Impact of melatonin effects on toxicology of vesicant chemical warfare agents: When science meets reality
Melatonin treatment for vesicants-induced damage
Abstract
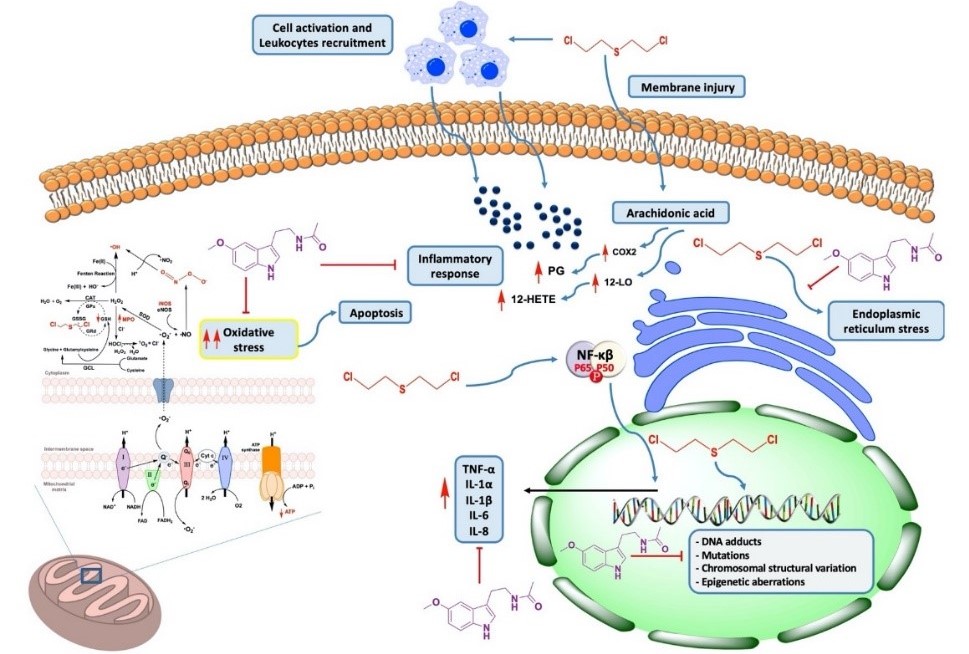
In this review we focused our attention on sulfur mustard [bis(2-chloroethyl) sulphide], the main vesicant chemical warfare agent (CWA), which has been widely used in different military conflicts, including World War I and the Iran-Iraq war. Moreover, the evolution of the recent Iraq and Syria conflicts suggests that terrorist groups are aware of the significant psychological and media effects that would be produced by the mere attempt to use CWAs. Sulfur mustard can produce the alkylation of macromolecules bearing sulfhydryl groups, such as DNA and proteins. This vesicant can also generate free radicals which can develop toxicity in the areas exposed, such as the eyes, skin, respiratory tract (inhalation) and gastrointestinal tract (ingestion). In this respect, we advance and propose three salvage mechanisms through which a broad-spectrum multipotent molecule, melatonin, could facilitate neutralization of the toxic damage induced by sulfur mustard radical scavenging. We also speculate that the long-term effects of varying severity can appear after acute poisoning. Besides, melatonin-based therapy strategies can modulate of epigenetic mechanisms and become very suitable for the clinical treatment of victimized patients. However, the utilization of melatonin as a “therapeutic bullet” addressed to counteract the vesicant CWAs needs much additional in vitro research as well as systematic animal studies and controlled translational trials.
References
2. López-Muñoz F, Alamo C, Guerra JA, et al. (2008) The development of neurotoxic agents as chemical weapons during the National Socialist period in Germany. Rev. Neurol. 47: 99-106.
3. Goldman M, Dacre JC (1989) Lewisite: its chemistry, toxicology, and biological effects. Rev Environ. Contam. Toxicol. 110: 75-115.
4. Smith M, Stone R, Guo R, et al. (2008) Vesicants and oxidative stress. Chemical warfare agents: chemistry, pharmacology, toxicology and therapeutics, eds Romano J Lukey B, Salem H (CRC Press, Boca Ratón), pp 247-292.
5. Newman-Taylor AJ, Morris AJ (1991) Experience with mustard gas casualties. Lancet 337: 242.
6. Requena L, Requena C, Sanchez M, et al. (1988) Chemical warfare. Cutaneous lesions from mustard gas. J. Am. Acad. Dermatol. 19: 529-536.
7. Gordon MK, DeSantis-Rodrigues A, Hahn R, et al. (2016) The molecules in the corneal basement membrane zone affected by mustard exposure suggest potential therapies. Ann. N. Y. Acad. Sci. 1378: 158-165.
8. Sunil VR, Patel KJ, Shen J, et al. (2011) Functional and inflammatory alterations in the lung following exposure of rats to nitrogen mustard. Toxicol. Appl. Pharmacol. 250: 10-18.
9. Sunil VR, Patel-Vayas K, Shen J, et al. (2011) Role of TNFR1 in lung injury and altered lung function induced by the model sulfur mustard vesicant, 2-chloroethyl ethyl sulfide. Toxicol. Appl. Pharmacol. 250: 245-255.
10. Khateri S, Ghanei M, Keshavarz S, et al. (2003) Incidence of lung, eye, and skin lesions as late complications in 34,000 Iranians with wartime exposure to mustard agent. J. Occup. Environ. Med. 45: 1136-1143.
11. Balali-Mood M, Hefazi M (2005) The pharmacology, toxicology, and medical treatment of sulphur mustard poisoning. Fundam. Clin. Pharmacol. 19: 297-315.
12. Pita R, Vidal-Asensi S (2010) Cutaneous and systemic toxicology of vesicants used in warfare. Actas Dermosifiliogr. 101: 7-18.
13. Somani S (1992) Toxicokinetics and toxicodynamics of mustard. Chemical warfare agents, eds Somani S. (Academic Press, San Diego, CA,), pp 13-50.
14. Davis KG, Aspera G (2001) Exposure to liquid sulfur mustard. Ann. Emerg. Med. 3: 653-656.
15. Menendez-Pelaez A, Reiter RJ (1993) Distribution of melatonin in mammalian tissues: the relative importance of nuclear versus cytosolic localization. J. Pineal Res. 15: 59-69.
16. Costa EJ, Shida CS, Biaggi MH, et al. (1997) How melatonin interacts with lipid bilayers: a study by fluorescence and ESR spectroscopies. FEBS Lett. 416: 103-106.
17. Foley HM, Steel AE (2019) Adverse events associated with oral administration of melatonin: A critical systematic review of clinical evidence. Complement. Ther. Med. 42: 65-81.
18. Luchetti F, Canonico B, Betti M, et al. (2010) Melatonin signaling and cell protection function. FASEB J. 24: 3603-3624.
19. Pita R, Marco-Contelles J, Ramos E, et al. (2013) Toxicity induced by chemical warfare agents: insights on the protective role of melatonin. Chem. Biol. Interact. 206: 134-142.
20. Galano A, Tan DX, Reiter RJ (2018) Melatonin: A versatile protector against oxidative DNA damage. Molecules 23: 530
21. Etemad L, Moshiri M, Balali-Mood M (2019) Advances in treatment of acute sulfur mustard poisoning - a critical review. Crit. Rev. Toxicol. 49: 191-214.
22. Borna H, Hosseini Qale Noe SH, Harchegani AB, et al. (2019) A review on proteomics analysis to reveal biological pathways and predictive proteins in sulfur mustard exposed patients: roles of inflammation and oxidative stress. Inhal. Toxicol. 31: 3-11.
23. Kehe K, Szinicz L (2005) Medical aspects of sulphur mustard poisoning. Toxicology 214: 198-209.
24. Gilman A, Philips FS (1946) The biological actions and therapeutic applications of the B-chloroethyl amines and sulfides. Science 103: 409-436.
25. Somani SM, Babu SR (1989) Toxicodynamics of sulfur mustard. Int. J. Clin. Pharmacol. Ther. Toxicol. 27: 419-435.
26. Orrenius S, McConkey DJ, Bellomo G, et al. (1989) Role of Ca2+ in toxic cell killing. Trends Pharmacol. Sci. 10: 281-285.
27. Miccadei S, Kyle ME, Gilfor D, et al. (1988) Toxic consequence of the abrupt depletion of glutathione in cultured rat hepatocytes. Arch. Biochem. Biophys. 265: 311-320.
28. Berger SJ, Sudar DC, Berger NA (1986) Metabolic consequences of DNA damage: DNA damage induces alterations in glucose metabolism by activation of poly (ADP-ribose) polymerase. Biochem. Biophys. Res. Commun. 134: 227-232.
29. Meier HL, Gross CL, Papirmeister B (1987) 2,2'-Dichlorodiethyl sulfide (sulfur mustard) decreases NAD+ levels in human leukocytes. Toxicol. Lett. 39: 109-122.
30. Papirmeister B, Gross CL, Meier HL, et al. (1985) Molecular basis for mustard-induced vesication. Fundam. Appl. Toxicol. 5: S134-149.
31. Ludlum DB, Austin-Ritchie P, Hagopian M, et al. (1994) Detection of sulfur mustard-induced DNA modifications. Chem. Biol. Interact. 91: 39-49.
32. Kilic E, Ortatatli M, Sezigen S, et al. (2018) Acute intensive care unit management of mustard gas victims: the Turkish experience. Cutan. Ocul. Toxicol. 37: 332-337.
33. Padley AP (2016) Gas: the greatest terror of the Great War. Anaesth. Intensive Care 44 Suppl: 24-30.
34. Sourdeval M, Lemaire C, Deniaud A, et al. (2006) Inhibition of caspase-dependent mitochondrial permeability transition protects airway epithelial cells against mustard-induced apoptosis. Apoptosis 11: 1545-1559.
35. Korkmaz A, Kunak ZI, Paredes SD, et al. (2008) The use of melatonin to combat mustard toxicity. REVIEW. Neuro. Endocrinol. Lett. 29: 614-619.
36. Pohanka M, Sobotka J, Jilkova M, et al. (2011) Oxidative stress after sulfur mustard intoxication and its reduction by melatonin: efficacy of antioxidant therapy during serious intoxication. Drug Chem. Toxicol. 34: 85-91.
37. McElroy CS, Day BJ (2016) Antioxidants as potential medical countermeasures for chemical warfare agents and toxic industrial chemicals. Biochem. Pharmacol. 100: 1-11.
38. Beigi Harchegani A, Khor A, Tahmasbpour E, et al. (2019) Role of oxidative stress and antioxidant therapy in acute and chronic phases of sulfur mustard injuries: a review. Cutan. Ocul. Toxicol. 38: 9-17.
39. Ucar M, Korkmaz A, Reiter RJ, et al. (2007) Melatonin alleviates lung damage induced by the chemical warfare agent nitrogen mustard. Toxicol. Lett. 173: 124-131.
40. Yaren H, Mollaoglu H, Kurt B, et al. (2007) Lung toxicity of nitrogen mustard may be mediated by nitric oxide and peroxynitrite in rats. Res. Vet. Sci. 83: 116-122.
41. Laskin JD, Black AT, Jan YH, et al. (2010) Oxidants and antioxidants in sulfur mustard-induced injury. Ann. N. Y. Acad. Sci. 1203: 92-100.
42. Kunak ZI, Macit E, Yaren H, et al. (2012) Protective effects of melatonin and S-methylisothiourea on mechlorethamine induced nephrotoxicity. J. Surg. Res. 175: e17-23.
43. Macit E, Yaren H, Aydin I, et al. (2013) The protective effect of melatonin and S-methylisothiourea treatments in nitrogen mustard induced lung toxicity in rats. Environ. Toxicol. Pharmacol. 36: 1283-1290.
44. Limson J, Nyokong T, Daya S (1998) The interaction of melatonin and its precursors with aluminium, cadmium, copper, iron, lead, and zinc: an adsorptive voltammetric study. J. Pineal Res. 24: 15-21.
45. Reiter RJ, Tan DX, Mayo JC, et al. (2003) Melatonin as an antioxidant: biochemical mechanisms and pathophysiological implications in humans. Acta Biochim. Pol. 50: 1129-1146.
46. Tarocco A, Caroccia N, Morciano G, et al. (2019) Melatonin as a master regulator of cell death and inflammation: molecular mechanisms and clinical implications for newborn care. Cell Death Dis. 10: 317.
47. Mouret S, Wartelle J, Batal M, et al. (2015) Time course of skin features and inflammatory biomarkers after liquid sulfur mustard exposure in SKH-1 hairless mice. Toxicol. Lett. 232: 68-78.
48. Carrascal L, Nunez-Abades P, Ayala A, et al. (2018) Role of melatonin in the inflammatory process and its therapeutic potential. Curr. Pharm. Des. 24: 1563-1588.
49. Markus RP, Fernandes PA, Kinker GS, et al. (2018) Immune-pineal axis - acute inflammatory responses coordinate melatonin synthesis by pinealocytes and phagocytes. Br. J. Pharmacol. 175: 3239-3250.
50. Jafari M (2007) Dose- and time-dependent effects of sulfur mustard on antioxidant system in liver and brain of rat. Toxicology 231: 30-39.
51. Naghii MR (2002) Sulfur mustard intoxication, oxidative stress, and antioxidants. Mil. Med. 167: 573-575.
52. Pant SC, Vijayaraghavan R, Kannan GM, et al. (2000) Sulphur mustard induced oxidative stress and its prevention by sodium 2,3-dimercapto propane sulphonic acid (DMPS) in mice. Biomed. Environ. Sci. 13: 225-232.
53. Beigi Harchegani A, Mirnam Niha M, Sohrabiyan M, et al. (2018) Cellular and molecular mechanisms of sulfur mustard toxicity on spermatozoa and male fertility. Toxicol. Res. (Camb) 7: 1029-1035.
54. Korkmaz A, Yaren H, Topal T, et al. (2006) Molecular targets against mustard toxicity: implication of cell surface receptors, peroxynitrite production, and PARP activation. Arch. Toxicol. 80: 662-670.
55. Pacher P, Beckman JS, Liaudet L (2007) Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 87: 315-424.
56. Ghabili K, Agutter PS, Ghanei M, et al. (2011) Sulfur mustard toxicity: history, chemistry, pharmacokinetics, and pharmacodynamics. Crit. Rev. Toxicol. 41: 384-403.
57. Szabo C (2003) Multiple pathways of peroxynitrite cytotoxicity. Toxicol. Lett. 140-141: 105-112.
58. Kehe K, Raithel K, Kreppel H, et al. (2008) Inhibition of poly(ADP-ribose) polymerase (PARP) influences the mode of sulfur mustard (SM)-induced cell death in HaCaT cells. Arch. Toxicol. 82: 461-470.
59. Korkmaz A, Kurt B, Yildirim I, et al. (2008) Effects of poly(ADP-ribose) polymerase inhibition in bladder damage caused by cyclophosphamide in rats. Exp. Biol Med. (Maywood) 233: 338-343.
60. Anderson G, Rodriguez M, Reiter RJ (2019) Multiple sclerosis: melatonin, orexin, and ceramide interact with platelet activation coagulation factors and gut-microbiome-derived butyrate in the circadian dysregulation of mitochondria in glia and immune cells. Int. J. Mol. Sci. 20: 5500.
61. Sinha Roy S, Mukherjee S, Kabir S, et al. (2005) Inhibition of cholinephosphotransferase activity in lung injury induced by 2-chloroethyl ethyl sulfide, a mustard analog. J. Biochem. Mol. Toxicol. 19: 289-297.
62. Anderson G, Maes M (2020) Gut dysbiosis dysregulates central and systemic homeostasis via suboptimal mitochondrial function: assessment, treatment and classification implications. Curr. Top. Med. Chem. Doi: 10.2174/1568026620666200131094445.
63. Jin CJ, Engstler AJ, Sellmann C, et al. (2016) Sodium butyrate protects mice from the development of the early signs of non-alcoholic fatty liver disease: role of melatonin and lipid peroxidation. Br. J. Nutr. 116: 1682-1693.
64. Pohanka M (2012) Antioxidants countermeasures against sulfur mustard. Mini. Rev. Med. Chem. 12: 742-748.
65. Pal A, Tewari-Singh N, Gu M, et al. (2009) Sulfur mustard analog induces oxidative stress and activates signaling cascades in the skin of SKH-1 hairless mice. Free Radic. Biol. Med. 47: 1640-1651.
66. Shohrati M, Ghanei M, Shamspour N, et al. (2008) Activity and function in lung injuries due to sulphur mustard. Biomarkers 13: 728-733.
67. Hosseini-khalili A, Haines DD, Modirian E, et al. (2009) Mustard gas exposure and carcinogenesis of lung. Mutat. Res. 678: 1-6.
68. Kumar O, Sugendran K, Vijayaraghavan R (2001) Protective effect of various antioxidants on the toxicity of sulphur mustard administered to mice by inhalation or percutaneous routes. Chem. Biol. Interact. 134: 1-12.
69. Ortiz GG, Benitez-King GA, Rosales-Corral SA, et al. (2008) Cellular and biochemical actions of melatonin which protect against free radicals: role in neurodegenerative disorders. Curr. Neuropharmacol. 6: 203-214.
70. Reiter RJ, Tan DX, Manchester LC, et al. (2003) Melatonin: detoxification of oxygen and nitrogen-based toxic reactants. Adv. Exp. Med. Biol. 527: 539-548.
71. Tan DX, Manchester LC, Terron MP, et al. (2007) One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 42: 28-42.
72. Mauriz JL, Collado PS, Veneroso C, et al. (2013) A review of the molecular aspects of melatonin's anti-inflammatory actions: recent insights and new perspectives. J. Pineal Res. 54: 1-14.
73. Maynard R (2007) Mustard gas. Chemical warfare agents: Toxicology and treatment, eds Marrs T, Maynard R, Sidell F (Wiley, New York), pp 375-407.
74. Malaviya R, Sunil VR, Cervelli J, et al. (2010) Inflammatory effects of inhaled sulfur mustard in rat lung. Toxicol. Appl. Pharmacol. 248: 89-99.
75. Chang YC, Wang JD, Svoboda KK, et al. (2013) Sulfur mustard induces an endoplasmic reticulum stress response in the mouse ear vesicant model. Toxicol. Appl. Pharmacol. 268: 178-187.
76. Fernandez A, Ordoñez R, Reiter RJ, et al. (2015) Melatonin and endoplasmic reticulum stress: relation to autophagy and apoptosis. J. Pineal Res. 59: 292-307.
77. Korkmaz A, Tan DX, Reiter RJ (2008) Acute and delayed sulfur mustard toxicity; novel mechanisms and future studies. Interdiscip. Toxicol. 1: 22-26.
78. Rodriguez C, Mayo JC, Sainz RM, et al. (2004) Regulation of antioxidant enzymes: a significant role for melatonin. J. Pineal Res. 36: 1-9.
79. Wolfe GA, Petteys SM, Phelps JF, et al. (2019) Sulfur mustard exposure: review of acute, subacute, and long-term effects and their management. J. Spec. Oper. Med. 19: 81-86.
80. Tahmasbpour E, Reza Emami S, Ghanei M, et al. (2015) Role of oxidative stress in sulfur mustard-induced pulmonary injury and antioxidant protection. Inhal. Toxicol. 27: 659-672.
81. Balali-Mood M, Hefazi M, Mahmoudi M, et al. (2005) Long-term complications of sulphur mustard poisoning in severely intoxicated Iranian veterans. Fundam. Clin. Pharmacol. 19: 713-721.
82. Balali-Mood M, Hefazi M (2006) Comparison of early and late toxic effects of sulfur mustard in Iranian veterans. Basic Clin. Pharmacol. Toxicol. 99: 273-282.
83. Shohrati M, Peyman M, Peyman A, et al. (2007) Cutaneous and ocular late complications of sulfur mustard in Iranian veterans. Cutan. Ocul. Toxicol. 26: 73-81.
84. Shahriary A, Ghanei M, Rahmani H (2017) The systemic nature of mustard lung: Comparison with COPD patients. Interdiscip. Toxicol. 10: 114-127.
85. Rowell M, Kehe K, Balszuweit F, et al. (2009) The chronic effects of sulfur mustard exposure. Toxicology 263: 9-11.
86. Korkmaz A, Yaren H, Kunak ZI, et al. (2008) Epigenetic perturbations in the pathogenesis of mustard toxicity; hypothesis and preliminary results. Interdiscip. Toxicol. 1: 236-241.
87. Mirsadraee M, Mozaffari A, Attaran D (2011) Prevalence of asthma in children of chemical warfare victims. Iran J. Pediatr. 21: 294-300.
88. Khan F, Niaz K, Ismail Hassan F, et al. (2017) An evidence-based review of the genotoxic and reproductive effects of sulfur mustard. Arch. Toxicol. 91: 1143-1156.
89. Feinberg AP (2013) The epigenetic basis of common human disease. Trans. Am. Clin. Climatol. Assoc. 124: 84-93.
90. Brookes E, Shi Y (2014) Diverse epigenetic mechanisms of human disease. Annu. Rev. Genet. 48: 237-268.
91. Yan H, Tian S, Slager SL, et al. (2016) Genome-wide epigenetic studies in human disease: a primer on -omic technologies. Am. J. Epidemiol. 183: 96-109.
92. Hodjat M, Rahmani S, Khan F, et al. (2017) Environmental toxicants, incidence of degenerative diseases, and therapies from the epigenetic point of view. Arch. Toxicol. 91: 2577-2597.
93. Imani S, Panahi Y, Salimian J, et al. (2015) Epigenetic: A missing paradigm in cellular and molecular pathways of sulfur mustard lung: a prospective and comparative study. Iran J. Basic Med. Sci. 18: 723-736.
94. Rahmani S, Abdollahi M (2017) Novel treatment opportunities for sulfur mustard-related cancers: genetic and epigenetic perspectives. Arch. Toxicol. 91: 3717-3735.
95. Panahi Y, Fattahi A, Zarei F, et al. (2018) Next-generation sequencing approaches for the study of genome and epigenome toxicity induced by sulfur mustard. Arch. Toxicol. 92: 3443-3457.
96. Yego EC, Dillman JF, 3rd (2013) Cytokine regulation by MAPK activated kinase 2 in keratinocytes exposed to sulfur mustard. Toxicol. In Vitro 27: 2067-2075.
97. Tahmasbpour E, Ghanei M, Qazvini A, et al. (2016) Gene expression profile of oxidative stress and antioxidant defense in lung tissue of patients exposed to sulfur mustard. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 800-801: 12-21.
98. Antequera F, Bird A (2018) CpG islands: A historical perspective. Methods Mol. Biol. 1766: 3-13.
99. Steinritz D, Schmidt A, Balszuweit F, et al. (2016) Epigenetic modulations in early endothelial cells and DNA hypermethylation in human skin after sulfur mustard exposure. Toxicol. Lett. 244: 95-102.
100. Simons T, Steinritz D, Bolck B, et al. (2018) Sulfur mustard-induced epigenetic modifications over time - a pilot study. Toxicol. Lett. 293: 45-50.
101. Dong X, Weng Z (2013) The correlation between histone modifications and gene expression. Epigenomics 5: 113-116.
102. Stillman B (2018) Histone modifications: Insights into their influence on gene expression. Cell 175: 6-9.
103. Venkatesh S, Workman JL (2015) Histone exchange, chromatin structure and the regulation of transcription. Nat. Rev. Mol. Cell. Biol. 16: 178-189.
104. Strakovsky RS, Pan YX (2012) In utero oxidative stress epigenetically programs antioxidant defense capacity and adulthood diseases. Antioxid. Redox Signal. 17: 237-253.
105. Alles J, Fehlmann T, Fischer U, et al. (2019) An estimate of the total number of true human miRNAs. Nucleic Acids Res. 47: 3353-3364.
106. O'Brien J, Hayder H, Zayed Y, et al. (2018) Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front. Endocrinol. (Lausanne) 9: 402.
107. Deppe J, Steinritz D, Santovito D, et al. (2016) Upregulation of miR-203 and miR-210 affect growth and differentiation of keratinocytes after exposure to sulfur mustard in normoxia and hypoxia. Toxicol. Lett. 244: 81-87.
108. Schmidt A, Steinritz D, Thiermann H, et al. (2016) Alteration of miRNA expression in early endothelial cells after exposure with sub-lethal sulfur mustard concentrations. Toxicol. Lett. 244: 88-94.
109. Schmidt A, Steinritz D, Thiermann H (2016) Development of the sulfur mustard resistant keratinocyte cell line HaCaT/SM. Toxicol. Lett. 244: 44-48.
110. Rothmiller S, Wolf M, Worek F, et al. (2018) Alteration of miRNA expression in a sulfur mustard resistant cell line. Toxicol. Lett. 293: 38-44.
111. Schmidt A, Wolf M, Rothmiller S, et al. (2018) Cytostatic resistance profile of the sulfur mustard resistant keratinocyte cell line HaCaT/SM. Toxicol. Lett. 293: 16-20.
112. Khafaei M, Samie S, Mowla SJ, et al. (2015) Evaluation of miR-9 and miR-143 expression in urine specimens of sulfur mustard exposed patients. Interdiscip. Toxicol. 8: 169-174.
113. Korkmaz A, Reiter RJ (2008) Epigenetic regulation: a new research area for melatonin? J. Pineal Res. 44: 41-44.
114. Sharma R, Ottenhof T, Rzeczkowska PA, et al. (2008) Epigenetic targets for melatonin: induction of histone H3 hyperacetylation and gene expression in C17.2 neural stem cells. J. Pineal Res. 45: 277-284.
115. Korkmaz A, (2009) Epigenetic actions of melatonin. J. Pineal Res. 46: 117-118.
116. Korkmaz A, Rosales-Corral S, Reiter RJ (2012) Gene regulation by melatonin linked to epigenetic phenomena. Gene 503: 1-11.
117. Hardeland R (2014) Melatonin, noncoding RNAs, messenger RNA stability and epigenetics--evidence, hints, gaps and perspectives. Int. J. Mol. Sci. 15: 18221-18252.
118. Tain YL, Huang LT, Hsu CN (2017) Developmental programming of adult disease: reprogramming by melatonin? Int. J. Mol. Sci. 18: 426.
119. Bahna SG, Niles LP (2017) Epigenetic induction of melatonin MT1 receptors by valproate: Neurotherapeutic implications. Eur. Neuropsychopharmacol. 27: 828-832.
120. Tain YL, Huang LT, Hsu CN, et al. (2014) Melatonin therapy prevents programmed hypertension and nitric oxide deficiency in offspring exposed to maternal caloric restriction. Oxid. Med. Cell. Longev. 2014: 283180.
121. Irmak MK, Topal T, Oter S (2005) Melatonin seems to be a mediator that transfers the environmental stimuli to oocytes for inheritance of adaptive changes through epigenetic inheritance system. Med. Hypotheses 64: 1138-1143.
122. Mousavi SS, Vahedi E, Shohrati M, et al. (2017) Nocturnal serum melatonin levels in sulfur mustard exposed patients with sleep disorders. J. R. Army Med. Corps. 163: 411-415.
123. Mousavi SS, Shohrati M, Vahedi E, et al. (2018) Effect of melatonin administration on sleep quality in sulfur mustard exposed patients with sleep disorders. Iran J. Pharm. Res. 17: 136-144.
124. Sulkava S, Ollila HM, Alasaari J, et al. (2017) Common genetic variation near melatonin receptor 1a gene linked to job-related exhaustion in shift workers. Sleep 40: 1-10.
125. Lahtinen A, Puttonen S, Vanttola P, et al. (2019) A distinctive DNA methylation pattern in insufficient sleep. Sci. Rep. 9: 1193.
126. Nourani MR, Mahmoodzadeh Hosseini H, Imani Fooladi AA (2015) Comparative transcriptional and translational analysis of heme oxygenase expression in response to sulfur mustard. J. Recept. Signal Transduct. Res. 35: 479-484.
127. Kassambara A, Klein B, Moreaux J (2009) MMSET is overexpressed in cancers: link with tumor aggressiveness. Biochem. Biophys. Res. Commun. 379: 840-845.
128. Guo M, Peng Y, Gao A, et al. (2019) Epigenetic heterogeneity in cancer. Biomark. Res. 7: 23.
129. Zhao Z, Shilatifard A (2019) Epigenetic modifications of histones in cancer. Genome Biol. 20: 245.
130. Ahuja N, Sharma AR, Baylin SB (2016) Epigenetic therapeutics: A new weapon in the war against cancer. Annu. Rev. Med. 67: 73-89.
131. Fardi M, Solali S, Farshdousti Hagh M (2018) Epigenetic mechanisms as a new approach in cancer treatment: An updated review. Genes Dis. 5: 304-311.
132. Roberti A, Valdes AF, Torrecillas R, et al. (2019) Epigenetics in cancer therapy and nanomedicine. Clin. Epigenetics 11: 81.
133. Haim A, Zubidat AE (2015) Artificial light at night: melatonin as a mediator between the environment and epigenome. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 370: 20140121
134. Schwimmer H, Metzer A, Pilosof Y, et al. (2014) Light at night and melatonin have opposite effects on breast cancer tumors in mice assessed by growth rates and global DNA methylation. Chronobiol. Int. 31: 144-150.
135. Lee SE, Kim SJ, Yoon HJ, et al. (2013) Genome-wide profiling in melatonin-exposed human breast cancer cell lines identifies differentially methylated genes involved in the anticancer effect of melatonin. J. Pineal Res. 54: 80-88.
136. Chappell G, Pogribny IP, Guyton KZ, et al. (2016) Epigenetic alterations induced by genotoxic occupational and environmental human chemical carcinogens: A systematic literature review. Mutat. Res. Rev. Mutat. Res. 768: 27-45.
137. Zubel T, Hochgesand S, John H, et al. (2019) A mass spectrometric platform for the quantitation of sulfur mustard-induced nucleic acid adducts as mechanistically relevant biomarkers of exposure. Arch. Toxicol. 93: 61-79.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


