Melatonin modifies tumor hypoxia and metabolism by inhibiting HIF-1α and energy metabolic pathway in the in vitro and in vivo models of breast cancer
Melatonin and tumor hypoxia in breast cancer
Abstract
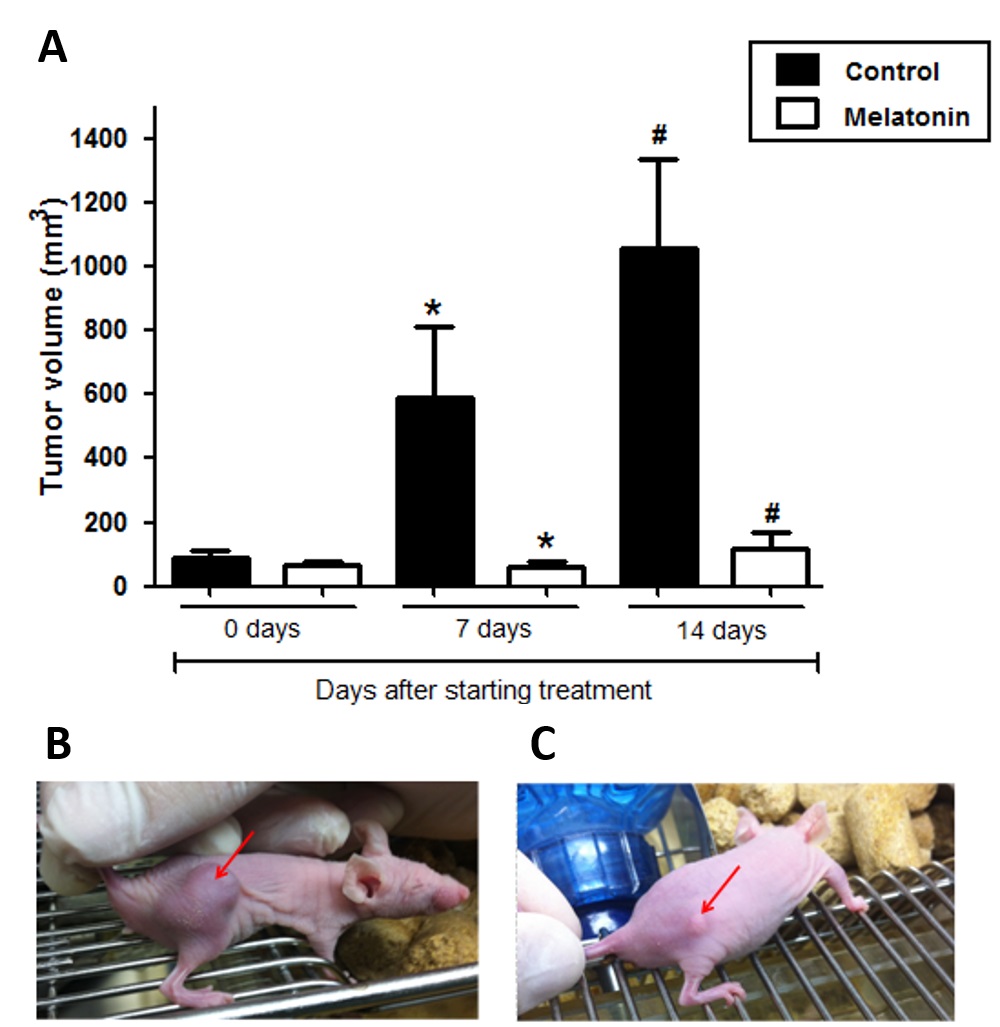
Breast cancer is the most common cancer among women and has a high mortality rate. Adverse conditions in the tumor microenvironment, such as hypoxia and acidosis, may exert selective pressure on the tumor, selecting subpopulations of tumor cells with advantages for survival in this environment. In this context, therapeutic agents that can modify these conditions, and consequently the intratumoral heterogeneity need to be explored. Melatonin, in addition to its physiological effects, exhibits important anti-tumor actions which may associate with modification of hypoxia and Warburg effect. In this study, we have evaluated the action of melatonin on tumor growth and tumor metabolism by different markers of hypoxia and glucose metabolism (HIF-1α, glucose transporters GLUT1 and GLUT3 and carbonic anhydrases CA-IX and CA-XII) in triple negative breast cancer model. In an in vitro study, gene and protein expressions of these markers were evaluated by quantitative real-time PCR and immunocytochemistry, respectively. The effects of melatonin were also tested in a MDA-MB-231 xenograft animal model. Results showed that melatonin treatment reduced the viability of MDA-MB-231 cells and tumor growth in Balb/c nude mice (p <0.05). The treatment significantly decreased HIF-1α gene and protein expression concomitantly with the expression of GLUT1, GLUT3, CA-IX and CA-XII (p <0.05). These results strongly suggest that melatonin down-regulates HIF-1α expression and regulates glucose metabolism in breast tumor cells, therefore, controlling hypoxia and tumor progression.
References
2. Flamant L, Notte A, Ninane N, Raes M, Michiels C (2010) Anti-apoptotic role of HIF-1 and AP-1 in paclitaxel exposed breast cancer cells under hypoxia. Mol. Cancer 9: 19-28.
3. Guerrab A El, et al. (2017) Quantification of hypoxia-related gene expression as a potential approach for clinical outcome prediction in breast cancer. PLoS One 12: e0175960.
4. Carmeliet P, Jain R K (2011), Molecular mechanisms and clinical applications of angiogenesis. Nature 473: 298–307.
5. Vordermark D (2010) Hypoxia-specific targets in cancer therapy: Role of splice variants. BMC Med. 8: 45.
6. Gilkes D (2016), Implications of Hypoxia in Breast Cancer Metastasis to Bone. Int. J. Mol. Sci. 17: pii: E1669.
7. Manoochehri Khoshinani H, Afshar S, Najafi R (2016), Hypoxia: A Double-Edged Sword in Cancer Therapy. Cancer Invest. 34: 536–545.
8. Chen C, Lou T (2017) Hypoxia inducible factors in hepatocellular carcinoma. Oncotarget 8: 46691–46703.
9. Karakashev S V, Reginato M J (2015) Progress toward overcoming hypoxia-induced resistance to solid tumor therapy. Cancer Manag. Res. 7: 253–64.
10. Hu Z, Sun R, Curtis C (2017) A population genetics perspective on the determinants of intra-tumor heterogeneity. Biochim. Biophys. Acta - Rev. Cancer 1867: 109–126.
11. Semenza G L (2019) Pharmacologic Targeting of Hypoxia-Inducible Factors. Annu. Rev. Pharmacol. Toxicol. 59, 379–403 (2019).
12. Ke Q, Costa M (2006) Hypoxia-Inducible Factor-1 (HIF-1). Mol. Pharmacol. 70: 1469–1480.
13. Soni S, Padwad Y S (2017) HIF-1 in cancer therapy: two decade long story of a transcription factor. Acta Oncol. (Madr). 56: 503–515.
14. Gabellieri C, Eykyn T R, Leach M O (2007) Conformational exchange in pimonidazole - a hypoxia marker. Magn. Reson. Chem. 45: 621–623.
15. Ow C P C, Ullah M M, Ngo J P, Sayakkarage A, Evans R G (2019) Detection of cellular hypoxia by pimonidazole adduct immunohistochemistry in kidney disease: methodological pitfalls and their solution. Am. J. Physiol. Physiol. 317: F322–F332.
16. Busk M, et al. (2013) PET imaging of tumor hypoxia using 18 F-labeled pimonidazole. Acta Oncol. (Madr). 52: 1300–1307.
17. Mascini N E, et al. (2016) Mass Spectrometry Imaging of the Hypoxia Marker Pimonidazole in a Breast Tumor Model. Anal. Chem. 88: 3107–3114.
18. Lu Z L et al. (2019) Construction of a GLUT-1 and HIF-1α gene knockout cell model in HEp-2 cells using the CRISPR/Cas9 technique. Cancer Manag. Res. 11: 2087–2096.
19. Pan H, Xia X, Pan H (2012) Active autophagy in the tumor microenvironment: A novel mechanism for cancer metastasis (Review). Oncol. Lett. 5: 411–416.
20. Shi Y, Liu S, Ahmad S, Gao Q (2018) Targeting Key Transporters in Tumor Glycolysis as a Novel Anticancer Strategy. Curr. Top. Med. Chem. 18: 454–466.
21. Chen X, et al. (2017) Predictive value of glucose transporter-1 and glucose transporter-3 for survival of cancer patients: A meta-analysis. Oncotarget 8: 13206–13213.
22. Park H S, et al. (2016) Hypoxia induces glucose uptake and metabolism of adipose-derived stem cells. Mol. Med. Rep. 14: 4706–4714.
23. Pinheiro C, et al. (2011) GLUT1 and CAIX expression profiles in breast cancer correlate with adverse prognostic factors and MCT1 overexpression. Histol. Histopathol. 26: 1279–1286.
24. Ancey P B, Contat C, Meylan E (2018) Glucose transporters in cancer – from tumor cells to the tumor microenvironment. FEBS J. 285: 2926–2943.
25. Madunić I V, Madunić J, Breljak D, Karaica D, Sabolić I (2018) Sodium-glucose cotransporters: New targets of cancer therapy? Arh. Hig. Rada Toksikol. 69: 278–285.
26 Schawartz L, Supuran C T, Alfrouk K O (2017) The Warburg Effect and the hallmarks of Cancer. Anticancer Agents Med. Chem.17: 164-170.
27. Paškevičiūtė M, Petrikaitė V (2019) Overcoming transporter-mediated multidrug resistance in cancer: failures and achievements of the last decades. Drug Deliv. Transl. Res. 9: 379–393.
28. Chiche J, et al. (2009) Hypoxia-Inducible Carbonic Anhydrase IX and XII Promote Tumor Cell Growth by Counteracting Acidosis through the Regulation of the Intracellular pH. Cancer Res. 69: 358–368.
29. Mboge M Y, et al. (2019) A non-catalytic function of carbonic anhydrase IX contributes to the glycolytic phenotype and pH regulation in human breast cancer cells. Biochem. J. 476: 1497–1513.
30. Qian J, Rankin E B (2019) Hypoxia-induced phenotypes that mediate tumor heterogeneity. Adv. Med. Biology, 1135, 43-55.
31. Reiter R J, et al. (2017) Melatonin, a full service anti-cancer agent: Inhibition of initiation, progression and metastasis. Int. J. Mol. Sci. 18: pii: E843.
32. S. Bhattacharya S,, Patel K K, Dehari D, Agrawal A K, Singh S (2019) Melatonin and its ubiquitous anticancer effects. Mol. Cell. Biochem. https:/doi.org/10.1007/s11010-019-03617-5.
33. Jardim-Perassi B V, et al. (2019) RNA-Seq transcriptome analysis shows anti-tumor actions of melatonin in a breast cancer xenograft model. Sci. Rep. 9: 966.
34. Proietti S, Cucina A, Minini M, Bizzarri M (2017) Melatonin, mitochondria, and the cancer cell. Cell. Mol. Life Sci. 74: 4015–4025.
35. Moradkhani F (2019) Immunoregulatory role of melatonin in cancer. J. Cell. Physiol https:/doi.org/10.1002/jcp.29036.
36. Colombo J, Maciel J M W, Ferreira L C, da Silva R F, Zuccari D A P de C (2016) Effects of melatonin on HIF-1α and VEGF expression and on the invasive properties of hepatocarcinoma cells. Oncol. Lett. 12: 231–237.
37. Al-Rasheed N M, et al. (2017) Original research paper. Pulmonary prophylactic impact of melatonin and/or quercetin: A novel therapy for inflammatory hypoxic stress in rats. Acta Pharm. 67: 125-135.
38. Sanchez-Sanchez A M, et al.(2015) Melatonin cytotoxicity is associated to Warburg effect inhibition in Ewing sarcoma cells. PLoS One 10: e0135420 .
39. Jardim-Perassi B V, et al. (2014) Effect of melatonin on tumor growth and angiogenesis in xenograft model of breast cancer. PLoS One 9: e85311.
40. Vriend J, Reiter R J (2016) Melatonin and the von Hippel-Lindau/HIF-1 oxygen sensing mechanism: A review. Biochim. Biophys. Acta 1865: 176-183.
41. Park J W, Hwang M S, Suh S I, Baek W K (2009) Melatonin down-regulates HIF-1 alpha expression through inhibition of protein translation in prostate cancer cells. J. Pineal Res. 46: 415–421.
42. Sohn E J, Won G, Lee J, Lee S, Kim S H (2015) Upregulation of miRNA3195 and miRNA374b Mediates the Anti-Angiogenic Properties of Melatonin in Hypoxic PC-3 Prostate Cancer Cells. J. Cancer 6: 19-28.
43. Sorace A G, et al. (2017) Quantitative [(18)F]FMISO PET Imaging Shows Reduction of Hypoxia Following Trastuzumab in a Murine Model of HER2+ Breast Cancer. Mol. Imaging Biol. 19: 130-137.
44. Liverani C et al. (2019) A biomimetic 3D model of hypoxia-driven cancer progression. Sci. Rep. 9: 12263.
45. Sanchez-Sanchez A M, et al. (2015) Melatonin Cytotoxicity is associated to Warburg Effect inhibition in Ewing Sarcoma cells. PLoS One 10: e0135420.
46. Park H S, et al. (2016) Hypoxia induces glucose uptake and metabolism of adipose-derived stem cells. Mol. Med. Rep. 14: 4706-4714.
47. Mayo J C, et al. (2018) Melatonin uptake by cells: An answer to its relationship with glucose? Molecules 23: pii: E1999 .
48. Hevia D, et al. (2015) Melatonin uptake through glucose transporters: a new target for melatonin inhibition of cancer. J. Pineal Res. 58, 234–250.
49. Kocdor, M A et al. (2013) Progressive increase of glucose transporter-3 (GLUT-3) expression in estrogen-induced breast carcinogenesis. Clin. Transl. Oncol. 15: 55-64.
50. Liu Y, et al. (2009) The expression and significance of HIF-1alpha and GLUT-3 in glioma. Brain Res. 1304, 149-54.
51. Schlößer H A, et al. (2017) Glucose transporters 1, 3, 6, and 10 are expressed in gastric cancer and glucose transporter 3 is associated with UICC stage and survival. Gastric Cancer 20: 83–91.
52. Chen X, et al. (2017) Predictive value of glucose transporter-1 and glucose transporter-3 for survival of cancer patients: A meta-analysis. Oncotarget 8: 13206-13213.
53. Labak C M, et al. (2016) Glucose transport: meeting the metabolic demands of cancer, and applications in glioblastoma treatment. Am. J. Cancer Res. 6: 1599-608 (2016).
54. Jamali S, et al. (2015) Hypoxia-induced carbonic anhydrase IX facilitates lactate flux in human breast cancer cells by non-catalytic function. Sci. Rep. 5: 13605.
55. Ames S, Pastorekova S, Becker H M (2018) The proteoglycan-like domain of carbonic anhydrase IX mediates non-catalytic facilitation of lactate transport in cancer cells. Oncotarget 9: 27940-27957.
56. Parks S K, Cormerais Y, Durivault J, Pouyssegur J (2017) Genetic disruption of the pHi-regulating proteins Na+/H+ exchanger 1 (SLC9A1) and carbonic anhydrase 9 severely reduces growth of colon cancer cells. Oncotarget 8: 10225-10237.
57. Sowa T, et al. (2017) Hypoxia-inducible factor 1 promotes chemoresistance of lung cancer by inducing carbonic anhydrase IX expression. Cancer Med. 6: 288-297.
58. Crooke A, Huete-Toral F, Martínez-Águila A, Martín-Gil A, Pintor J (2012) Involvement of carbonic anhydrases in the ocular hypotensive effect of melatonin analogue 5-MCA-NAT. J. Pineal Res. 52: 265-70.
59. Siddiqui M H, et al. (2019) Exogenous melatonin counteracts NaCl-induced damage by regulating the antioxidant system, proline and carbohydrates metabolism in tomato seedlings. Int. J. Mol. Sci. 20: pii: E353
60. Tafreshi NK, et al. (2016) Evaluation of CAIX and CAXII Expression in Breast Cancer at Varied O2 Levels: CAIX is the Superior Surrogate Imaging Biomarker of Tumor Hypoxia. Mol. Imaging Biol. 18: 219-31.
61. Meehan J, et al. (2017) Inhibition of pH regulation as a therapeutic strategy in hypoxic human breast cancer cells. Oncotarget 8: 42857-42875.
62. Chu C Y, et al. (2016) CA IX is upregulated in CoCl2-induced hypoxia and associated with cell invasive potential and a poor prognosis of breast cancer. Int. J. Oncol. 48: 271-80.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


