Onset in late adolescence of schizophrenia: Could melatonin modulate this debut?
Melatonin at the onset of schizophrenia
Abstract
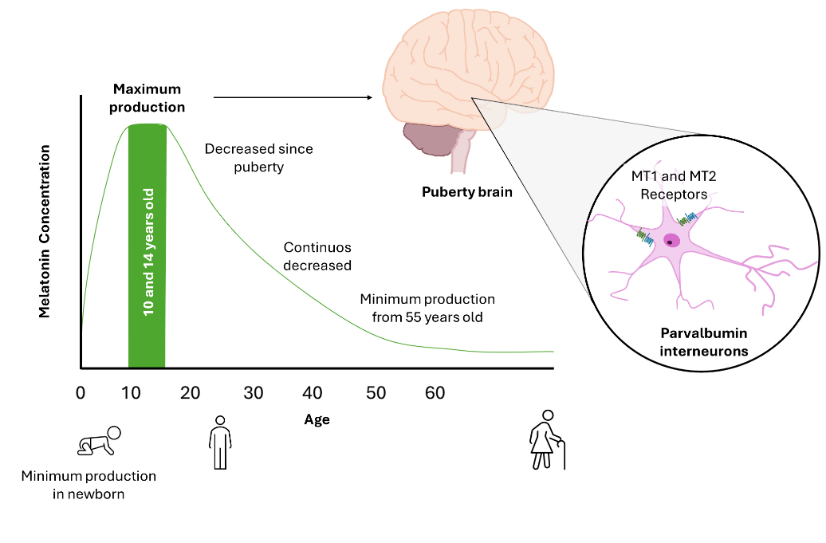
Schizophrenia, one of the most serious and widespread mental disorders in the world, makes its debut often in late adolescence and early adulthood, which allows us to focus our attention on those brain areas that still retain plasticity during this period. Parvalbumin interneurons, GABAergic and inhibitory, in both cortical and hippocampal areas, maintain their plasticity and are particularly vulnerable to oxidative stress due to their high energy requirements. Evidence has shown that their damage favors the triggering of schizophrenia by altering the neurobehavioral development of individuals. These neurons have melatonin receptors of MT1 and MT2, and the cytoprotective role of melatonin has been reported on these neurons. However, the role of this indolamine played in adolescence in protecting parvalbumin interneurons, reducing their oxidative stress and/or preventing their disappearance, which could prevent the onset of schizophrenia, is not yet known. The importance of this activity and its implications on patient therapy require the urgent studies
References
2. Turetsky BI, et al. (2007) Facial emotion recognition in schizophrenia: when and why does it go awry? Schizophr. Res. 94: 253–263.
3. Saykin AJ, et al. (1994) Neuropsychological deficits in neuroleptic naive patients with first-episode schizophrenia. Arch. Gen. Psychiatry 51: 124–131.
4. Turetsky B, et al. (1995) Frontal and temporal lobe brain volumes in schizophrenia. Relationship to symptoms and clinical subtype. Arch. Gen. Psychiatry 52: 1061–1070.
5. Marwaha S, et al. (2007) Rates and correlates of employment in people with schizophrenia in the UK, France and Germany. Br. J. Psychiatry 191: 30–37.
6. Hjorthøj C, Stürup AE, McGrath JJ, Nordentoft M (2017) Years of potential life lost and life expectancy in schizophrenia: a systematic review and meta-analysis. Lancet Psychiatry 4: 295–301.
7. Hor K, Taylor M (2010) Suicide and schizophrenia: a systematic review of rates and risk factors. J Psychopharmacol 24: 81–90.
8. Sullivan EM, O’Donnell P (2012) Inhibitory interneurons, oxidative stress, and schizophrenia. Schizophr Bull 38: 373–376.
9. Xerri C (2008) Imprinting of idiosyncratic experience in cortical sensory maps: neural substrates of representational remodeling and correlative perceptual changes. Behav. Brain Res. 192: 26–41.
10. Curto Y, et al. (2024) Erythropoietin restrains the inhibitory potential of interneurons in the mouse hippocampus. Mol. Psychiatry. https://doi.org/10.1038/s41380-024-02528-2.
11. Petilla Interneuron Nomenclature Group, et al. (2008) Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat. Rev. Neurosci. 9: 557–568.
12. Kolb B, Mychasiuk R, Muhammad A, Gibb R (2013) Brain plasticity in the developing brain. Prog. Brain Res. 207: 35–64.
13. Kolb B, Gibb R (2015) Plasticity in the prefrontal cortex of adult rats. Front. Cell Neurosci. 9: 15.
14. Bicks LK, Koike H, Akbarian S, Morishita H (2015) Prefrontal cortex and social cognition in mouse and man. Front. Psychol. 6: 1805.
15. Anand KS, Dhikav V (2012) Hippocampus in health and disease: An overview. Ann. Indian Acad. Neurol. 15: 239–246.
16. Hunziker U, Largo R, Zachmann M, Prader A (1986) Compensatory maturational deceleration of growth or “catch-down growth” in patients with congenital adrenal hyperplasia after delayed initiation of therapy. Eur. J. Pediatr. 144: 550–553.
17. Steullet P, et al. (2017) Oxidative stress-driven parvalbumin interneuron impairment as a common mechanism in models of schizophrenia. Mol. Psychiatry 22: 936–943.
18. Khadimallah I, et al. (2022) Mitochondrial, exosomal miR137-COX6A2 and gamma synchrony as biomarkers of parvalbumin interneurons, psychopathology, and neurocognition in schizophrenia. Mol. Psychiatry 27: 1192–1204.
19. Perez SM, Boley A, Lodge DJ (2019) Region specific knockdown of Parvalbumin or Somatostatin produces neuronal and behavioral deficits consistent with those observed in schizophrenia. Transl. Psychiatry 9: 264.
20. Elam HB, Perez SM, Donegan JJ, Eassa NE, Lodge DJ (2024) Knockdown of Lhx6 during embryonic development results in neurophysiological alterations and behavioral deficits analogous to schizophrenia in adult rats. Schizophr. Res. 267: 113–121.
21. Kann O (2016) The interneuron energy hypothesis: Implications for brain disease. Neurobiol. Dis. 90: 75–85.
22. Kann O, Huchzermeyer C, Kovács R, Wirtz S, Schuelke M (2011) Gamma oscillations in the hippocampus require high complex I gene expression and strong functional performance of mitochondria.Brain 134: 345–358.
23. Santos-Silva T, et al. (2024) Adolescent stress-induced ventral hippocampus redox dysregulation underlies behavioral deficits and excitatory/inhibitory imbalance related to schizophrenia. Schizophr. Bull. sbae033. https://doi.org/10.1093/schbul/sbae033.
24. Santos-Silva T, et al. (2024) Perineuronal nets as regulators of parvalbumin interneuron function: Factors implicated in their formation and degradation. Basic. Clin. Pharmacol. Toxicol. 134: 614–628.
25. Gibel-Russo R, Benacom D, Di Nardo AA (2022) Non-cell-autonomous factors implicated in parvalbumin interneuron maturation and critical periods. Front. Neural. Circuits. 16: 875873.
26. Bagherifard A, et al. (2023) Melatonin and bone-related diseases: an updated mechanistic overview of current evidence and future prospects. Osteoporos. Int. 34: 1677–1701.
27. Targhazeh N, et al. (2022) Oncostatic activities of melatonin: Roles in cell cycle, apoptosis, and autophagy. Biochimie 202: 34–48.
28. Luo C, et al. (2019) The multiple protective roles and molecular mechanisms of melatonin and its precursor N-acetylserotonin in targeting brain injury and liver damage and in maintaining bone health. Free Radic. Biol. Med. 130: 215–233.
29. Pérez-Martínez Z, Boga JA, Potes Y, Melón S, Coto-Montes A (2024) Effect of melatonin on herpesvirus type 1 replication. Int J Mol Sci 25: 4037.
30. Hosseinzadeh A, et al. (2024) Therapeutic potential of melatonin in targeting molecular pathways of organ fibrosis. Pharmacol. Rep. 76: 25–50.
31. Li T, et al. (2019) Exogenous melatonin as a treatment for secondary sleep disorders: A systematic review and meta-analysis. Front. Neuroendocrinol. 52: 22–28.
32. Hosseinzadeh A. et al. (2024) Melatonin and oral diseases: possible therapeutic roles based on cellular mechanisms. Pharmacol Rep. 76 (3):487-503.
33. Mehrzadi S, et al. (2023) Protective and therapeutic potential of melatonin against intestinal diseases: updated review of current data based on molecular mechanisms. Expert. Rev. Gastroenterol. Hepatol. 17: 1011–1029.
34. Potes Y, et al. (2023) Benefits of the neurogenic potential of melatonin for treating neurological and neuropsychiatric disorders. Int. J. Mol. Sci. 24: 4803.
35. Thomas L, Purvis CC, Drew JE, Abramovich DR, Williams LM (2002) Melatonin receptors in human fetal brain: 2-[(125)I]iodomelatonin binding and MT1 gene expression. J. Pineal Res. 33: 218–224.
36. Jin Y, Choi J, Won J, Hong Y (2018) The Relationship between autism spectrum disorder and melatonin during fetal development. Molecules 23: 198.
37. Zhang Z, van Praag H (2015) Maternal immune activation differentially impacts mature and adult-born hippocampal neurons in male mice. Brain Behav. Immun. 45: 60–70.
38. Chitimus DM, et al. (2020) Melatonin’s impact on antioxidative and anti-inflammatory reprogramming in homeostasis and disease. Biomolecules 10: 1211.
39. Cowman M, et al. (2024) Measures of social and occupational function in early psychosis: A systematic review and meta-analysis. Schizophr. Bull. 50: 266–285.
40. Galván-Arrieta T, et al. (2017) The role of melatonin in the neurodevelopmental etiology of schizophrenia: A study in human olfactory neuronal precursors. J. Pineal Res. 63: e12421.
41. Waldhauser F, et al. (1984) Fall in nocturnal serum melatonin during prepuberty and pubescence. Lancet 1: 362–365.
42. Amminger GP, et al. (2010) Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch. Gen. Psychiatry 67: 146–154.
43. Hashimoto T, et al. (2003) Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J. Neurosci. 23: 6315–6326.
44. Das A, et al. (2013) Overexpression of melatonin membrane receptors increases calcium-binding proteins and protects VSC4.1 motoneurons from glutamate toxicity through multiple mechanisms. J. Pineal Res. 54: 58–68.
45. Reiter RJ, Sharma R, Rosales-Corral S, de Campos Zuccari DAP, de Almeida Chuffa LG (2022) Melatonin: A mitochondrial resident with a diverse skill set. Life Sci. 301: 120612.
46. Ma Z, et al. (2017) Melatonin and mitochondrial function during ischemia/reperfusion injury. Cell. Mol. Life Sci. 74: 3989–3998.
47. Bastos MAV, et al. (2019) Pineal gland and schizophrenia: A systematic review and meta-analysis. Psychoneuroendocrinology 104: 100–114.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


