Specificity and nanomolar potency of melatonin on G-protein coupled melatonin MT1 and MT2 receptors expressed in HEK-293T human embryo kidney cells
The potency of melatonin on MT1 and MT2 receptors
Abstract
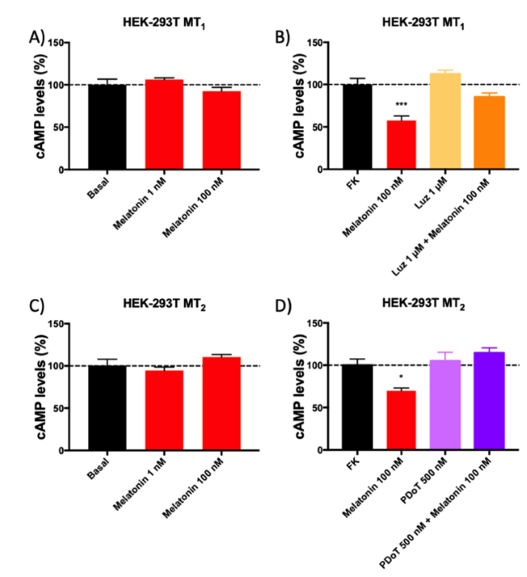
This is a pre-registered study, i.e. a study whose hypotheses and experiments designed to address these hypotheses have been deposited in a database before starting the experiments. The study aims at assessing the Gs versus Gi coupling and the potency of melatonin in the human version of melatonin MT1 and MT2 G-protein-coupled receptors expressed in HEK-293T cells. The results show that these receptors are Gi but not Gs coupled. By using a standard procedure of modulation of 0.5 µM forskolin-induced cAMP levels, it was found that the potency on MT2 receptor-mediated actions is in the low nanomolar range, but the potency on MT1 receptor is in the high nanomolar range. The potency of melatonin to stimulate the MT2 receptor is similar to that of a selective agonist, N-[2-(2-methoxy-6H-isoindolo[2,1-a]indol-11-yl)ethyl]butanamide (IIK7). Overall, the data on the potency of melatonin on its receptors will provide a new look for melatonin research. It is important to consider this finding for appropriately addressing physiological or therapeutic effects based on melatonin potency. Thus, the low doses of melatonin used in the existing prolonged release preparations or in other supplements should be revisited.
References
2. Lerner AB, Case JD, Takahashi (1960) Isolation of melatonin and 5-methoxyindole-3-acetic acid from bovine pineal glands. J. Biol. Chem. 235: 1992–1997.
3. Emet, M, Ozcan, H, Ozel L, Yayla M, Halici Z. Hacimuftuoglu A (2016) A review of melatonin, its receptors and drugs. Euras. J. Med. 48: 135–141.
4. Crooke A, Huete-Toral F, Colligris B, Pintor J (2017) The role and therapeutic potential of melatonin in age-related ocular diseases. J. Pineal Res. 63: e12430.
5. Cecon E, Oishi A, Jockers R (2017) Melatonin receptors: molecular pharmacology and signalling in the context of system bias. Br. J. Pharmacol.175: 3263–3280.
6. Carracedo G, Carpena C, Concepción P, Díaz V, García-García M, Jemni N, et al. (2017) Presence of melatonin in human tears. J. Optometry 10: 3–4.
7. Zaccara G, Schmidt D (2018) Antiepileptic drugs in clinical development: Differentiate or Die? Curr. Pharm. Des. 23: 5593–5605.
8. Wade AG, Ford I, Crawford G, McConnachie A, Nir T, Laudon M, et al. (2010) Nightly treatment of primary insomnia with prolonged release melatonin for 6 months: a randomized placebo controlled trial on age and endogenous melatonin as predictors of efficacy and safety. BMC Med. 8: 51.
9. Gringras P, Nir T, Breddy J, Frydman-Marom A, Findling RL (2017) Efficacy and safety of pediatric prolonged-release melatonin for insomnia in children with autism spectrum disorder. J. Am. Acad. Child. Adol. Psych. 56: 948–957.
10. Alston M, Cain SW, Rajaratnam SMW (2019) Advances of melatonin-based therapies in the treatment of disturbed sleep and mood. Handbook Exp. Pharmacol. 253: 305-319.
11. Low TL, Choo FN, Tan SM (2020) The efficacy of melatonin and melatonin agonists in insomnia – An umbrella review. J. Psychiatr. Res. 121: 10–23.
12. Atkin T, Comai S, Gobbi G (2018) Drugs for insomnia beyond benzodiazepines: Pharmacology, clinical applications, and discovery. Pharmacol. Rev. 70: 197–245.
13. Alexander SP, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA et al. (2017) The concise guide to Pharmacology 2017/18: G protein-coupled receptors. Br. J. Pharmacol. 174: S17–S129.
14. Huete-Toral F, Crooke A, Martínez-Águila A, Pintor J, Martinez-Aguila A, Pintor J. et al. (2015) Melatonin receptors trigger cAMP production and inhibit chloride movements in nonpigmented ciliary epithelial cells. J. Pharmacol. Exxp. Ther. 352: 119–128.
15. Vanecek J. (1998) Cellular mechanisms of melatonin action. Physiol. Rev. 78: 687–721.
16. Brydon L, Roka F, Petit L, De Coppet P, Tissot M, Barrett P. et al. (1999) Dual signaling of human Mel1a melatonin receptors via G(i2), G(i3), and G(q/11) proteins. Mol. Endocrinol. 13: 2025–2038.
17. Dubocovich ML (1995) Melatonin receptors: Are there multiple subtypes? Trends Pharmacol. Sci. 16: 50–56.
18. Blask DE, Hill SM, Dauchy RT, Xiang S, Yuan L, Duplessis T, et al. (2011) Circadian regulation of molecular, dietary, and metabolic signaling mechanisms of human breast cancer growth by the nocturnal melatonin signal and the consequences of its disruption by light at night. J. Pineal Res. 51: 259–269.
19. Sugden D, Davidson K, Hough KA, Teh MT (2004) Melatonin, melatonin receptors and melanophores: a moving story. Pigment Cell Res. 17: 454–460.
20. Hardeland R (2009) Melatonin: Signaling mechanisms of a pleiotropic agent. BioFactors. 35: 183–192.
21. Pintor J, Martin L, Pelaez T, Hoyle CHV, Peral A (2001) Involvement of melatonin MT 3 receptors in the regulation of intraocular pressure in rabbits. Eur. J. Pharmacol. 416: 251–254.
22. Mailliet F, Ferry G, Vella F, Thiam K, Delagrange P, Boutin JA (2004) Organs from mice deleted for NRH: quinone oxidoreductase 2 are deprived of the melatonin binding site MT3. FEBS Lett. 578: 116–120.
23. Lavoie J, Rosolen SG, Chalier C, Hebert M (2013) Negative impact of melatonin ingestion on the photopic electroretinogram of dogs. Neurosci. Lett. 543: 78–83.
24. de Sampaio F, Mesquita FP, de Sousa PR, Silva JL, Alves CN (2014) The melatonin analog 5-MCA-NAT increases endogenous dopamine levels by binding NRH:quinone reductase enzyme in the developing chick retina. Int. J. Dev. Neurosci. 38: 119–126.
25. Wiechmann AF, Sherry DM (2013) Role of melatonin and its receptors in the vertebrate retina. Int. Rev. Cell. Mol. Biol. 300: 211–242.
26. Duncan MJ, Takahashi JS, Dubocovich ML (1986) Characterization of 2-[125I]iodomelatonin binding sites in hamster brain. Eur. J. Pharmacol. 132: 333–334.
27. Duncan MJ, Takahashi JS, Dubocovich ML (1988) 2-[125I]Iodomelatonin binding sites in hamster brain membranes: pharmacological characteristics and regional distribution. Endocrinol. 122: 1825–1833.
28. Duncan MJ, Takahashi JS, Dubocovich ML (1989) characteristics and autoradiographic localization of 2-[125I]Iodomelatonin binding sites in Djungarian hamster brain. Endocrinol. 125: 1011–1018.
29. MacKenzie RS, Melan MA, Passey DK, Witt-Enderby PA (2002) Dual coupling of MT(1) and MT(2) melatonin receptors to cyclic AMP and phosphoinositide signal transduction cascades and their regulation following melatonin exposure. Biochem. Pharmacol. 63: 587-595.
30. Ahmed R, Mahavadi S, Al-Shboul O, Bhattacharya S, Grider JR, Murthy KS (2013) Characterization of signaling pathways coupled to melatonin receptors in gastrointestinal smooth muscle. Regul. Peptides, 184: 96–103.
31. Alkozi HA, Navarro G, Aguinaga D, Reyes-Resina I, Sanchez-Naves J, Pérez de Lara, M.J. et al. (2019) Adrenergic-melatonin heteroreceptor complexes are key in controlling ion homeostasis and intraocular eye pressure and their disruption contributes to hypertensive glaucoma. BioRxiv, Cold Spring Harbor Laboratory. 636688. https://doi.org/10.1101/636688.
32. Foster ED, and Deardorff A (2017) Open Science Framework (OSF). J. Medic. Library Assoc. University Library System, University of Pittsburgh. 105.
33. Franco R, Navarro G, Rivas-Santisteban R, Awad Alkozi H (2019) Potency of melatonin at G-protein-coupled MT1 and MT2 receptors. Available at: OsfIo/W7qxh.
34. Graham FL, Smiley J, Russell WC, Nairn R (1977) Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 36: 59–72.
35. Dyson MR (2016) Fundamentals of expression in mammalian cells. Adv. Exp. Med. Biol. 896: 217-224.
36. Nettleship JE, Watson PJ, Rahman-Huq N, Fairall L, Posner MG, Upadhyay A, et al. (2015) Transient expression in HEK 293 cells: an alternative to E. coli for the production of secreted and intracellular mammalian proteins. Methods Mol. Biol. 1258: 209-222
37. Hu J, Han J, Li H, Zhang X, Liu LL, Chen F, et al. (2018) Human embryonic kidney 293 cells: a vehicle for biopharmaceutical manufacturing, structural biology, and electrophysiology. Cells Tissues Organs. S. Karger AG. p. 1–8.
38. Hinz S, Navarro G, Borroto-Escuela D, Seibt BF, Ammon C, de Filippo E, et al. (2018) Adenosine A2A receptor ligand recognition and signaling is blocked by A2B receptors. Oncotarget 9: 13593–13611.
39. Navarro G, Borroto-Escuela D, Angelats E, Etayo I, Reyes-Resina I, Pulido-Salgado M, et al. (2018) Receptor-heteromer mediated regulation of endocannabinoid signaling in activated microglia. Role of CB1 and CB2 receptors and relevance for Alzheimer’s disease and levodopa-induced dyskinesia. Brain, Behav. Immun. 67: 139–151.
40. Reyes-Resina I, Navarro G, Aguinaga D, Canela EI, Schoeder CT, Załuski M, et al. (2018) Molecular and functional interaction between GPR18 and cannabinoid CB2G-protein-coupled receptors. Relevance in neurodegenerative diseases. Biochem. Pharmacol. 157: 169-179.
41. Liu J, Clough SJ, Hutchinson AJ, Adamah-Biassi EB, Popovska-Gorevski M, Dubocovich ML (2016) MT 1 and MT 2 Melatonin Receptors: A Therapeutic Perspective . Ann. Rev. Pharmacol. Toxicol. 56: 361–383.
42. Shiu SY, Pang B, Tam CW, Yao KM (2010) Signal transduction of receptor-mediated antiproliferative action of melatonin on human prostate epithelial cells involves dual activation of Galpha(s) and Galpha(q) proteins. J. Pineal. Res. 49: 301-311.
43. Lai FP, Mody SM, Yung LY, Kam JY, Pang CS, Pang SF, Wong YH (2002) Molecular determinants for the differential coupling of Galpha(16) to the melatonin MT1, MT2 and Xenopus Mel1c receptors. J. Neurochem. 80: 736-745.
44. Mody SM, Ho MK, Joshi SA, Wong YH, (2000) Incorporation of Galpha(z)-specific sequence at the carboxyl terminus increases the promiscuity of galpha(16) toward G(i)-coupled receptors. Mol. Pharmacol. 57: 13-23.
45. Conway S, Drew JE, Canning SJ, Barrett P, Jockers R, Strosberg AD, et al. (1997) Identification of Mel1a melatonin receptors in the human embryonic kidney cell line HEK293: evidence of G protein-coupled melatonin receptors which do not mediate the inhibition of stimulated cyclic AMP levels. FEBS Lett. 407: 121–126.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


