and Alzheimer's disease - An updated review. Aging Med. (Milton). 6 (1): 71–81. https://doi.org/10.1002/agm2.12221.
21. Karasek M, Reiter RJ (2002) Melatonin and aging. Neuro. Endo. Letts. 23(Sup 1):14–16.
22. Reiter RJ, Sharma R, Cucielo MS, Tan DX, Rosales-Corral S, Gancitano G, de Almeida Chuffa LG (2023) Brain washing and neural health: role of age, sleep, and the cerebrospinal fluid melatonin rhythm. Cell. Mol. Life. Sci. 80 (4): 88. https://doi.org/10.1007/s00018-023-04736-5.
23. Reiter RJ, Sharma R, Ma, Q, Rosales-Corral SA, Acuna-Castroviejo D, Escames G (2019) Inhibition of mitochondrial pyruvate dehydrogenase kinase: a proposed mechanism by which melatonin causes cancer cells to overcome aerobic glycolysis, limit tumor growth and reverse insensitivity to chemotherapy. Melatonin Res. 2 (3): 105-119. doi:10.32794/mr11250033.
24. Anderson G (2019) Daytime orexin and night-time melatonin regulation of mitochondria melatonin roles in circadian oscillations systemically and centrally in breast cancer symptomatology. Melatonin Res. 2 (4): 1-8; doi: 10.32794/mr11250037.
25. Zheng Z, Zhang S, Zhang H, Gao Z, Wang X, Liu X, Xue C, Yao L, Lu G (2022) Mechanisms of autoimmune cell in DA neuron apoptosis of Parkinson's disease: Recent advancement. Oxid. Med. Cell. Longev. 2022: 7965433. https://doi.org/10.1155/2022/7965433.
26. Raza S, Rajak S, Srivastava J, Tewari A, Gupta P, Chakravarti B, Ghosh S, Chaturvedi CP, Sinha RA (2022) ULK1 inhibition attenuates telomerase activity in hepatic cells. Biochim. Biophys. Acta. Mol. Cell. Res. 1869 (12): 119355. https://doi.org/10.1016/j.bbamcr.2022.119355.
27. Dong L, Sun Q, Qiu H, Yang K, Xiao B, Xia T, Wang A, Gao H, Zhang S (2023) Melatonin protects against developmental PBDE-47 neurotoxicity by targeting the AMPK/mitophagy axis. J. Pineal Res. 75 (1): e12871. https://doi.org/10.1111/jpi.12871.
28. Maestroni GJ, Conti A, Pierpaoli W (1986) Role of the pineal gland in immunity. circadian synthesis and release of melatonin modulates the antibody response and antagonizes the immunosuppressive effect of corticosterone. J. Neuroimmunol. 13 (1): 19-30. doi: 10.1016/0165-5728(86)90047-0.
29. Pierpaoli W, Maestroni GJ (1987) Melatonin: a principal neuroimmunoregulatory and anti-stress hormone: its anti-aging effects. Immunol. Lett. 16 (3-4): 355-361. doi: 10.1016/0165-2478(87)90169-6.
30. Lesnikov VA, Korneva EA, Dall'ara A, Pierpaoli W (1992) The involvement of pineal gland and melatonin in immunity and aging: II. Thyrotropin-releasing hormone and melatonin forestall involution and promote reconstitution of the thymus in anterior hypothalamic area (AHA)-lesioned mice. Int. J. Neurosci. 62 (1-2): 141-53. doi: 10.3109/00207459108999767.
31. Williams WR (2018). Dampening of neurotransmitter action: molecular similarity within the melatonin structure. Endocr. Regul. 52 (4): 199-207. doi: 10.2478/enr-2018-0025.
32. Kanelakis KC, Morishima Y, Dittmar KD, Galigniana MD, Takayama S, Reed JC, Pratt WB (1999) Differential effects of the hsp70-binding protein BAG-1 on glucocorticoid receptor folding by the hsp90-based chaperone machinery. J. Biol. Chem. 274 (48): 34134–34140. https://doi.org/10.1074/jbc.274.48.34134.
33. Luo S, Hou Y, Zhang Y, Feng L, Hunter RG, Yuan P, Jia Y, Li H, Wang G, K Manji H, S McEwen B, Xiao C, Bao H, Du J (2021) Bag-1 mediates glucocorticoid receptor trafficking to mitochondria after corticosterone stimulation: Potential role in regulating affective resilience. J. Neurochem. 158 (2): 358–372. https://doi.org/10.1111/jnc.15211.
34. Tobeiha M, Jafari A, Fadaei S, Mirazimi SMA, Dashti F, Amiri A, Khan H, Asemi Z, Reiter RJ, Hamblin MR, Mirzaei H (2022) Evidence for the benefits of melatonin in cardiovascular disease. Front. Cardiovasc. Med. 9: 888319. https://doi.org/10.3389/fcvm.2022.888319.
35. Davoodvandi A, Nikfar B, Reiter RJ, Asemi Z (2022) Melatonin and cancer suppression: insights into its effects on DNA methylation. Cell Mol Biol Lett. 27 (1): 73. https://doi.org/10.1186/s11658-022-00375-z.
36. Winge I, McKinney JA, Ying M, D'Santos, CS, Kleppe R, Knappskog, PM, Haavik J (2008) Activation and stabilization of human tryptophan hydroxylase 2 by phosphorylation and 14-3-3 binding. Biochem. J. 410 (1): 195–204. https://doi.org/10.1042/BJ20071033.
37. Aleshin VA, Artiukhov AV, Kaehne T, Graf AV, Bunik VI (2021) Daytime dependence of the activity of the rat brain pyruvate dehydrogenase corresponds to the mitochondrial sirtuin 3 level and acetylation of brain proteins, all regulated by thiamine administration decreasing phosphorylation of PDHA Ser293. Int. J. Mol. Sci. 22 (15): 8006. https://doi.org/10.3390/ijms22158006.
38. Li HY, Cai ZY (2022) SIRT3 regulates mitochondrial biogenesis in aging-related diseases. J. Biomed. Res. 37 (2): 77-88. https://doi.org/10.7555/JBR.36.20220078.
39. Bai Y, Yang Y, Gao Y, Lin D, Wang Z, Ma J (2021) Melatonin postconditioning ameliorates anoxia/reoxygenation injury by regulating mitophagy and mitochondrial dynamics in a SIRT3-dependent manner. Eur J Pharmacol. 904: 174157. https://doi.org/10.1016/j.ejphar.2021.174157.
40. Anderson G, Maes M (2020) Gut dysbiosis dysregulates central and systemic homeostasis via suboptimal mitochondrial function: assessment, treatment and classification implications. Curr. Top. Med. Chem. 20 (7): 524–539. https://doi.org/10.2174/1568026620666200131094445.
41. Jin CJ, Engstler AJ, Sellmann C, Ziegenhardt D, Landmann M, Kanuri G, Lounis H, Schröder M, Vetter W, Bergheim I (2016) Sodium butyrate protects mice from the development of the early signs of non-alcoholic fatty liver disease: role of melatonin and lipid peroxidation. Br. J. Nutr. 116 (10): 1682–1693. https://doi.org/10.1017/S0007114516004025.
42. Mokkawes T, de Visser SP (2023) Melatonin activation by cytochrome P450 isozymes: How does CYP1A2 compare to CYP1A1? Int. J. Mol. Sci. 24 (4): 3651. https://doi.org/10.3390/ijms24043651.
43. Ma X, Idle JR, Krausz KW, Gonzalez FJ (2005) Metabolism of melatonin by human cytochromes p450. Drug. Metab. Dispos. 33 (4): 489–494. https://doi.org/10.1124/dmd.104.002410.
44. Ferreira ZS, Garcia CR, Spray DC, Markus RP (2003) P2Y(1) receptor activation enhances the rate of rat pinealocyte-induced extracellular acidification via a calcium-dependent mechanism. Pharmacology 69 (1): 33–37. https://doi.org/10.1159/000071264.
45. Souza-Teodoro LH, Dargenio-Garcia L, Petrilli-Lapa CL, Souza Eda S, Fernandes PA, Markus RP, Ferreira ZS (2016) Adenosine triphosphate inhibits melatonin synthesis in the rat pineal gland. J. Pineal Res. 60 (2): 242–249. https://doi.org/10.1111/jpi.12309.
46. Sousa KS, Quiles CL, Muxel SM, Trevisan IL, Ferreira ZS, Markus RP (2022) Brain damage-linked ATP promotes P2X7 receptors mediated pineal N-acetylserotonin release. Neuroscience 499: 12–22. https://doi.org/10.1016/j.neuroscience.2022.06.039.
47. Jang SW, Liu X, Pradoldej S, Tosini G, Chang Q, Iuvone PM, Ye K (2010) N-acetylserotonin activates TrkB receptor in a circadian rhythm. Proc. Natl. Acad. Sci. USA. 107 (8): 3876–3881. https://doi.org/10.1073/pnas.0912531107.
48. Kang JH, Guo XD, Wang YD, Kang XW (2023) Neuroprotective effects of N-acetylserotonin and its derivative. Neuroscience 517: 18–25. https://doi.org/10.1016/j.neuroscience.2023.02.017.
49. Yoo DY, Nam SM, Kim W, Lee CH, Won MH, Hwang IK, Yoon YS (2011) N-acetylserotonin increases cell proliferation and differentiating neuroblasts with tertiary dendrites through upregulation of brain-derived neurotrophic factor in the mouse dentate gyrus. J. Vet. Med. Sci. 73 (11): 1411–1416. https://doi.org/10.1292/jvms.11-0123.
50. Anderson G, Reiter RJ (2019) Glioblastoma: Role of mitochondria N-acetylserotonin/melatonin ratio in mediating effects of miR-451 and aryl hydrocarbon receptor and in coordinating wider biochemical changes. Int. J. Tryptophan. Res. 12: 1178646919855942. https://doi.org/10.1177/1178646919855942.
51. Wang S, Duan H, Li B, Hong W, Li X, Wang Y, Guo ZC (2022) BDNF and TrKB expression levels in patients with endometriosis and their associations with dysmenorrhoea. J. Ovarian. Res. 15 (1): 35. https://doi.org/10.1186/s13048-022-00963-9.
52. Anderson G (2019) Endometriosis pathoetiology and pathophysiology: Roles of vitamin A, estrogen, immunity, adipocytes, gut microbiome and melatonergic pathway on mitochondria regulation. Biomol. Concepts. 10 (1): 133–149. https://doi.org/10.1515/bmc-2019-0017.
53. Park S, Ham J, Yang C, Park W, Park H, An G, Song J, Hong T, Park SJ, Kim HS, Song G, Lim W (2023) Melatonin inhibits endometriosis development by disrupting mitochondrial function and regulating tiRNAs. J. Pineal. Res. 74 (1): e12842. https://doi.org/10.1111/jpi.12842.
54. Anderson G, Maes M (2017) Interactions of tryptophan and its catabolites with melatonin and the alpha 7 nicotinic receptor in central nervous system and psychiatric disorders: Role of the aryl hydrocarbon receptor and direct mitochondria regulation. Int. J. Tryptophan Res. 10: 1178646917691738. https://doi.org/10.1177/1178646917691738.
55. Markus RP, Silva CL, Franco DG, Barbosa EM Jr, Ferreira ZS (2010) Is modulation of nicotinic acetylcholine receptors by melatonin relevant for therapy with cholinergic drugs? Pharmacol. Ther. 126 (3): 251–262. https://doi.org/10.1016/j.pharmthera.2010.02.009.
56. Barbotin AL, Mimouni NEH, Kuchcinski G, Lopes R, Viard R, Rasika S, Mazur D, Silva MSB, Simon V, Boursier A, Pruvo JP, Yu Q, Candlish M, Boehm U, Bello FD, Medana C, Pigny P, Dewailly D, Prevot V, Catteau-Jonard S, Giacobini P (2023) Hypothalamic neuroglial plasticity is regulated by anti-Müllerian hormone and disrupted in polycystic ovary syndrome. EbioMedicine 90: 104535. https://doi.org/10.1016/j.ebiom.2023.104535.
57. Imbernon M, Saponaro C, Helms HCC, Duquenne M, Fernandois D, Deligia E, Denis RGP, Chao DHM, Rasika S, Staels B, Pattou F, Pfrieger FW, Brodin B, Luquet S, Bonner C, Prevot V (2022) Tanycytes control hypothalamic liraglutide uptake and its anti-obesity actions. Cell Metab. 34 (7): 1054–1063.e7. https://doi.org/10.1016/j.cmet.2022.06.002.
58. Desroziers E (2022) Unusual suspects: Glial cells in fertility regulation and their suspected role in polycystic ovary syndrome. J. Neuroendocrinol. 34 (6): e13136. https://doi.org/10.1111/jne.13136.
59. Guo L, Qi YJ, Tan H, Dai D, Balesar R, Sluiter A, van Heerikhuize J, Hu SH, Swaab DF, Bao AM (2022) Different oxytocin and corticotropin-releasing hormone system changes in bipolar disorder and major depressive disorder patients. EBioMedicine 84: 104266. https://doi.org/10.1016/j.ebiom.2022.104266.
60. Vanuytsel T, van Wanrooy S, Vanheel H, Vanormelingen C, Verschueren S, Houben E, Salim Rasoel S, Tόth J, Holvoet L, Farré R, Van Oudenhove L, Boeckxstaens G, Verbeke K, Tack J (2014) Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut 63 (8): 1293–1299. https://doi.org/10.1136/gutjnl-2013-305690.
61. Wolkowitz OM (1994) Prospective controlled studies of the behavioral and biological effects of exogenous corticosteroids. Psychoneuroendocrinology 19 (3): 233–255. https://doi.org/10.1016/0306-4530(94)90064-7.
62. Fenton CG, Crastin A, Martin CS, Suresh S, Montagna I, Hussain B, Naylor AJ, Jones SW, Hansen MS, Gorvin CM, Price M, Filer A, Cooper MS, Lavery GG, Raza K, Hardy RS (2022) 11β-Hydroxysteroid dehydrogenase type 1 within osteoclasts mediates the bone protective properties of therapeutic corticosteroids in chronic inflammation. Int. J. Mol. Sci. 23 (13): 7334. https://doi.org/10.3390/ijms23137334.
63. Kim SH, Hong JY, Bae S, Lee H, Wi YM, Ko JH, Kim B, Joo EJ, Seok H, Shi HJ, Yoo JR, Hyun M, Kim HA, Jang S, Mun SJ, Kim J, Kim MC, Jung DS, Kim SH, Peck KR (2022) Risk factors for coronavirus disease 2019 (COVID-19)-associated pulmonary aspergillosis in critically ill patients: A nationwide, multicenter, retrospective cohort study. J. Korean Med. Sci. 37 (18): e134. https://doi.org/10.3346/jkms.2022.37.e134.
64. Hasan ZT, Atrakji DMQYMAA, Mehuaiden DAK (2022) The effect of melatonin on thrombosis, sepsis and mortality rate in COVID-19 Patients. Int. J. Infect. Dis. 114: 79–84. https://doi.org/10.1016/j.ijid.2021.10.012.
65. Anderson G, Carbone, A, Mazzoccoli G (2021) Tryptophan metabolites and aryl hydrocarbon receptor in severe acute respiratory syndrome, coronavirus-2 (SARS-CoV-2) pathophysiology. Int. J. Mol. Sci. 22 (4): 1597. https://doi.org/10.3390/ijms22041597.
66. Yamada K, Sato H, Sakamaki K, Kamada M, Okuno Y, Fukuishi N, Furuta K, Tanaka S (2019) Suppression of IgE-independent degranulation of murine connective tissue-type mast cells by Dexamethasone. Cells 8 (2): 112. https://doi.org/10.3390/cells8020112.
67. Feng Q, Xu M, Yu YY, Hou Y, Mi X, Sun YX, Ma S, Zuo XY, Shao LL, Hou M, Zhang XH, Peng J (2017) High-dose dexamethasone or all-trans-retinoic acid restores the balance of macrophages towards M2 in immune thrombocytopenia. J. Thromb. Haemost. 15 (9): 1845–1858. https://doi.org/10.1111/jth.13767.
68. Picard K, Bisht K, Poggini S, Garofalo S, Golia MT, Basilico B, Abdallah F, Ciano Albanese N, Amrein I, Vernoux N, Sharma K, Hui CW, C Savage J, Limatola C, Ragozzino D, Maggi L, Branchi I, Tremblay MÈ (2021) Microglial-glucocorticoid receptor depletion alters the response of hippocampal microglia and neurons in a chronic unpredictable mild stress paradigm in female mice. Brain Behav. Immun. 97: 423–439. https://doi.org/10.1016/j.bbi.2021.07.022.
69. Diao L, Hierweger AM, Wieczorek A, Arck PC, Thiele K (2021) Disruption of glucocorticoid action on CD11c+ dendritic cells favors the generation of CD4+ regulatory T cells and improves fetal development in mice. Front. Immunol. 12: 729742. https://doi.org/10.3389/fimmu.2021.729742.
70. Paolino S, Cutolo M, Pizzorni C (2017) Glucocorticoid management in rheumatoid arthritis: morning or night low dose? Reumatologia 55 (4): 189–197. https://doi.org/10.5114/reum.2017.69779
71. Adami G, Fassio A, Rossini M, Bertelle D, Pistillo F, Benini C, Viapiana O, Gatti D (2023) Tapering glucocorticoids and risk of flare in rheumatoid arthritis on biological disease-modifying antirheumatic drugs (bDMARDs). RMD Open. 9 (1): e002792. https://doi.org/10.1136/rmdopen-2022-002792.
72. Law R, Clow A (2020) Stress, the cortisol awakening response and cognitive function. Int. Rev. Neurobiol. 150: 187–217. https://doi.org/10.1016/bs.irn.2020.01.001.
73. Ennis GE, Moffat SD, Hertzog C (2016) The cortisol awakening response and cognition across the adult lifespan. Brain. Cogn. 105: 66–77. https://doi.org/10.1016/j.bandc.2016.04.001.
74. Trevino CM, Geier T, Morris R, Cronn S, deRoon-Cassini T (2022) Relationship between decreased cortisol and development of chronic pain in traumatically injured. J. Surg. Res. 270: 286–292. https://doi.org/10.1016/j.jss.2021.08.040.
75. Bagnato G, Cordova F, Sciortino D, Miceli G, Bruno A, Ferrera A, Sangari D, Coppolino G, Muscatello MRA, Pandolfo G, Zoccali RA, Roberts WN (2018) Association between cortisol levels and pain threshold in systemic sclerosis and major depression. Rheumatol. Int. 38 (3): 433–441. https://doi.org/10.1007/s00296-017-3866-3.
76. Paananen M, O'Sullivan P, Straker L, Beales D, Coenen P, Karppinen J, Pennell C, Smith A (2015) A low cortisol response to stress is associated with musculoskeletal pain combined with increased pain sensitivity in young adults: a longitudinal cohort study. Arthritis Res. Ther. 17: 355. https://doi.org/10.1186/s13075-015-0875-z.
77. Thomas SJ, Larkin T (2020) Cognitive distortions in relation to plasma cortisol and oxytocin levels in major depressive disorder. Front. Psychiatry 10: 971. https://doi.org/10.3389/fpsyt.2019.00971.
78. Hua G, Paulen L, Chambon P (2016) GR SUMOylation and formation of an SUMO-SMRT/NCoR1-HDAC3 repressing complex is mandatory for GC-induced IR nGRE-mediated transrepression. Proc. Natl. Acad. Sci. USA. 113 (5): E626–E634. https://doi.org/10.1073/pnas.1522821113.
79. Li ZY, Jiang YM, Liu YM, Guo Z, Shen SN, Liu XM, Pan RL (2014) Saikosaponin D acts against corticosterone-induced apoptosis via regulation of mitochondrial GR translocation and a GR-dependent pathway. Prog. Neuropsychopharmacol. Biol. Psychiatry 53: 80–89. https://doi.org/10.1016/j.pnpbp.2014.02.010.
80. Kokkinopoulou I, Moutsatsou P (2021) Mitochondrial glucocorticoid receptors and their actions. Int. J. Mol. Sci. 22 (11): 6054. https://doi.org/10.3390/ijms22116054.
81. Li ZY, Li QZ, Chen L, Chen BD, Zhang C, Wang X, Li WP (2016) HPOB, an HDAC6 inhibitor, attenuates corticosterone-induced injury in rat adrenal pheochromocytoma PC12 cells by inhibiting mitochondrial GR translocation and the intrinsic apoptosis pathway. Neurochem. Int. 99: 239–251. https://doi.org/10.1016/j.neuint.2016.08.004.
82. Kim M, Lee HA, Cho HM, Kang SH, Lee E, Kim IK (2018) Histone deacetylase inhibition attenuates hepatic steatosis in rats with experimental Cushing's syndrome. Korean. J. Physiol. Pharmacol. 22 (1): 23–33. https://doi.org/10.4196/kjpp.2018.22.1.23.
83. Zhang L, Chen C, Qi J (2020) Activation of HDAC4 and GR signaling contributes to stress-induced hyperalgesia in the medial prefrontal cortex of rats. Brain Res. 1747: 147051. https://doi.org/10.1016/j.brainres.2020.147051.
84. Kuzmochka C, Abdou HS, Haché RJ, Atlas E (2014) Inactivation of histone deacetylase 1 (HDAC1) but not HDAC2 is required for the glucocorticoid-dependent CCAAT/enhancer-binding protein α (C/EBPα) expression and preadipocyte differentiation. Endocrinology 155 (12): 4762–4773. https://doi.org/10.1210/en.2014-1565.
85. Vishwas DK, Mukherjee A, Haldar C (2013) Melatonin improves humoral and cell-mediated immune responses of male golden hamster following stress induced by dexamethasone. J. Neuroimmunol. 259 (1-2): 17–25. https://doi.org/10.1016/j.jneuroim.2013.03.002.
86. Giudice A, Aliberti SM, Barbieri A, Pentangelo P, Bisogno I, D'Arena G, Cianciola E, Caraglia M, Capunzo M (2022) Potential mechanisms by which glucocorticoids induce breast carcinogenesis through Nrf2 inhibition. Front. Biosci. (Landmark Ed) 27 (7): 223. https://doi.org/10.31083/j.fbl2707223.
87. Pal Chowdhury J, Haldar C (2022) Stress associated ovarian dysfunctions in a seasonal breeder Funambulus pennanti: Role of glucocorticoids and possible amelioration by melatonin. Gen. Comp. Endocrinol. 316: 113962. https://doi.org/10.1016/j.ygcen.2021.113962.
88. Shi XT, Zhu HL, Xu XF, Xiong YW, Dai LM, Zhou GX, Liu WB, Zhang YF, Xu DX, Wang H (2021) Gestational cadmium exposure impairs placental angiogenesis via activating GC/GR signaling. Ecotoxicol. Environ. Saf. 224: 112632. https://doi.org/10.1016/j.ecoenv.2021.112632.
89. Zhou J, Zhang J, Luo X, Li M, Yue Y, Laudon M, Jia Z, Zhang R (2017) Neu-P11, a novel MT1/MT2 agonist, reverses diabetes by suppressing the hypothalamic-pituitary-adrenal axis in rats. Eur. J. Pharmacol. 812: 225–233. https://doi.org/10.1016/j.ejphar.2017.07.001.
90. Quiros I, Mayo JC, Garcia-Suarez O, Hevia D, Martin V, Rodríguez C, Sainz RM (2008) Melatonin prevents glucocorticoid inhibition of cell proliferation and toxicity in hippocampal cells by reducing glucocorticoid receptor nuclear translocation. J. Steroid Biochem. Mol. Biol. 110 (1-2): 116–124. https://doi.org/10.1016/j.jsbmb.2008.02.009.
91. Singh AK, Haldar C (2016) Melatonin modulates glucocorticoid receptor mediated inhibition of antioxidant response and apoptosis in peripheral blood mononuclear cells. Mol. Cell. Endocrinol. 436: 59–67. https://doi.org/10.1016/j.mce.2016.07.024.
92. Kim MJ, Choi GE, Chae CW, Lim JR, Jung YH, Yoon JH, Park JY, Han HJ (2023) Melatonin-mediated FKBP4 downregulation protects against stress-induced neuronal mitochondria dysfunctions by blocking nuclear translocation of GR. Cell. Death Dis. 14 (2): 146. https://doi.org/10.1038/s41419-023-05676-5.
93. Sun L, Huang L, Nguyen P, Bisht KS, Bar-Sela G, Ho AS, Bradbury CM, Yu W, Cui H, Lee S, Trepel JB, Feinberg AP, Gius D (2008) DNA methyltransferase 1 and 3B activate BAG-1 expression via recruitment of CTCFL/BORIS and modulation of promoter histone methylation. Cancer Res. 68 (8): 2726–2735. https://doi.org/10.1158/0008-5472.CAN-07-6654.
94. Ma F, Zhang M, Gong W, Weng M, Quan Z (2015) MiR-138 Suppresses cell proliferation by targeting Bag-1 in gallbladder carcinoma. PloS One 10 (5): e0126499. https://doi.org/10.1371/journal.pone.0126499.
95. Hou G, Chen H, Yin Y, Pan Y, Zhang X, Jia F (2020) MEL Ameliorates post-SAH cerebral vasospasm by affecting the expression of eNOS and HIF1α via H19/miR-138/eNOS/NO and H19/miR-675/HIF1α. Mol. Ther. Nucleic. Acids 19: 523–532. https://doi.org/10.1016/j.omtn.2019.12.002.
96. Chen Z, Huai Y, Chen G, Liu S, Zhang Y, Li D, Zhao F, Chen X, Mao W, Wang X, Yin C, Yang C, Xu X, Ru K, Deng X, Hu L, Li Y, Peng S, Zhang G, Lin X, Qian A (2022) MiR-138-5p targets MACF1 to aggravate aging-related bone loss. Int. J. Biol. Sci. 18 (13): 4837–4852. https://doi.org/10.7150/ijbs.71411.
97. Brás JP, Bravo J, Freitas J, Barbosa MA, Santos SG, Summavielle T, Almeida MI (2020) TNF-alpha-induced microglia activation requires miR-342: impact on NF-kB signaling and neurotoxicity. Cell. Death Dis. 11 (6): 415. https://doi.org/10.1038/s41419-020-2626-6.
98. Sun J, Pan LM, Chen LB, Wang Y (2017) LncRNA XIST promotes human lung adenocarcinoma cells to cisplatin resistance via let-7i/BAG-1 axis. Cell Cycle 16 (21): 2100–2107. https://doi.org/10.1080/15384101.2017.1361071.
99. Gao W, Zhang Y, Yuan L, Huang F, Wang YS (2023) Long non-coding RNA H19-overexpressing exosomes ameliorate UVB-induced photoaging by upregulating SIRT1 via sponging miR-138. Photochem. Photobiol. https://doi.org/10.1111/php.13801.
100. Taheri M, Askari A, Behzad Moghadam K, Hussen BM, Ghafouri-Fard S, Kiani A (2023) A review on the role of NCK1 Antisense RNA 1 (NCK1-AS1) in diverse disorders. Pathol. Res. Pract. 245: 154451. https://doi.org/10.1016/j.prp.2023.154451.
101. Xu Z, Zhang F, Xu H, Yang F, Zhou G, Tong M, Li Y, Yang S (2022) Melatonin affects hypoxia-inducible factor 1α and ameliorates delayed brain injury following subarachnoid hemorrhage via H19/miR-675/HIF1A/TLR4. Bioengineered 13 (2): 4235–4247. https://doi.org/10.1080/21655979.2022.2027175.
102. Tang H, Zhong H, Liu W, Wang Y, Wang Y, Wang L, Tang S, Zhu H (2022) Melatonin alleviates hyperglycemia-induced cardiomyocyte apoptosis via regulation of long non-coding RNA H19/miR-29c/MAPK axis in diabetic cardiomyopathy. Pharmaceuticals (Basel) 15 (7): 821. https://doi.org/10.3390/ph15070821.
103. Tan DX, Manchester LC, Liu X, Rosales-Corral SA, Acuna-Castroviejo D, Reiter RJ (2013) Mitochondria and chloroplasts as the original sites of melatonin synthesis: a hypothesis related to melatonin's primary function and evolution in eukaryotes. J. Pineal Res. 54 (2): 127–138. https://doi.org/10.1111/jpi.12026.
104. Wang SH, Zhu XL, Wang F, Chen SX, Chen ZT, Qiu Q, Liu WH, Wu MX, Deng BQ, Xie Y, Mai JT, Yang Y, Wang JF, Zhang HF, Chen YX (2021) LncRNA H19 governs mitophagy and restores mitochondrial respiration in the heart through Pink1/Parkin signaling during obesity. Cell Death Dis. 12 (6): 557. https://doi.org/10.1038/s41419-021-03821-6.
105. Gerasymchuk M, Cherkasova V, Kovalchuk O, Kovalchuk I (2020) The role of microRNAs in organismal and skin aging. Int. J. Mol. Sci. 21 (15): 5281. https://doi.org/10.3390/ijms21155281.
106. Cai Y, Sheng Z, Chen Y, Wang J (2019) LncRNA HMMR-AS1 promotes proliferation and metastasis of lung adenocarcinoma by regulating MiR-138/sirt6 axis. Aging 11 (10): 3041–3054. https://doi.org/10.18632/aging.101958.
107. Liu Q, Cui W, Yang C, Du LP (2021) Circular RNA ZNF609 drives tumor progression by regulating the miR-138-5p/SIRT7 axis in melanoma. Aging 13 (15): 19822–19834. https://doi.org/10.18632/aging.203394.
108. Wang M, Sun H, Yao Y, Tang X, Wu B (2019) MicroRNA-217/138-5p downregulation inhibits inflammatory response, oxidative stress and the induction of neuronal apoptosis in MPP+-induced SH-SY5Y cells. Am. J. Transl. Res. 11 (10): 6619–6631.
109. Zhang F, Yang Y, Chen X, Liu Y, Hu Q, Huang B, Liu Y, Pan Y, Zhang Y, Liu D, Liang R, Li G, Wei Q, Li L, Jin L (2021) The long non-coding RNA βFaar regulates islet β-cell function and survival during obesity in mice. Nat. Commun. 12 (1): 3997. https://doi.org/10.1038/s41467-021-24302-6.
110. Luan B, Sun C (2018) MiR-138-5p affects insulin resistance to regulate type 2 diabetes progression through inducing autophagy in HepG2 cells by regulating SIRT1. Nutr. Res. 59: 90–98. https://doi.org/10.1016/j.nutres.2018.05.001.
111. Matsushita K, Okita H, Suzuki A, Shimoda K, Fukuma M, Yamada T, Urano F, Honda T, Sano M, Iwanaga S, Ogawa S, Hata J, Umezawa A (2003) Islet cell hyperplasia in transgenic mice overexpressing EAT/mcl-1, a bcl-2 related gene. Mol. Cell Endocrinol. 203 (1-2): 105–116. https://doi.org/10.1016/s0303-7207(03)00095-9.
112. Suksri K, Semprasert N, Junking M, Kutpruek S, Limjindaporn T, Yenchitsomanus PT, Kooptiwut S (2021) Dexamethasone induces pancreatic β-cell apoptosis through upregulation of TRAIL death receptor. J. Mol. Endocrinol. 67 (3): 95–106. https://doi.org/10.1530/JME-20-0238.
113. Delangre E, Liu J, Tolu S, Maouche K, Armanet M, Cattan P, Pommier G, Bailbé D, Movassat J (2021) Underlying mechanisms of glucocorticoid-induced β-cell death and dysfunction: a new role for glycogen synthase kinase 3. Cell Death Dis. 12 (12): 1136. https://doi.org/10.1038/s41419-021-04419-8.
114. do Carmo Buonfiglio D, Peliciari-Garcia RA, do Amaral FG, Peres R, Nogueira TC, Afeche SC, Cipolla-Neto J (2011) Early-stage retinal melatonin synthesis impairment in streptozotocin-induced diabetic wistar rats. Invest. Ophthalmol. Vis. Sci. 52 (10): 7416–7422. https://doi.org/10.1167/iovs.10-6756.
115. Pei HF, Hou JN, Wei FP, Xue Q, Zhang F, Peng CF, Yang Y, Tian Y, Feng J, Du J, He L, Li XC, Gao EH, Li D, Yang YJ (2017) Melatonin attenuates postmyocardial infarction injury via increasing Tom70 expression. J. Pineal Res. 62 (1): 10.1111/jpi.12371. https://doi.org/10.1111/jpi.12371.
116. Sun H, Zheng M, Liu J, Fan W, He H, Huang F (2023) Melatonin promoted osteogenesis of human periodontal ligament cells by regulating mitochondrial functions through the translocase of the outer mitochondrial membrane 20. J. Periodontal. Res. 58 (1): 53–69. https://doi.org/10.1111/jre.13068.
117. Nasoni MG, Carloni S, Canonico B, Burattini S, Cesarini E, Papa S, Pagliarini M, Ambrogini P, Balduini W, Luchetti F (2021) Melatonin reshapes the mitochondrial network and promotes intercellular mitochondrial transfer via tunneling nanotubes after ischemic-like injury in hippocampal HT22 cells. J. Pineal Res. 71 (1): e12747. https://doi.org/10.1111/jpi.12747.
118. Karra AG, Sioutopoulou A, Gorgogietas V, Samiotaki M, Panayotou G, Psarra AG (2022) Proteomic analysis of the mitochondrial glucocorticoid receptor interacting proteins reveals pyruvate dehydrogenase and mitochondrial 60 kDa heat shock protein as potent binding partners. J. Proteomics 257: 104509. https://doi.org/10.1016/j.jprot.2022.104509.
119. Kinkel MD, Yagi R, McBurney D, Nugent A, Horton WE Jr (2004) Age-related expression patterns of Bag-1 and Bcl-2 in growth plate and articular chondrocytes. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 279 (2): 720–728. https://doi.org/10.1002/ar.a.20063.
120. Li H, Liu M, Zhang C (2022) Women with polycystic ovary syndrome (PCOS) have reduced melatonin concentrations in their follicles and have mild sleep disturbances. BMC Womens Health 22 (1):7 9. https://doi.org/10.1186/s12905-022-01661-w.
121. Anderson G, Jacob A, Bellivier F, Geoffro PA (2016) Bipolar Disorder: The role of the kynurenine and melatonergic pathways. Curr. Pharm. Des. 22 (8): 987–1012. https://doi.org/10.2174/1381612822666151214105314.
122. Sharifi M, Rajabpoor Nikoo N, Badehnoosh B, Shafabakhsh R, Asemi R, Reiter RJ, Asemi Z (2023) Therapeutic effects of melatonin on endometriosis, targeting molecular pathways: Current knowledge and future perspective. Pathol. Res. Pract. 243: 154368. https://doi.org/10.1016/j.prp.2023.154368.
123. Porniece Kumar M, Cremer AL, Klemm P, Steuernagel L, Sundaram S, Jais A, Hausen AC, Tao J, Secher A, Pedersen TÅ, Schwaninger M, Wunderlich FT, Lowell BB, Backes H, Brüning JC (2021). Insulin signalling in tanycytes gates hypothalamic insulin uptake and regulation of AgRP neuron activity. Nat. Metab. 3 (12): 1662–1679. https://doi.org/10.1038/s42255-021-00499-0.
124. Ahmad F, Sachdeva P, Sarkar J, Izhaar R (2022) Circadian dysfunction and Alzheimer's disease - An updated review. Aging Med. (Milton) 6 (1): 71-81. doi: 10.1002/agm2.12221.
125. Anderson G (2022) Amyotrophic Lateral Sclerosis pathoetiology and pathophysiology: roles of astrocytes, gut microbiome, and muscle interactions via the mitochondrial melatonergic pathway, with disruption by glyphosate-based herbicides. Int. J. Mol. Sci. 24 (1): 587. https://doi.org/10.3390/ijms24010587.
126. Huang Y, Liu Q, Huang G, Wen J, Chen G (2022) Hypothalamic kisspeptin neurons regulates energy metabolism and reproduction under chronic stress. Front. Endocrinol. (Lausanne) 13: 844397. https://doi.org/10.3389/fendo.2022.844397.
127. Walker SE, Zanoletti O, Guillot de Suduiraut I, Sandi C (2017) Constitutive differences in glucocorticoid responsiveness to stress are related to variation in aggression and anxiety-related behaviors. Psychoneuroendocrinology 84: 1-10. doi: 10.1016/j.psyneuen.2017.06.011.
128. Shiuchi T, Otsuka A, Shimizu N, Chikahisa S, Séi H (2021) Feeding rhythm-induced hypothalamic agouti-related protein elevation via glucocorticoids leads to insulin resistance in skeletal muscle. Int. J. Mol. Sci. 22 (19): 10831. https://doi.org/10.3390/ijms221910831.
129. Givalois L, Arancibia S, Alonso G, Tapia-Arancibia L (2004) Expression of brain-derived neurotrophic factor and its receptors in the median eminence cells with sensitivity to stress. Endocrinology 145 (10): 4737–4747. https://doi.org/10.1210/en.2004-0616.
130. Anderson G (2022) Tumor microenvironment and metabolism: Role of the mitochondrial melatonergic pathway in determining intercellular interactions in a new dynamic homeostasis. Int. J. Mol. Sci. 24 (1): 311. https://doi.org/10.3390/ijms24010311.
131. Zhu K, Zhang Y, Zhang J, Zhou F, Zhang L, Wang S, Zhu Q, Liu Q, Wang X, Zhou L (2020) Acetylation of Hsp90 reverses dexamethasone-mediated inhibition of insulin secretion. Toxicol. Lett. 320: 19-27. doi: 10.1016/j.toxlet.2019.11.022.
132. Steidemann MM, Liu J, Bayes K, Castro LP, Ferguson-Miller S, LaPres JJ (2023) Evidence for crosstalk between the aryl hydrocarbon receptor and the translocator protein in mouse lung epithelial cells. Exp. Cell. Res. 429 (1): 113617. https://doi.org/10.1016/j.yexcr.2023.113617.
133. Magrì A, Lipari CLR, Risiglione P, Zimbone S, Guarino F, Caccamo A, Messina A (2023) ERK1/2-dependent TSPO overactivation associates with the loss of mitophagy and mitochondrial respiration in ALS. Cell Death Dis. 14 (2): 122. https://doi.org/10.1038/s41419-023-05643-0.
134. Mafi A, Rismanchi H, Gholinezhad Y, Mohammadi MM, Mousavi V, Hosseini SA, Milasi YE, Reiter RJ, Ghezelbash B, Rezaee M, Sheida A, Zarepour F, Asemi Z, Mansournia MA, Mirzaei H (2023) Melatonin as a regulator of apoptosis in leukaemia: molecular mechanism and therapeutic perspectives. Front. Pharmacol. 14: 1224151. doi: 10.3389/fphar.2023.1224151.
135. Sedighi Pashaki A, Sheida F, Moaddab Shoar L, Hashem T, Fazilat-Panah D, Nemati Motehaver A, Ghanbari Motlagh A, Nikzad S, Bakhtiari M, Tapak L, Keshtpour Amlashi Z, Javadinia SA, Keshtpour Amlashi Z (2023) A randomized, controlled, parallel-group, trial on the long-term effects of melatonin on fatigue associated with breast cancer and its adjuvant treatments. Integr. Cancer Ther. 22: 15347354231168624. doi: 10.1177/15347354231168624.
136. Xue KH, Jiang YF, Bai JY, Zhang DZ, Chen YH, Ma JB, Zhu ZJ, Wang X, Guo P (2023) Melatonin suppresses Akt/mTOR/S6K activity, induces cell apoptosis, and synergistically inhibits cell growth with sunitinib in renal carcinoma cells via reversing Warburg effect. Redox. Rep. 28 (1): 2251234. doi: 10.1080/13510002.2023.2251234.
137. Lingas EC (2023) A narrative review of the carcinogenic effect of night shift and the potential protective role of melatonin. Cureus 15 (8): e43326. doi: 10.7759/cureus.43326.
138. Noureddine LM, Ablain J, Surmieliova-Garnès A, Jacquemetton J, Pham TH, Marangoni E, Schnitzler A, Bieche I, Badran B, Trédan O, Hussein N, Le Romancer M, Poulard C (2023) PRMT5 triggers glucocorticoid-induced cell migration in triple-negative breast cancer. Life. Sci. Alliance 6 (10): e202302009. doi: 10.26508/lsa.202302009.
139. Mohammadi S, Zahmatkesh M, Asgari Y, Aminyavari S, Hassanzadeh G (2023) Evaluation of hippocampal arylalkylamine N-acetyltransferase activity in amyloid-β neurotoxicity. J. Mol. Endocrinol. 71 (2): e220161. doi: 10.1530/JME-22-0161.
140. Cardinali DP, Brusco LI, Liberczuk C, Furio AM (2002) The use of melatonin in Alzheimer's disease. Neuro. Endocrinol. Lett. 23 (1):20-23.
141. Jean-Louis G, von Gizycki H, Zizi F (1998) Melatonin effects on sleep, mood, and cognition in elderly with mild cognitive impairment. J. Pineal Res. 25 (3):177-83. doi: 10.1111/j.1600-079x.1998.tb00557.x.
142. Leone V, Gibbons SM, Martinez K, Hutchison AL, Huang EY, Cham CM, Pierre JF, Heneghan AF, Nadimpalli A, Hubert N, Zale E, Wang Y, Huang Y, Theriault B, Dinner AR, Musch MW, Kudsk KA, Prendergast BJ, Gilbert JA, Chang EB (2015) Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe 17 (5): 681-9. doi: 10.1016/j.chom.2015.03.006.
143. van der Velpen IF, de Feijter M, Raina R, Özel F, Perry M, Ikram MA, Vernooij MW, Luik AI (2023) Psychosocial health modifies associations between HPA-axis function and brain structure in older age. Psych. neuroendocrinology 153: 106106. doi: 10.1016/j.psyneuen.2023.106106.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


