Cell polarization, migration and tissue repair: A promising field for future melatonin research
Polarization, migration and repair
Abstract
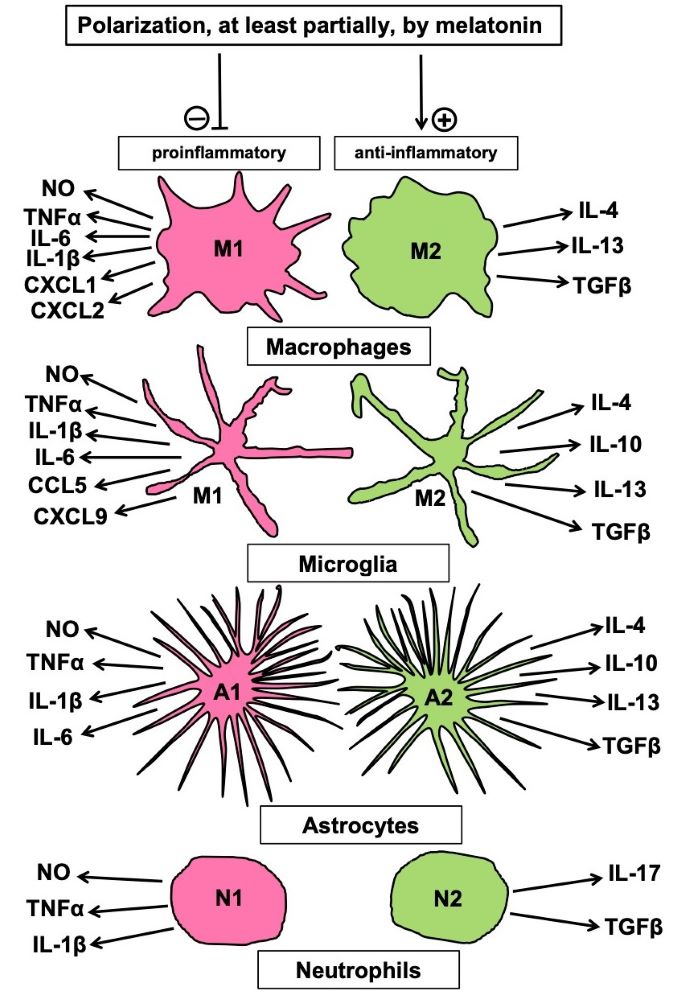
Melatonin has been shown to support the repair of various tissue injuries. Wound healing is a complicated process that comprises several different cellular activities and regulation mechanisms, such as activation and programming of stem cells, interaction of different cell types, polarization of cells, especially concerning the alternative of pro- vs. anti-inflammatory behavior, migration of cells to the site of replacement, with guidance by other cells and modified extracellular matrix, as observed in the formation of biobridges. In most of these processes, melatonin acts as a decisive modulator, but details depend on tissue and cell types and have not been completely identified. Many aspects will require a considerable amount of work for understanding, in this context, the role of melatonin on a comprehensive basis. Moreover, the modulation of important cell properties has remained partially unknown or has only poorly considered in recent work. For instance, pro- or anti-inflammatory polarization of cells has been described in various cell types, not only in macrophages, in which melatonin is a major regulator, but also in microglia, in astrocytes and in neutrophils. Even in fibroblasts, polarization has been observed and concerns the alternative of inflammatory or fibrotic behavior. Notably, polarized cells that support healing in normal tissue seem to also protect tumors, whereas inflammatory phenotypes show antitumor activities. With regard to antitumor properties of melatonin, it seems necessary to clarify whether melatonin polarizes cells differently in the tumor microenvironment, compared to normal tissue, in which it promotes healing.
References
2. Hardeland R, Cardinali DP, Srinivasan V, Spence SW, Brown GM, Pandi-Perumal SR (2011) Melatonin – A pleiotropic, orchestrating regulator molecule. Prog. Neurobiol. 93: 350-384.
3. Xia Y, Zeng S, Zhao Y, Zhu C, Deng B, Zhu G, et al. (2019) Melatonin in macrophage biology: Current understanding and future perspectives. J. Pineal Res. 66: e12547.
4. Hardeland R (2018) Melatonin and inflammation —Story of a double-edged blade. J. Pineal Res. 65: e12525.
5. Carrillo-Vico A, Guerrero JM, Lardone PJ, Reiter RJ (2005) A review of the multiple actions of melatonin on the immune system. Endocrine 27: 189-200.
6. Hardeland R (2013) Melatonin and the theories of aging: a critical appraisal of melatonin’s role in antiaging mechanisms. J. Pineal Res. 55: 325-356.
7. Hardeland R, Cardinali DP, Brown GM, Pandi-Perumal SR (2015) Melatonin and brain inflammaging. Prog. Neurobiol. 127-128: 46-63.
8. Reiter RJ, Mayo JC, Tan D-X, Sainz RM, Alatorre-Jimenez M, Qin L (2016) Melatonin as an antioxidant: under promises but over delivers. J. Pineal Res. 61: 253-278.
9. Gensel J, Zhang B (2015) Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. 1619: 1-11.
10. Zhang Q, Sioud M. (2023) Tumor-associated macrophage subsets: shaping polarization and targeting. Int. J. Mol. Sci. 24: 7493.
11. Arora S, Dev K, Agarwal B, Das P, Syed MA (2018) Macrophages: Their role, activation and polarization in pulmonary diseases. Immunology 223: 383-396.
12. Hu W, Lin J, Lian X, Yu F, Liu W, Wu Y, et al. (2019) M2a and M2b macrophages predominate in kidney tissues and M2 subpopulations were associated with the severity of disease of IgAN patients. Clin. Immunol. 205: 8-15.
13. Pugazhenthi K, Kapoor M, Clarkson AN, Hall I, Appleton I (2008) Melatonin accelerates the process of wound repair in full-thickness incisional wounds. J. Pineal Res. 44: 387-396.
14. Avila-Ponce de León U, Vázquez-Jiménez A, Matadamas-Guzman M, Pelayo R, Resendis-Antonio O (2021) Transcriptional and microenvironmental landscape of macrophage transition in cancer: A Boolean analysis. Front. Immunol. 12: 642842
15. Lanoix D, Lacasse AA, Reiter RJ, Vaillancourt C (2012) Melatonin: the smart killer: the human trophoblast as a model. Mol. Cell. Endocrinol. 348: 1-11.
16. Slominski A, Hardeland R, Zmijewski MA, Slominski SM, Reiter RJ, Paus R (2018) Melatonin: A cutaneous perspective on its production, metabolism, and functions. J. Invest. Dermatol. 138: 490-499.
17. Bjørklund G, Dadar M, Aaseth J, Chirumbolo S (2020) Thymosin β4: a multi-faceted tissue repair stimulating protein in heart injury. Curr. Med. Chem. 25: 6294-6305.
18. Liu W, Yu M, Xie D, Wang L, Ye C, Zhu Q, et al. (2020) Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem Cell Res. Ther. 11: 259.
19. Hardeland R (2022) Melatonin and the programming of stem cells. Int. J. Mol. Sci. 23: 1971.
20. Hardeland R (2021) Melatonin and microglia. Int. J. Mol. Sci. 22: 8296.
21. Reiter RJ, Sharma R, Tan D-X, Chuffa LGA, da Silva DGH, Slominski AT, et al. (2024) Dual sources of melatonin and evidence for different primary functions. Front. Endocrinol. (Lausanne) 15: 1414463.
22. Markus RP, Cecon E, Pires-Lapa MA (2013) Immune-pineal axis: nuclear factor kappaB (NF-kB) mediates the shift in the melatonin source from pinealocytes to immune competent cells. Int. J. Mol. Sci. 14: 10979-10997.
23. Markus RP, Fernandes PA, Kinker GS, da Silveira Cruz-Machado S, Marçola M (2018) Immune-pineal axis - acute inflammatory responses coordinate melatonin synthesis by pinealocytes and phagocytes. Br. J. Pharmacol. 175: 3239-3250.
24. Markus RP, Sousa KS, da Silveira Cruz-Machado S, Fernandes PA, Ferreira ZS (2021) Possible role of pineal and extra-pineal melatonin in surveillance, immunity, and first-line defense. Int. J. Mol. Sci. 22: 12143.
25. Radio NM, Doctor JS, Witt-Enderby PA (2006) Melatonin enhances alkaline phosphatase activity in differentiating human adult mesenchymal stem cells grown in osteogenic medium via MT2 melatonin receptors and the MEK/ERK1/2 signaling cascade. J. Pineal Res. 40: 332-342.
26. Luchetti F, Canonico B, Bartolini D, Arcangeletti M, Ciffolilli S. Murdolo G, et al. (2014) Melatonin regulates mesenchymal stem cell differentiation: a review. J. Pineal Res. 56: 382-397.
27. Lai M, Jin Z, Tang Q. Lu M (2017) Sustained release of melatonin from TiO2 nanotubes for modulating osteogenic differentiation of mesenchymal stem cells in vitro. J. Biomater. Sci. Polym. Ed. 28: 1651-1664.
28. Dong P, Gu X, Zhu G, Li M. Ma B, Zi Y (2018) Melatonin induces osteoblastic differentiation of mesenchymal stem cells and promotes fracture healing in a rat model of femoral fracture via neuropeptide Y/neuropeptide Y receptor Y1 signaling. Pharmacology 2018, 102: 272-280.
29. Maria S, Samsonraj RM, Munmun F, Glas J, Silvestros M, Kotlarczyk MP, et al. (2018) Biological effects of melatonin on osteoblast/osteoclast cocultures, bone, and quality of life: Implications of a role for MT2 melatonin receptors, MEK1/2, and MEK5 in melatonin-mediated osteoblastogenesis. J. Pineal Res. 64: e12465.
30. Jiang T, Xia C, Chen X, Hu Y, Wang Y, Wu J, et al. (2019) Melatonin promotes the BMP9-induced osteogenic differentiation of mesenchymal stem cells by activating the AMPK/beta-catenin signalling pathway. Stem Cell Res. Ther. 10: 408.
31. Xu L, Liu Y, Sun Y, Wang B, Xiong Y, Lin W, et al. (2017). Tissue source determines the differentiation potentials of mesenchymal stem cells: a comparative study of human mesenchymal stem cells from bone marrow and adipose tissue. Stem Cell Res. Ther. 8: 275.
32. Wang Y, Barthez M, Chen D (2023) Mitochondrial regulation in stem cells. Trends Cell Biol. 2023: S0962-8924(23)00207-6.
33. Hardeland R, Poeggeler B, Pappolla MA (2009) Mitochondrial actions of melatonin ― an endeavor to identify their adaptive and cytoprotective mechanisms. Proc. Sax. Acad. Sci., Math. Nat. Class, 65 (3): pp. 14-31.
34. Acuña Castroviejo D, López LC, Escames G, López A, García JA, Reiter RJ (2011) Melatonin-mitochondria interplay in health and disease. Curr. Top. Med. Chem. 11: 221-240.
35. Hardeland R (2017) Melatonin and the electron transport chain. Cell. Mol. Life Sci. 74: 3883-3896.
36. Reiter RJ, Tan D-X, Rosales-Corral S, Galano A, Zhou XJ, Xu B (2018) Mitochondria: central organelles for melatonin's antioxidant and anti-aging actions. Molecules 23: 509.
37. Prado NJ, Ferder L, Manucha W, Diez ER (2018) Anti-inflammatory effects of melatonin in obesity and hypertension. Curr. Hypertens. Rep. 20: 45.
38. Tan D-X, Hardeland R (2020) Targeting host defense system and rescuing compromised mitochondria to increase tolerance against pathogens by melatonin may impact outcome of deadly virus infection pertinent to COVID-19. Molecules 25: 4410.
39. Stacchiotti A, Favero G, Rodella LF (2020) Impact of melatonin on skeletal muscle and exercise. Cells 9: 288.
40. Hardeland R (2018) Recent findings in melatonin research and their relevance to the CNS. Cent. Nerv. Syst. Agents Med. Chem. 18: 102-114.
41. Xu S, Li L, Wu J, An S, Fang H, et al. (2021) Melatonin attenuates sepsis-induced small-intestine injury by upregulating SIRT3-mediated oxidative-stress inhibition, mitochondrial protection, and autophagy induction. Front. Immunol.12: 625627.
42. Hardeland R (2021): Sirtuins, melatonin, and the relevance of circadian oscillators.In: Sirtuin Biology in Medicine. Targeting New Avenues of Care in Development, Aging, and Disease (Maiese K, ed.), Academic Press, London – San Diego, CA – Cambridge, MA – Oxford, pp. 137-151.
43. Niu B, Li B, Wu C, Wu J, Yan Y, Shang R, et al. (2016) Melatonin promotes goat spermatogonia stem cells (SSCs) proliferation by stimulating glial cell line-derived neurotrophic factor (GDNF) production in Sertoli cells. Oncotarget 7: 77532-77542.
44. Bai C, Gao Y, Zhang X, Yang W, Guan W (2018) Melatonin promotes self-renewal of nestin-positive pancreatic stem cells through activation of the MT2/ERK/SMAD/nestin axis. Artif. Cells Nanomed. Biotechnol. 46: 62-74.
45. Gao Y, Ma L, Bai C, Zhang X, Yang W (2019) Melatonin promotes self-renewal and nestin expression in neural stem cells from the retina. Histol. Histopathol. 34: 645-654
46. Kwon HS, Koh SH (2020) Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl. Neurodegener. 9: 42.
47. Liddelow SA, Barres BA (2017) Reactive astrocytes: production, function, and therapeutic potential. Immunity 46: 957-967.
48. Oksanen M, Lehtonen S, Jaronen M, Goldsteins G, Hamalainen RH, Koistinaho J (2019) Astrocyte alterations in neurodegenerative pathologies and their modeling in human induced pluripotent stem cell platforms. Cell. Mol. Life Sci. 76: 2739-2760.
49. Tiwari-Woodruff S, Morales LB, Lee R, Voskuhl RR (2007) Differential neuroprotective and antiinflammatory effects of estrogen receptor (ER) alpha and ERbeta ligand treatment. Proc. Natl. Acad. Sci. USA 104: 14813-14818.
50. Metcalfe S, Anselmi N, Escobar A, Visser MB, Kay JG (2021) Innate phagocyte polarization in the oral cavity. Front. Immunol. 12: 768479.
51. Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. (2009) Polarization of tumor-associated neutrophil phenotype by TGF-β: “N1” versus “N2” TAN. Cancer Cell 16: 183-194.
52. Yu T, Tang Q, Chen X, Fan W, Zhou Z, Huang W, et al. (2021) TGF-β1 and IL-17A comediate the protumor phenotype of neutrophils to regulate the epithelial-mesenchymal transition in oral squamous cell carcinoma. J. Oral Pathol. Med. 50: 383-361.
53. Kieler M, Hofmann M, Schabbauer G (2021) More than just protein building blocks: how amino acids and related metabolic pathways fuel macrophage polarization. FEBS J. 288: 3694-3714.
54. Chen H, Yang W,Xue X, Li Y, Jin Z, Ji Z (2022) Integrated analysis revealed an inflammatory cancer-associated fibroblast-based subtypes with promising implications in predicting the prognosis and immunotherapeutic response of bladder cancer patients. Int. J. Mol. Sci. 23: 15970.
55. Ramakrishnan S (2022) HIF-2 in cancer-associated fibroblasts polarizes macrophages and creates an immunosuppressive tumor microenvironment in pancreatic cancer. Gastroenterology 162: 1835-1837.
56. Ledoult E, Jendoubi M, Collet A, Guerrier T, Largy A, Speca S, et al. (2022) Simple gene signature to assess murine fibroblast polarization. Sci. Rep. 12: 11748.
57. Schneider MR, Schmidt-Ullrich R, Paus R (2009) The hair follicle as a dynamic miniorgan. Curr. Biol.19: R132-R142.
58. Qiang L, Sample A, Liu H, Wu X, He Y-Y (2017) Epidermal SIRT1 regulates inflammation, cell migration, and wound healing. Sci. Rep. 7:14110.
59. Chen X, Tong G, Fan J, Shen Y, Wang N. Gong W, et al. (2022) FGF21 promotes migration and differentiation of epidermal cells during wound healing via SIRT1-dependent autophagy. Br. J. Pharmacol. 179:1102-1121.
60. Zhu R, Liu C, Gundersen GG (2018) Nuclear positioning in migrating fibroblasts. Semin. Cell Dev. Biol. 82: 41-50.
61. Patschan D, Hildebrandt A, Rinneburger J, Wessels JT, Patschan S, Becker JU, et al. (2012) The hormone melatonin stimulates renoprotective effects of "early outgrowth" endothelial progenitor cells in acute ischemic kidney injury. Am. J. Physiol. Renal Physiol. 302: F1305-F1312.
62. Zhang J, Li L, Yu J, Fan Zhang F, Shi J, Li M, et al. (2023) Autophagy-modulated biomaterial: A robust weapon for modulating the wound environment to promote skin wound healing. Int. J. Nanomedicine 18: 2567-2588.
63. Tan SS, Zhan W, Poon CJ, Han X, Marre D, Boodhun S, et al. (2018) Melatonin promotes survival of nonvascularized fat grafts and enhances the viability and migration of human adipose-derived stem cells via down-regulation of acute inflammatory cytokines. J. Tissue Eng. Regen. Med. 12: 382-392.
64. Woodley JP, Lambert DW, Ortega Asencio I (2022) Understanding fibroblast behavior in 3D biomaterials. Tissue Eng. Part B Rev. 28: 569-578.
65. Tajiri N, Kaneko Y, Shinozuka K, Ishikawa H, Yankee E, McGrogan M, et al. (2013) Stem cell recruitment of newly formed host cells via a successful seduction? Filling the gap between neurogenic niche and injured brain site. PLoS One 2013, 8: e74857.
66. Tajiri N, Duncan K, Antoine A, Pabon M,Acosta SA, de la Pena I, et al. (2014) Stem cell-paved biobridge facilitates neural repair in traumatic brain injury. Front. Syst. Neurosci. 8: 116.
67. Sullivan R, Duncan K, Dailey T, Kaneko Y, Tajiri N, Borlongan CV (2015) A possible new focus for stroke treatment - migrating stem cells. Expert Opin. Biol. Ther. 15: 949-958.
68. Duncan K, Gonzales-Portillo GS, Acosta SA, Kaneko Y, Borlongan CV, Tajiri N (2015) Stem cell-paved biobridges facilitate stem transplant and host brain cell interactions for stroke therapy. Brain Res. 1623: 160-165.
69. Crowley MG, Tajiri N (2017) Exogenous stem cells pioneer a biobridge to the advantage of host brain cells following stroke: New insights for clinical applications. Brain Circ. 3: 130-134.
70. Lee JY, Xu K, Nguyen H, Guedes VA, Borlongan CV, Acosta SA (2017) Stem cell-induced biobridges as possible tools to aid neuroreconstruction after CNS injury. Front. Cell Dev. Biol. 5: 51.
71. Liska MG, Crowley MG, Nguyen H, Borlongan CV (2017) Biobridge concept in stem cell therapy for ischemic stroke. J. Neurosurg. Sci. 61:173-179.
72. Osier N, McGreevy E, Pham L, Puccio A, Ren D, Conley YP, et al. (2018) Melatonin as a therapy for traumatic brain injury: A review of published evidence. Int. J. Mol. Sci. 19: 1539.
73. Blum B, Kaushal S, Khan S, Kim JH, Alvarez Villalba CL (2021) Melatonin in Traumatic Brain Injury and Cognition. Cureus 13: e17776.
74. Li D, He T, Zhang Y, Liu J, Zhao H, Wang D, et al. (2023) Melatonin regulates microglial polarization and protects against ischemic stroke-induced brain injury in mice. Exp. Neurol. 367: 114464.
75. Hardeland R (2009) Melatonin: Signaling mechanisms of a pleiotropic agent. BioFactors 35: 183-192.
76. Hardeland R (2017) The expanding functions of melatonin and their consequences to signaling. In: Mini-Reviews in Recent Melatonin Research (Hardeland R, ed.), Cuvillier, Göttingen, pp. 26-42.
77. Hardeland R (2018) Extended signaling by melatonin. Cell Cell. Life Sci. J. 3: 000123.
78. Hardeland R (2014) Melatonin, noncoding RNAs, messenger RNA stability and epigenetics ― evidence, hints, gaps and perspectives. Int. J. Mol. Sci. 15: 18221-18252.
79. Zhao L, An R, Yang Y, Yang X, Liu H, Yue L, et al. (2015) Melatonin alleviates brain injury in mice subjected to cecal ligation and puncture via attenuating inflammation, apoptosis, and oxidative stress: the role of SIRT1 signaling. J. Pineal Res. 59: 230-239.
80. Carloni S, Favrais G, Saliba E, Albertini MC, Chalon S, Longini M, et al. (2016) Melatonin modulates neonatal brain inflammation through endoplasmic reticulum stress, autophagy, and miR-34a/silent information regulator 1 pathway. J. Pineal Res. 61: 370-380.
81. Shakibaei M, Shayan P, Busch F, Aldinger C, Buhrmann C, Lueders C, et al. (2012) Resveratrol mediated modulation of sirt-1/Runx2 promotes osteogenic differentiation of mesenchymal stem cells: potential role of Runx2 deacetylation. PLoS ONE 7: e35712.
82. Denu RA, Hematti P (2016) Effects of oxidative stress on mesenchymal stem cell biology. Oxid. Med. Cell. Longev. 2016: 2989076.
83. Yuan H-F, Zhai C, Yan X-L, Zhao DD, Wang J-X, Zeng Q, et al.(2012) SIRT1 is required for long-term growth of human mesenchymal stem cells. J. Mol. Med. 90: 389-400.
84. Shi R, Jin Y, Hu W, Lian W, Cao C, Han S, et al. (2020) Exosomes derived from mmu_circ_0000250-modified adipose-derived mesenchymal stem cells promote wound healing in diabetic mice by inducing miR-128-3p/SIRT1-mediated autophagy. Am. J. Physiol. Cell Physiol. 318: C848-C856.
85. Christovam AC, Theodoro V, Mendonça FAS, Esquisatto MAM, Dos Santos GMT, do Amaral MEC. (2019) Activators of SIRT1 in wound repair: an animal model study. Arch. Dermatol. Res. 311: 193-201.
86. Zou J, Duan Y, Wang Y, Liu A, Chen Y, Guo D, et al. (2022) Phellopterin cream exerts an anti-inflammatory effect that facilitates diabetes-associated cutaneous wound healing via SIRT1. Phytomedicine 107: 154447.
87. Beegum F, Anuranjana PV, George KT, Divya KP, Begum F, Krishnadas N, et al. (2022) Sirtuins as therapeutic targets for improving delayed wound healing in diabetes. J. Drug Target 30: 911-926.
88. Fu Y, Wang Y, Liu Y, Tang C, Cai J, Chen G, et al. (2022) p53/sirtuin 1/NF-κB signaling axis in chronic inflammation and maladaptive kidney repair after cisplatin nephrotoxicity. Front. Immunol. 13: 925738.
89. Perrone S, Carloni S, Dell'Orto VG, Filonzi L, Beretta V, Petrolini C, et al. (2023). Hypoxic ischemic brain injury: animal models reveal new mechanisms of melatonin-mediated neuroprotection. Rev. Neurosci. 35 (3): 331-339.
90. Zhao W, Zhang R, Zang C, Zhang L, Zhao R, Li Q, et al. (2022) Exosome derived from mesenchymal stem cells alleviates pathological scars by inhibiting the proliferation, migration and protein expression of fibroblasts via delivering miR-138-5p to target SIRT1. Int. J. Nanomedicine 17: 4023-4038.
91. Wang Y, Barthez M, Chen D (2023) Mitochondrial regulation in stem cells. Trends Cell Biol. [online ahead of print, Oct 31]; doi: 10.1016/j.tcb.2023.10.003.
92. Zou Y, Zhang J, Xu J, Fu L, Xu Y, Wang X, et al. (2021) SIRT6 inhibition delays peripheral nerve recovery by suppressing migration, phagocytosis and M2-polarization of macrophages. Cell. Biosci. 11: 210.
93. Mendivil-Perez M, Soto-Mercado V, Guerra-Librero A, Fernandez-Gil BI, Florido J, Shen YQ, et al. (2017) Melatonin enhances neural stem cell differentiation and engraftment by increasing mitochondrial function. J. Pineal Res. 63: e12415.
94. Zhang S, Chen S, Li Y, Liu Y (2017) Melatonin as a promising agent of regulating stem cell biology and its application in disease therapy. Pharmacol. Res. 117: 252-260.
95. Lee MS, Yin TC, Sung PH, Chiang JY, Sun CK, Yip HK (2017) Melatonin enhances survival and preserves functional integrity of stem cells: A review. J. Pineal Res. 62: e12372.
96. Hu C, Li L (2019) Melatonin plays critical role in mesenchymal stem cell-based regenerative medicine in vitro and in vivo. Stem Cell Res. Ther. 10: 13.
97. Zhao L, Hu C, Zhang P, Jiang H, Chen J (2020) Melatonin preconditioning is an effective strategy for mesenchymal stem cell-based therapy for kidney disease. J. Cell. Mol. Med. 24: 25-33.
98. Abdel-Kawi SH, Hashem KS (2022) Administration of melatonin in diabetic retinopathy es effective and improves the efficacy of mesenchymal stem cell treatment. Stem Cells Int. 2022: 6342594.
99. Fang XY, Zhao DW, Zhang C, Ge HF, Zhang XY, Zhao FC, et al. (2022) A three-dimensional matrix system containing melatonin and neural stem cells repairs damage from traumatic brain injury in rats. Neural Regen. Res. 17: 2512-2517.
100. Qin W, Wang J, Hu Q, Qin R, Ma N, Zheng F, et al. (2023) Melatonin-pretreated human umbilical cord mesenchymal stem cells improved endometrium regeneration and fertility recovery through macrophage immunomodulation in rats with intrauterine adhesions. Biol. Reprod. 109: 918-937.
101. Luchetti F, Carloni S, Nasoni MG, Reiter RJ, Balduini W (2023) Melatonin, tunneling nanotubes, mesenchymal cells, and tissue regeneration. Neural Regen. Res. 18: 760-762.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


