Constant light exposure terminates pregnancy in rats with pineal gland dysfunction, low melatonin level and pro-inflammatory response
Constant light terminates rat pregnancy
Abstract
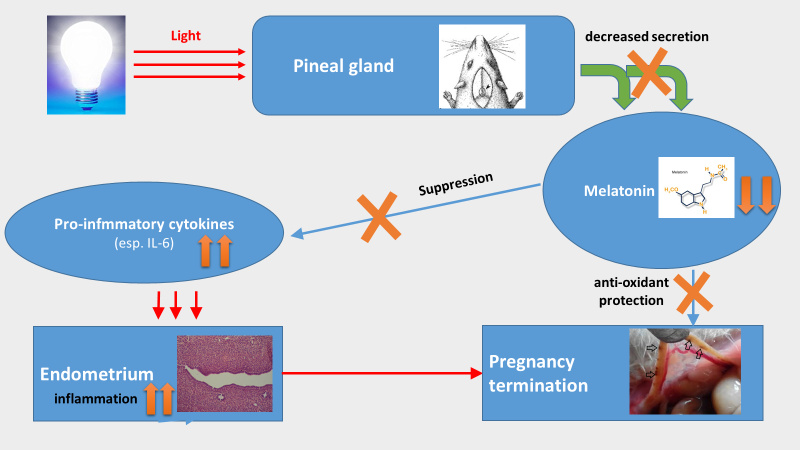
In the current study, the prolonged light exposure at night on the function of pineal gland, melatonin production, pro-inflammatory response and progress of pregnancy in pregnant rats were investigated. A long term (entire gestation stage) of 24 h light exposure not only modify the morphologies of pinealocytes by decreasing their nucleus/cytoplasm ratio and mitochondrial numbers but also reduce the level of circulating melatonin. The biological consequences for this constant light exposure in pregnant rats were the elevated the pro-inflammatory response indicated by the increased production of IL-6 and finally, the termination of pregnancy compared to their controlled counterparts under the normal light/dark cycle. The result showed that the pregnancy was terminated at the early stage of embryo development. The report, for the first time, established a potential association among the pineal function, pro-inflammatory reaction and pregnant progress under the influence of light exposure. This observation has a high relevant to the rise in human infertility since humans have overexposed to the light at night with the increased light pollution globally.
References
2. Pishak VP (2002) Shyshkopodibne tilo – mistse i rol' u khronorytmolohichnii orhanizatsii fiziolohichnykh funktsii (Pineal gland and its role and place in chrono-rhythmic organization of physiologic functions). Bukovyns'kyi medychnyi visnyk. 6 (3-4): 4-6. (in Ukrainian).
3. Pishak VP, Bulyk RIe (2006) Mekhanizmy uchasti shyshkopodibnoi zalozy v zabezpechenni tsyrkadiannoi rytmichnosti fiziolohichnykh funktsii (Mechanisms of participation of a pineal gland in the maintenance of circadian rhythmicity of the physiological functions). Bukovyns'kyi medychnyi visnyk. 10 (4): 4-7. (in Ukrainian).
4. Asif M, Pervez, AR (2019) Role of Melatonin and Plant-Growth-Promoting Rhizobacteria in the Growth and Development of Plants. Clean Soil Air Water. 479 (6): 1800459. doi: 10.1002/clen.201800459.
5. Yildirim A, Tamer SA, Sahin D, Bagriacik F, Kahraman MM et al. (2019) The effects of antibiotics and melatonin on hepato-intestinal inflammation and gut microbial dysbiosis induced by a short term high-fat diet consumption in rats. Br. J. Nutr. 20: 1-31. doi: 10.1017/S0007114519001466.
6. Berbets A, Koval H, Barbe A, Albota O, Yuzko O (2019) Melatonin decreases and cytokines increase in women with placental insufficiency. J. Matern. Fetal Neonatal Med. 25: 1-6. doi: 10.1080/14767058.2019.1608432.
7. Mokhtari F, Asbagh FA, Azmoodeh O, Bakhtiyari M, Almasi-Hashiani A (2019) Effects of melatonin administration on chemical pregnancy rates of polycystic ovary syndrome patients undergoing intrauterine insemination: a randomized clinical trial. Int. J. Fert. Ster. 13 (3): 225-229. doi: 10.22074/ijfs.2019.5717.
8. Moghani-Ghoroghi F, Moshkdanian G, Sehat M, et al. (2018) Melatonin pretreated blastocysts along with calcitonin administration improved implantation by upregulation of heparin binding-epidermal growth factor expression in murine endometrium. Cell J. 19 (4): 599-606. doi: 10.22074/cellj.2018.4737.
9. Takayama H, Nakamura Y, Tamura H (2003) Pineal gland (melatonin) affects the parturition time but not luteal function and fetal growth, in pregnant rats. Endocr. J. 50 (1): 37-43. DOI: 10.1507/endocrj.50.37.
10. Richter HG, Hansell JA, Raut Sh, Giussani DA (2009) Melatonin improves placental efficiency and birth weight and increases the placental expression of antioxidant enzymes in undernourished pregnancy. J. Pineal Res. 46: 357-364. doi: 10.1111/j.1600-079X.2009.00671.x.
11. Shimada M, Seki H, Samejima M, Hayase M, Shirai F (2016) Salivary melatonin levels and sleep-wake rhythms in pregnant women with hypertensive and glucose metabolic disorders: A prospective analysis. BioSci. Trends 10 (1): 34-41. doi: 10.5582/bst.2015.01123.
12. Song YK, Wu H, Wang XG, Haire A, Zhang XS, Zhang, JL et al. (2019) Melatonin improves the efficiency of super-ovulation and timed artificial insemination in sheep. Peer J. 7: e6750. doi: 10.7717/peerj.6750.
13. Abecia, JA; Forcada, F; Vazquez, MI; Muino-Blanco, T; Cebrian-Perez, JA; Perez-Pe, R; Casao, A (2019) Role of melatonin on embryo viability in sheep. Reprod. Fert. Dev. 31 (1): 82-92. doi: 10.1071/RD18308.
14. Tamura H, Takasaki A, Taketani T, Tanabe M, Kizuka F, Lee L, Tamura I et al. (2012) The role of melatonin as an antioxidant in the follicle. J. Ovar. Res. 5 (5): doi:10.1186/1757-2215-5-5.
15. Talpur HS, Chandio IB, Brohi RD, Worku T, Rehman Z, Bhattarai D et al. (2018) Research progress on the role of melatonin and its receptors in animal reproduction: A comprehensive review. Reprod. Dom. Anim. 53: 831–49. doi: 10.1111/rda.13188.
16. Soliman A, Lacasse A, Lanoix D, Sagrillo-Fagundes L, Boulard V, Vaillancourt C (2015) Placental melatonin system is present throughout pregnancy and regulates villous trophoblast differentiation. J. Pineal Res. 59 (1): 38-46. doi: 10.1111/jpi.12236.
17. Esroy OF, Özkan N, Özsoy Z (2016) Effects of melatonin on cytokine release and healing of colonic anastomoses in an experimental sepsis model. Ulus. Travma. Acil. Cerrahi. Derg. 22 (4): 315-21. doi: 10.5505/tjtes.2015.49465.
18. Woo-Jin Yi, Tae Sung Kim (2017) Melatonin protects mice against stress-induced inflammation through enhancement of M2 macrophage polarization. Int. Immunopharmacol. 48: 146-58. doi: 10.1016/j.intimp.2017.05.006.
19. Gang X, Jon D, Tiecheng, Fangyun T, Jimo B (2018) Chronic circadian advance shifts abolish melatonin secretion for days in rats. Neurobiol. Sleep Circad. Rhythms 5: 78–83. doi: 10.1016/j.nbscr.2018.02.002.
20. Tan DX, Xu B, Zhou X, Reiter RJ (2018) Pineal calcification, melatonin production, aging, associated health consequences and rejuvenation of the pineal gland. Molecules 23 (2). pii: E301. doi: 10.3390/molecules23020301.
21. Lynch HJ, Rivest RW, Ronsheim PM, Wurtman RJ (1981) Light intensity and the control of melatonin secretion in rats. Neuroendocrinol. 33 (3): 181-185. doi: 10.1159/000123226.
22. Wideman CH, Murphy HM (2009) Constant light induces alterations in melatonin levels, food intake, feed efficiency, visceral adiposity, and circadian rhythms in rats. Nutr. Neurosci. 12 (5): 233-240. doi: 10.1179/147683009X423436.
23. Zapadnyuk IP, Zapadnyuk VI, Zakhariya EA, Zapadnyuk BV (1984) Laboratornye zhivotnye: razvedenie, soderzhanie, ispol'zovanie v eksperimente (Laboratory animals: breeding, keeping, use in experiment). Kyiv: Vishcha shkola. (in Russian).
24. Lillie RD (1954) Histopathologic technique and practical histochemistry. Ed.2. Blakiston, New York, USA.
25. Kus I, Sarsilmaz M, Ozen OA, Turkoglu AO, Pekmez H, Songur A, Kelestimur H (2004) Light and electron microscopic examination of pineal gland in rats exposed to constant light and constant darkness. Neuroendocrinol. Lett. 25 (1/2): 102–108.
26. Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 17: 208-212. doi: 10.1083/jcb.17.1.208.
27. Tan DX, Manchester LC, Fuentes-Broto L, Paredes SD, Reiter RJ (2011) Significance and application of melatonin in the regulation of brown adipose tissue metabolism: relation to human obesity. Obes. Rev. 12 (3): 167-188. doi: 10.1111/j.1467-789X.2010.00756.x.
28. Obayashi K, Saeki K, Iwamoto J, Ikada Y, Kurumatani N (2013) Exposure to light at night and risk of depression in the elderly. J. Affect. Disord. 151 (1): 331-336. doi: 10.1016/j.jad.2013.06.018.
29. Simko F, Pechanova O, Repova Bednarova K, et al. (2014) Hypertension and cardiovascular remodelling in rats exposed to continuous light: protection by ACE-inhibition and melatonin. Mediators Inflamm. 2014: 703175. doi: 10.1155/2014/703175.
30. Cardinali DP, Vigo DE (2017) Melatonin, mitochondria, and the metabolic syndrome. Cell Mol. Life Sci. 74 (21): 3941-3954. doi: 10.1007/s00018-017-2611-0.
31. Cipolla-Neto J, Amaral FG, Afeche SC, Tan DX, Reiter RJ (2014) Melatonin, energy metabolism, and obesity: a review. J. Pineal Res. 56 (4): 371-381. doi: 10.1111/jpi.12137.
32. Kim YJ, Park MS, Lee E, Choi JW (2016) High incidence of breast cancer in light-polluted areas with spatial effects in Korea. Asian Pac. J. Cancer Prev. 17 (1): 361-367. doi: 10.7314/apjcp.2016.17.1.361.
33. James P, Bertrand KA, Hart JE, Schernhammer ES, Tamimi RM, Laden F (2017) Outdoor light at night and breast cancer incidence in the Nurses' Health Study II. Environ. Health Perspect. 125 (8): 087010. doi: 10.1289/EHP935.
34. Stevens RG, Brainard GC, Blask DE, Lockley SW, Motta ME (2014) Breast cancer and circadian disruption from electric lighting in the modern world. CA. Cancer J. Clin. 64 (3): 207-218. doi: 10.3322/caac.21218.
35. Stevens RG, Blask DE, Brainard GC, Hansen J, Lockley SW, Provencio I, Rea MS, Reinlib L (2007) Meeting report: the role of environmental lighting and circadian disruption in cancer and other diseases. Environ. Health Perspect. 115: 1357–1362.
36. Malek I, Haim A (2019) Bright artificial light-at-night is associated with increased body mass, poor reproductive success, and compromised disease tolerance in Australian budgerigars (Melopsittacus undulatus). Integr. Zool. 31: doi: 10.1111/1749-4877.12409.
37. Russ A, Reitemeier S, Weissmann A, Gottschalk J, Einspanier A, Klenke R (2015) Seasonal and urban effects on the endocrinology of a wild passerine. Ecol. Evol. 19; 5 (23): 5698-5710. doi: 10.1002/ece3.1820.
38. Robert KA1, Lesku JA, Partecke J, Chambers B (2015) Artificial light at night desynchronizes strictly seasonal reproduction in a wild mammal. Proc. Biol. Sci. 282 (1816): 20151745. doi: 10.1098/rspb.2015.1745.
39. Reiter RJ (1974) Influence of pinealectomy on the breeding capability of hamsters maintained under natural photoperiodic and temperature conditions. Neuroendocrinol. 13: 366–370. doi: 10.1159/000122222.
40. Billyard AJ1, Eggett DL, Franz KB (2006) Dietary magnesium deficiency decreases plasma melatonin in rats. Magnes Res. 19 (3): 157-161. doi: 10.1684/mrh.2006.0002.
41. Dauchy RT, Wren MA et al. (2015) The influence of red light exposure at night on circadian metabolism and physiology in Sprague-Dawley rats. J. Am. Assoc. Lab. Anim. Sci. 54 (1): 40-50.
42. Dini P, Ducheyne K, Lemahieu I, Wambacq W, Vandaele H, Daels P. (2019) Effect of environmental factors and changes in the body condition score on the onset of the breeding season in mares. Reprod. Domest. Anim. 54 (7): 987-995. doi: 10.1111/rda.13452.
43. Mura MC, Luridiana S, Farci F, Di Stefano MV, Daga C, Pulinas L, Starič J, Carcangiu V. (2017) Melatonin treatment in winter and spring and reproductive recovery in Sarda breed sheep. Anim. Reprod. Sci. 185: 104–108. doi: 10.1016/j.anireprosci.2017.08.009.
44. Frost D, Zucker I (1983) Photoperiod and melatonin influence seasonal gonadal cycles in the grasshopper mouse (Onychomys leucogaster). J. Reprod. Fertil. 69 (1): 237-244. doi:10.1530/jrf.0.0690237.
45. Williams HL (1984) The effect of feeding melatonin during late summer on the onset of the breeding season of sheep. Br. Vet. J. 140 (4): 407-408.
46. Zhang L, Zhang Z, Wang J, Lv D, Zhu T, Wang F, Tian X, Yao Y, Ji P, Liu G (2019) Melatonin regulates the activities of ovary and delays the fertility decline in female animals via MT1/AMPK pathway. J. Pineal Res. 66 (3): e12550. doi: 10.1111/jpi.12550.
47. Seron-Ferre M, Valenzuela GJ, Torres-Farfan C (2007) Circadian clocks during embryonic and fetal development. Birth Defects Res. C Embryo Today. 81: 204–214. doi: 10.1002/bdrc.20101.
48. Asgari Z, Ghasemian F, Ramezani M, Bahadori MH (2012) The effect of melatonin on the developmental potential and implantation rate of mouse embryos. Cell J. 14 (3): 203-208.
49. Tian, XZ, Wang, F, Zhang, L, Ji, PY, Wang, J, Lv, DY et al. (2017) Melatonin Promotes the In Vitro Development of Microinjected Pronuclear Mouse Embryos via Its Anti-Oxidative and Anti-Apoptotic Effects. Int. J. Mol. Sci. 18 (5). pii: E988. doi: 10.3390/ijms18050988.
50. Nishihara T, Hashimoto S, Ito K, Nakaoka Y, Matsumoto K, Hosoi Y, Morimoto Y (2014) Oral melatonin supplementation improves oocyte and embryo quality in women undergoing in vitro fertilization-embryo transfer. Gynecol. Endocrinol. 30 (5): 359-362. doi: 10.3109/09513590.2013.879856.
51. Choi J, Park SM, Lee E, Kim JH, Jeong YI, Lee JY, Park SW, Kim HS, Hossein MS, Jeong YW et al. (2008) Anti-apoptotic effect of melatonin on preimplantation development of porcine parthenogenetic embryos. Mol. Reprod. Dev. 75: 1127–1135. doi: 10.1002/mrd.20861.
52. Yildiz A (2015) Improving the conception rate of dairy cows by using a melatonin implant. Large Anim. Rev. 21 (5): 201-205.
53. Zhang L, Zhang ZZ, Wang F, Tian XZ, Ji PY, Liu GS (2017) Effects of melatonin administration on embryo implantation and offspring growth in mice under different schedules of photoperiodic exposure. Reprod. Biol. Endocrinol. 15: 78. doi: 10.1186/s12958-017-0297-7.
54. Zarazaga LA, Gatica MC, Gallego-Calvo L, Celi I, Guzman JL (2013) Short communication. Melatonin improves the reproductive performance of seasonal anoestrus goats exposed to buck effect during early post-partum. Span. J. Agricul. Res. 11 (4): 997-1003. doi: 10.5424/sjar/2013114-4115.
55. Wuliji, T; Litherland, A; Goetsch, AL; Sahlu, T; Puchala, R; Dawson, LJ; Gipson, T (2003) Evaluation of melatonin and bromocryptine administration in Spanish goats I. Effects on the out of season breeding performance in spring, kidding rate and fleece weight of does. Small Rumin. Res. 49 (1): 31-40. doi:10.1016/S0921-4488(03)00055-5.
56. Teixeira AA, Simoes MJ, Wanderley Teixeira V, Soares JJr (2004) Evaluation of the implantation in pinealectomized and/or submitted to the constant illumination rats. Int. J. Morphol. 22 (3): 189-194.
57. Malek I, Haim A (2019) Bright artificial light-at-night is associated with increased body mass, poor reproductive success, and compromised disease tolerance in Australian budgerigars (Melopsittacus undulatus). Integr. Zool. 31: doi: 10.1111/1749-4877.12409.
58. Li C, Zhou X (2015) Melatonin and male reproduction. Clin. Chim. Acta 446: 175-180. doi: 10.1016/j.cca.2015.04.029.
59. Casper RF, Gladanac B (2014) Circadian rhythm and its disruption: impact on reproductive function. Fertil. Steril. 102 (2): 319-20. doi: 10.1016/j.fertnstert.2014.04.053.
60. Reiter RJ, Tamura H, Tan DX, Xu XY (2014) Melatonin and the circadian system: contributions to successful female reproduction. Fertil. Steril. 102 (2): 321-328. doi: 10.1016/j.fertnstert.2014.06.014.
61. Tian XZ, Wang F, Zhang L, Ji PY, Wang J, Lv DY et al. (2017) Melatonin promotes the in vitro development of microinjected pronuclear mouse embryos via its anti-oxidative and anti-apoptotic effects. Int. J. Mol. Sci. 18 (5): 988. doi: 10.3390/ijms18050988.
62. Meng X, Li Y, Li S, Zhou Y, Gan R, Xu D, and Li H (2017) Dietary sources and bioactivities of melatonin. Nutrients 9 (4): 367. doi: 10.3390/nu9040367.
63. Herman AP, Bochenek J, Skipor J, Król K, Krawczyńska A, Antushevich H, Pawlina B, Marciniak E, and Tomaszewska-Zaremba D (2015) Interleukin-1β modulates melatonin secretion in ovine pineal gland: ex vivo study. Biomed. Res. Int. 526464. doi: 10.1155/2015/526464.
64. Liu X, Gong Y, Xiong K, Ye Y, Xiong Y, Zhuang Z, Luo Y, Jiang Q, He F (2013) Melatonin mediates protective effects on inflammatory response induced by interleukin-1 beta in human mesenchymal stem cells. J. Pineal Res. 55 (1): 14-25. doi: 10.1111/jpi.12045.
65. Chen S, Huang S, Chen J, Wang K, Yang Y, Liu P, Lin G, Sytwu H. (2016) Melatonin enhances interleukin-10 expression and suppresses chemotaxis to inhibit inflammation in situ and reduce the severity of experimental autoimmune encephalomyelitis. Inter. Immunopharmacol. 31: 169-177. doi: 10.1016/j.intimp.2015.12.020.
66. Haddadi GH, Fardid R (2014) Oral administration of melatonin modulates the expression of tumor necrosis factor-α (TNF-α) gene in irradiated rat cervical spinal cord. Rep. Pract. Oncol. Radiother. 20 (2): 123–7. doi: 10.1016/j.rpor.2014.11.003.
67. Tan DX, Reiter RJ. (2019) Mitochondria: the birth place, battle ground and the site of melatonin metabolism in cells. Melatonin Research. 2 (1): 44-66. doi:https://doi.org/https://doi.org/10.32794/mr11250011.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


