Protective effects of melatonin on mitochondrial injury and neonatal neuron apoptosis induced by maternal hypothyroidism
Impact of melatonin on mitochondrial outer membrane of neonatal brain
Abstract
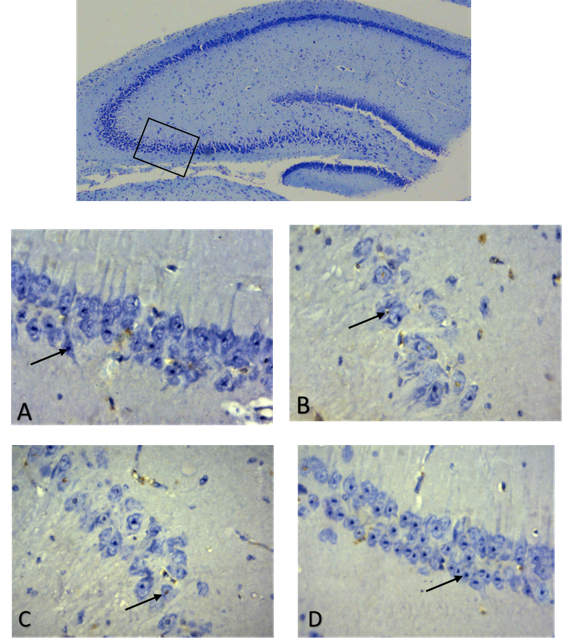
In the current study, we reported the beneficial effects of melatonin in preventing neonatal neuronal apoptosis induced by maternal hypothyroidism. During the gestation and early lactation stages, the mother rats were given propylthiouracil (PTU) to inhibit their thyroid gland activity which resulted in the increased serum TSH and reduced T4 levels. This maternal hypothyroidism caused neuronal apoptosis of their pups, particularly in the CA3 area of hippocampus. Melatonin administration preserved function of thyroid gland and significantly reduced the apoptosis. Further studies have uncovered the potentially protective mechanisms of melatonin, that is, as a mitochondrial targeted antioxidant, melatonin preserves the mitochondrial outer membrane, inhibits the release of cytochrome C from mitochondria to cytoplasm and down regulates the gene expressions of Bax, along with caspases 3 and 9. Thus, melatonin breaks the mitochondria related apoptotic pathway to suppress the neuronal apoptosis induced by the maternal hypothyroidism. Considering the limited remedies to effectively treat hypothyroidism associated neonatal brain damage, melatonin may provide an alternative method for this disorder.
References
2. Bernal J (2015) Thyroid hormones in brain development and function. In: Feingold KR et al. (ED). Endotext. https://www.ncbi.nlm.nih.gov/books/NBK285549.
3. Escobar GM, Obregon MJ, Escobar del Re F (2007) Iodine deficiency and brain development in the first half of pregnancy. Public Health Nutrition 10 (12A): 1554–1570 doi: 10.1017/S1368980007360928.
4. Jin J, Maren S (2015) Prefrontal-hippocampal interactions in memory and emotion. Front. Syst. Neurosci. 9: 170. doi:10.3389/fnsys.2015.00170.
5. Cherubini E, Miles R (2015) The CA3 region of the hippocampus: how is it? What is it for? How does it do it? Front. Cell Neurosci. 9: 19. doi:10.3389/fncel.2015.00019.
6. Amaral DG, Scharfman HE, Lavenex P (2007) The dentate gyrus: fundamental neuroanatomical organization (dentate gyrus for dummies). Prog. Brain Res. 163: 3–22. doi:10.1016/S0079-6123(07)63001-5.
7. Maruszak A, Thuret S (2014) Why looking at the whole hippocampus is not enough—a critical role for anteroposterior axis, subfield and activation analyses to enhance predictive value of hippocampal changes for Alzheimer’s disease diagnosis. Front. Cell Neurosci. 8: 95. https://doi.org/10.3389/fncel.2014.00095.
8. Le Duigou C, Simonnet J, Teleñczuk MT, Fricker D, Miles R (2014) Recurrent synapses and circuits in the CA3 region of the hippocampus: an associative network. Front. Cell Neurosci. 7: 262. doi:10.3389/fncel.2013.00262.
9. Hidayat M, Haider I, Khurram A, Lone KP (2019) The immunohistochemical localization of Bax in the brain of hypothyroid neonate during maternal melatonin intake. Int. J. Anat. Res. 7 (3): 6901-6905. doi: https://dx.doi.org/10.16965/ijar.2019.259.
10. Xiao Q, Nikodem VM (1998) Apoptosis in the developing cerebellum of the thyroid hormone deficient rat. Front. Bio-science 3 (1): A52-57.
11. Faustino LC, Ortiga-Carvalho TM (2014) Thyroid hormone role on cerebellar development and maintenance: a perspective based on transgenic mouse models. Front. Endocrinol. (Lausanne). 5: 75. doi:10.3389/fendo.2014.00075.
12. Cooke GE, Mullally S, Correia N, O'Mara SM, Gibney J (2014) Hippocampal volume is decreased in adults with hypothyroidism. Thyroid 24 (3): 433-40. doi: 10.1089/thy.2013.0058.
13. Nam SM, Kim JW, Yoo DY et al. (2018) Hypothyroidism increases cyclooxygenase-2 levels and pro-inflammatory response and decreases cell proliferation and neuroblast differentiation in the hippocampus. Mol. Med. Rep. 17 (4): 5782–5788. doi:10.3892/mmr.2018.8605.
14. Becker EB, Bonni A (2004) Cell cycle regulation of neuronal apoptosis in development and disease. Prog. Neurobiol. 72 (1):1-25.
15. Chatonnet F, Picou F, Fauquier T, Flamant F (2011) Thyroid hormone action in cerebellum and cerebral cortex development. J. Thyroid Res. 2011: 145762. doi:10.4061/2011/145762.
16. Osellame LD, Blacker TS, Duchen MR (2012) Cellular and molecular mechanisms of mitochondrial function. Best Pract. Res. Clin. Endocrinol. Metab. 26 (6): 711–723. doi:10.1016/j.beem.2012.05.003.
17. Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol. Pathol. 35 (4): 495–516. doi:10.1080/01926230701320337.
18. Chen X, Guo C, Kong J (2012) Oxidative stress in neurodegenerative diseases. Neural Regen. Res. 7 (5): 376–385. doi:10.3969/j.issn.1673-5374.2012.05.009.
19. Alemu A, Terefe B, Abebe M, Biadgo B (2016) Thyroid hormone dysfunction during pregnancy: A review. Int. J. Reprod. Biomed. (Yazd). 14 (11): 677–686.
20. Miranda A, Sousa N (2018) Maternal hormonal milieu influence on fetal brain development. Brain Behav. 8 (2): e00920. https://doi.org/10.1002/brb3.920.
21. Sahay RK, Nagesh VS (2012) Hypothyroidism in pregnancy. Ind. J. Endocrinol. Metab. 16 (3): 364–370. doi:10.4103/2230-8210.95667.
22. Dirar AM, Kalhan A (2018) Hypothyroidism during pregnancy: Controversy over screening and intervention, World J. Obstet. Gynecol. 7 (1): 1-16. doi: 10.5317/wjog.v7.i1.
23. Krajewski S, Krajewska M, Ellerby LM et al. (1999) Release of caspase-9 from mitochondria during neuronal apoptosis and cerebral ischemia. Proc. Natl. Acad. Sci. 96 (10): 5752-5757. https://doi.org/10.1073/pnas.96.10.5752.
24. Srinivasan V, Spence DW, Pandi-Perumal SR, Brown GM, Cardinali DP (2011) Melatonin in mitochondrial dysfunction and related disorders. Int. J. Alzheimers Dis. 2011: 326320. doi: 10.4061/2011/326320.
25. Wang C, Youle RJ (2009) The role of mitochondria in apoptosis. Ann. Rev. Genet. 43: 95–118. doi:10.1146/annurev-genet-102108-134850.
26. Rebecca A. Kirkland and James L (2001) Evidence for redox regulation of cytochrome c release during programmed neuronal death: antioxidant effects of protein synthesis and caspase inhibition. J. Neurosc. 21 (6): 1949-1963; doi: https://doi.org/10.1523/JNEUROSCI.21-06-01949.2001.
27. Skulachev VP, Anisimov VN, Antonenko YN (2009) An attempt to prevent senescence: A mitochondrial approach. BBA. 1787 (5): 437-461 https://doi.org/10.1016/j.bbabio.2008.12.008.
28. Jiang X, Wang X (2004) Cytochrome C-mediated apoptosis. Ann. Rev. Biochem. 73: 87-106.
29. Tan DX, Reiter RJ (2019) Mitochondria: the birth place, battle ground and the site of melatonin metabolism in cells. Melatonin Res. 2 (1): 44-66. doi: https://doi.org/10.32794/mr11250011.
30. Beriwal N, Namgyal T, Asmaa PS, Al Quraan M (2019) Role of immune-pineal axis in neurodegenerative diseases, unraveling novel hybrid dark hormone therapies. Heliyon. 5 (1): e01190. doi: 10.1016/j.heliyon.2019.e01190.
31. Reiter RJ, Tan DX, Rosales-Corral S, Galano A, Jou MJ, Acuna-Castroviejo D (2018) Melatonin mitigates mitochondrial meltdown: interactions with SIRT3. Int. J. Mol. Sci. 19 (8): 2439. doi:10.3390/ijms19082439.
32. Di Bella G, Mascia F, Gualano L, Di Bella L (2013) Melatonin anticancer effects: review. Int J. Mol. Sci.14 (2): 2410–2430. doi:10.3390/ijms14022410.
33. Bojková B, Kubatka P, Qaradakhi T, Zulli A and Kajo K (2018) Melatonin may increase anticancer potential of pleiotropic drugs, Int. J. Mol. Sci. 19: 3910. doi:10.3390/ijms19123910.
34. Tan DX, Manchester LC, Qin L, Reiter RJ (2016) Melatonin: a mitochondrial targeting molecule involving mitochondrial protection and dynamics. Int. J. Mol. Sci. 17 (12): 2124. doi:10.3390/ijms17122124.
35. García G, Martínez-Ballarín G, Acuña-Castroviejo D et al. (2011). Melatonin reduces membrane rigidity and oxidative damage in the brain of SAMP(8) mice. Neurobiol. Aging 32: 2045-2054. 10.1016/j.neurobiolaging.2009.12.013.
36. Tan DX, Manchester LC, Sanchez-Barcelo E, Mediavilla MD, Reiter RJ (2010) Significance of high levels of endogenous melatonin in mammalian cerebrospinal fluid and in the central nervous system. Curr. Neuropharmacol. 8 (3): 162–167. doi:10.2174/157015910792246182.
37. Wang DD, Jin MF, Zhao DJ, Ni H (2019) Reduction of mitophagy-related oxidative stress and preservation of mitochondria function using melatonin therapy in an HT22 hippocampal neuronal cell model of glutamate-induced excitotoxicity. Front. Endocrinol. (Lausanne).10: 550. doi:10.3389/fendo.2019.
38. Webster KA (2012) Mitochondrial membrane permeabilization and cell death during myocardial infarction: roles of calcium and reactive oxygen species. Future Cardiol. 8 (6): 863-884.
39. Schenkel LC, Bakovic M (2014) Formation and regulation of mitochondrial membranes. Int. J. Cell Bio. 2014: 709828.13. https://doi.org/10.1155/2014/709828.
40. Ferreira Cda S, Maganhin CC, Simões Rdos S, Girão MJ, Baracat EC (2010) Melatonin: cell death modulator. Rev. Assoc. Med. Bras. 56 (6): 715-718.
41. Wang LM, Suthana NA, Chaudhury D, Weaver DR, Colwell CS (2005) Melatonin inhibits hippocampal long-term potentiation. Eur. J. Neurosci. 22 (9): 2231–2237. doi:10.1111/j.1460-9568.2005.04408.
42. Cai J, Yang J, Dean P (1998) Mitochondrial control of apoptosis: the role of cytochrome C. BBA. 1366 (1–2): 139-149.
43. Cheng M, Huang H, Lin Y et al. (2019) BA6 Induces Apoptosis via Stimulation of Reactive Oxygen Species and Inhibition of Oxidative Phosphorylation in Human Lung Cancer Cells. Oxid. Med. Cell Longev. 2019: 6342104. https://doi.org/10.1155/2019/6342104.
44. Wang X (2009) The antiapoptotic activity of melatonin in neurodegenerative diseases CNS Neurosci. Ther. 15 (4): 345-357.
45. Vakifahmetoglu-Norberg H, Ouchida AT, Norberg (2017) The role of mitochondria in metabolism and cell death. BBRC. 482 (3): 426-431. doi: 10.1016/j.bbrc.2016.11.088.
46. Alkalby JM, Sarah JS (2013) Effect of propylthiouracil-induced hypothyroidism on reproductive efficiency of adult male rats. Bas. J. Vet. Res. 12 (2): 113-121.
47. Hidayat M (2012) Protective role of melatonin and insulin on streptozotocin induced nephrotoxicity in albino rats. Pak. J. Med.& Health Sci. 6 (3): 669-674.
48. Hua YC, Chunga MH, Lee TH (2018) An assay of optimal cytochrome c oxidase activity in fish gills. Anal. Biochem. 553: 38-45. doi: 10.1016/j.ab.2018.05.017.
49. Makino Y, Ichimura M (2007) Cytochrome C oxidase as a cause of variation in oxygen uptake rates among vegetables. J. Am. Soci. Horticul. Sci. 132 (2): 239-245.
50. Melendez-Ferro M, Rice MW, Roberts RC, Perez-Costas E (2013) An accurate method for the quantification of cytochrome C oxidase in tissue sections. J. Neurosci. Methods 214 (2): 156–162. doi:10.1016/j.jneumeth.2013.01.010.
51. Vilhjálmsdóttir J, Gennis RB (2018) The electron distribution in the “activated” state of cytochrome c oxidase. Sci. Rep. 8: 7502.doi:10.1038/s41598-018-25779.
52. Tait SW, Green DR (2013) Mitochondrial regulation of cell death. Cold Spring Harb. Perspect. Biol. 5 (9): a008706. doi:10.1101/cshperspect.a008706.
53. Kotiadis VN, Duchen MR, Osellame LD (2014) Mitochondrial quality control and communications with the nucleus are important in maintaining mitochondrial function and cell health. BBA. 1840 (4):1254-1265.
54. Chipuk JE, Bouchier-Hayes L, Green DR (2006) Mitochondrial outer membrane permeabilization during apoptosis: the innocent bystander scenario. Cell Death Differ. 13: 1396–1402.
55. Kaniak-Golik A, Skoneczna A (2015) Mitochondria–nucleus network for genome stability. Free Radic. Biol. Med. 82: 73-104.
56. Dowling AL, Martz GU, Leonard JL, Zoeller RT. (2000) Acute changes in maternal thyroid hormone induce rapid and transient changes in gene expression in fetal rat brain, J. Neurosci. 20 (6): 2255–2265.
57. Martín M, Macías M, Escames G, Reiter RJ (2000) Melatonin-induced increased activity of the respiratory chain complexes I and IV can prevent mitochondrial damage induced by ruthenium red in vivo. J. Pineal Res. 28: 242–248. doi: 10.1034/j.1600-079X.2000.280407.
58. Ahmed RG (2012). Maternal-fetal thyroid interactions, Thyroid Hormone, N.K. Agrawal (Ed.), ISBN: 978-953-51-0678-4. pp. 125-156. DOI: 10.5772/48076.
59. Stepien BK, Huttner WB (2019) Transport, metabolism, and function of thyroid hormones in the developing mammalian brain. Front. Endocrinol. (Lausanne). 10: 209. doi:10.3389/fendo.2019.00209.
60. Mogulkoc R, Baltaci AK (2003) The effect of intraperitoneal melatonin supplementation on the release of thyroid hormones and testosterone in rats with hyperthyroid. Neuro. Endocrinol. Lett. 24 (5): 345-347.
61. Suofu Y, Li W, Jean-Alphonse FG et al. (2017) Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc. Natl. Acad. Sci. 114 (38): E7997-E8006. doi: 10.1073/pnas.1705768114.
62. Rubenstein JL (2011) Annual research review: Development of the cerebral cortex: implications for neurodevelopmental disorders. J. Child Psychol. Psychiatry 52 (4): 339–355. doi:10.1111/j.1469-7610.2010.02307.x.
63. Prezioso G, Giannini C, Chiarelli F (2018) Effect of thyroid hormones on neurons and neurodevelopment. Horm. Res. Paediatr. 90: 73–81. https://doi.org/10.1159/000492129.
64. Patel J, Landers K, Li H, Mortimer RH, Richard K (2011) Thyroid hormones and fetal neurological development. J. Endocrinol. 209 (1): 1–8. doi: https://doi.org/10.1530/JOE-10-0444.
65. Rovet JF (2014) The role of thyroid hormones for brain development and cognitive function. Szinnai G (ed): Endocr. Dev. 26: 26-43. doi:10.1159/000363153.
66. Zoeller TR, Dowling AL, Herzig CT, Iannacone EA, Gauger KJ, Bansal R (2002) Thyroid hormone, brain development, and the environment. Environ. Health Perspect. 110: (3): 355–361. doi:10.1289/ehp.02110s3355.
67. D'Angelo G, Marseglia L, Manti S et al. (2016) Atopy and autoimmune thyroid diseases: melatonin can be useful? Ital. J. Pediatr. 42 (1): 95. doi:10.1186/s13052-016-0305-0.
68. Marin G, Santos F, Bernal M, Martinez G, Roman V et al. (2015). Melatonin in the thyroid gland: Regulation by thyroid-stimulating hormone and role in thyroglobulin gene expression. J. Physiol. Pharmacol. 66 (5): 643-652.
69. Zhang Y, Zhang WX, Zhang YJ et al. (2018) Melatonin for the treatment of spinal cord injury. Neural Regen. Res. 13 (10):1685–1692. doi:10.4103/1673-5374.238603.
70. Anderson G, and Mazzoccoli G (2019) Left ventricular hypertrophy: roles of mitochondria CYP1B1 and melatonergic pathways in co-ordinating wider pathophysiology. Int. J. Mol. Sci. 20 (16): 4068.
71. Musshoff U, Riewenherm D, Berger E, Fauteck JD, Speckmann EJ (2002) Melatonin receptors in rat hippocampus: molecular and functional investigations. Hippocampus 12 (2): 165-173.
72. Graziella A, Carlo VL, Ragazzi E, Steinhart H, Varesio L (2003) Developments in tryptophan and serotonin metabolism Springer-Verlag US. https://doi.org/10.1007/978-1-4615-0135-0.
73. Lacoste B, Angeloni D, Dominguez-Lopez S, Calderoni S et al. (2015) J. Pineal Res. 58: 397–417 doi:10.1111/jpi.12224.
74. Valdés-Tovar M, Estrada-Reyes R, Solís-Chagoyán H et al. (2018) Circadian modulation of neuroplasticity by melatonin: a target in the treatment of depression. Br. J. Pharmacol. 175 (16): 3200–3208. doi:10.1111/bph.14197.
75. Tan DX, Manchester LC, Reiter RJ, Qi W, Kim SJ, El-Sokkary GH (1998) Melatonin protects hippocampal neurons in vivo against kainic acid-induced damage in mice. J. Neurosci. Res. 54 (3): 382-389.
76. Zhu Y, Li M, Wang X et al. (2011) Caspase cleavage of cytochrome c1 disrupts mitochondrial function and enhances cytochrome c release. Cell Res. 22 (1):127-141.
77. Farooqui AA, Ong WY, Horrocks LA (2008) Possible mechanisms of neural injury caused by glutamate and its receptors. In: Neurochemical Aspects of Excitotoxicity. Springer, NY.
78. Mansouri A, Demeilliers C, Amsellem S, Pessayre D, Fromenty B (2001) Acute ethanol administration oxidatively damages and depletes mitochondrial DNA in mouse liver, brain, heart, and skeletal muscles: protective effects of antioxidants. J. Pharmacol. Exp. Ther. 298: 737-743.
79. Jou MJ (2011) Melatonin preserves the transient mitochondrial permeability transition for protection during mitochondrial Ca(2+) stress in astrocyte. J. Pineal. Res. 50 (4): 427-435. doi: 10.1111/j.1600-079X.2011.00861.
80. Tiong YL, Ng KY, Koh RY, Ponnudurai G, Chye SM (2019). Melatonin prevents oxidative stress-induced mitochondrial dysfunction and apoptosis in high glucose-treated schwann cells via upregulation of Bcl2, NF-κB, mTOR, Wnt signalling pathways. Antioxidants (Basel) 8 (7): 198. doi:10.3390/antiox8070198.
81. Perdomo J, Cabrera J, Estévez F, Loro J, Reiter RJ, Quintana J (2013) Melatonin induces apoptosis through a caspase-dependent but reactive oxygen species-independent mechanism in human leukemia Molt-3 cells. J. Pineal Res. 55 (2): 195-206. doi: 10.1111/jpi.12062.
82. Kakkar P, Singh BK (2007) Mitochondria: A hub of redox activities and cellular distress control. Mol. Cell Biochem. 305 (1-2): 235-253. doi: 10.1007/s11010-007-9520-8.
83. Baghcheghi Y, Salmani H, Beheshti F, Hosseini M (2017) Contribution of brain tissue oxidative damage in hypothyroidism-associated learning and memory impairments. Adv. Biomed. Res. 6: 59.doi:10.4103/2277-9175.206699.
84. Habib S, Ali A (2011) Biochemistry of nitric oxide. Ind. J. Clin. Biochem. 26 (1): 3-17.
85. Turrens JF (2003). Mitochondrial formation of reactive oxygen species. J. Physiol. 552 (2): 335-344.
86. Pacher P, Beckman JS, Liaudet L (2007) Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 87 (1): 315–424. doi:10.1152/physrev.00029.2006.
87. Guo C, Sun L, Chen X, Zhang D (2013) Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen. Res. 8 (21): 2003–2014. doi:10.3969/j.issn.1673-5374.2013.21.009.
88. Nita M, Grzybowski A (2016) The Role of the reactive oxygen species and oxidative stress in the patho mechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxid. Med. Cell. Longev. 2016: 3164734. doi:10.1155/2016/3164734.
89. Panel M, Ghaleh B, Morin D (2018) Mitochondria and aging: A role for the mitochondrial transition pore? Aging Cell 17 (4): e12793. doi:10.1111/acel.12793.
90. Šileikytė J, Forte M (2019) The Mitochondrial Permeability Transition in Mitochondrial Disorders. Oxid. Med. Cell. Longev. 2019: 3403075. doi:10.1155/2019/3403075.
91. Görlach A, Bertram K, Hudecova S, Krizanova O (2015) Calcium and ROS: A mutual interplay. Redox Biol. 6: 260–271. doi:10.1016/j.redox.2015.08.010.
92. Fischer TW, Zmijewski MA, Wortsman J, Slominski A (2007) Melatonin maintains mitochondrial membrane potential and attenuates activation of initiator (casp-9) and effector caspases (casp-3/casp-7) and PARP in UVR-exposed HaCaT keratinocytes. J. Pineal Res. 44 (4): 397-407.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


