The potential of melatonin in the prevention and attenuation of oxidative hemolysis and myocardial injury from cd147 SARS-CoV-2 spike protein receptor binding
Melatonin for hemolysis in COVID-19
Abstract
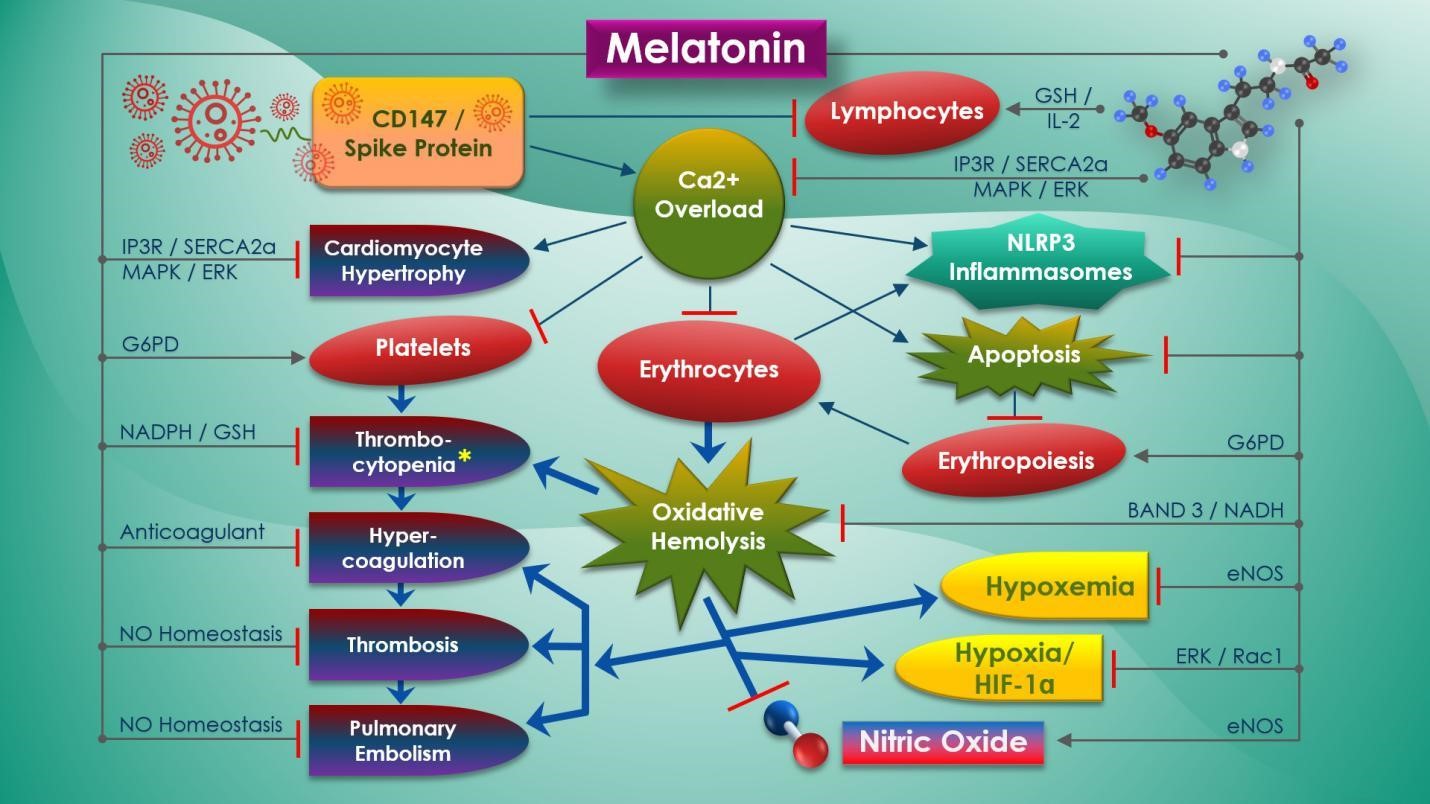
The rapid escalation of pandemic health threats associated with the novel, pathogenic SARS-CoV-2 coronavirus poses unprecedented challenges as proven effective vaccines and drugs have yet to be produced. Refractory hypoxemia and myocardial injury have been observed as two of the major causes of fatality in COVID-19 patients. SARS-CoV-2 spike (S) protein binding to broadly expressed CD147 receptors on erythrocytes causes oxidative hemolysis that may result in refractory hypoxemia and myocardial injury. Both of these life-threatening conditions are further exacerbated by imbalance in ACE2 from spike (S) protein receptor binding. Dysregulation in the CD147-cyclophilin A signaling pathway, together with altered calcium signaling from SARS-CoV-2 ion channel activities, may contribute to hypercoagulation, thrombosis, and cardiac remodeling resulting in heart failure. Melatonin is an ancient pleiotropic molecule with recognized antioxidant properties that is essential for the protection of erythrocytes from oxidative hemolysis. Found in erythrocytes, melatonin can reverse hemolytic anemia, normalize heme synthesis, restore lymphocytes and platelet counts, and reduce vessel permeability during an acute hemolytic crisis by maintaining intracellular calcium homeostasis and reduction of oxidative stress. In acute hypoxic conditions, melatonin is cardioprotective via blunting of cardiopulmonary response to hypoxia and suppressing hypoxia pathways. Melatonin normalizes endothelial-dependent nitric oxide production to prevent multiple organ damage from hypercoagulability, thrombosis, and hypertension associated with oxidative hemolysis and ACE2 deficiency, protecting cardiomyocytes from hypertrophy. This review discusses the full potential of melatonin as a safe and effective therapeutic intervention for the prevention and attenuation of hemoglobinopathies, refractory hypoxemia and myocardial injury during critical COVID-19 infections.
References
2. Ortega JT, Serrano ML, Pujol FH (2020) Role of changes in SARS-CoV-2 spike protein in the interaction with the human ACE2 receptor: An in silico analysis. EXCLI J. 19: 410–417. doi:10.17179/excli2020-1167.
3. Beniac DR, deVarennes SL, Andonov A, He R, Booth TF (2007) Conformational reorganization of the SARS coronavirus spike following receptor binding: implications for membrane fusion. PloS one 2 (10): e1082. https://doi.org/10.1371/journal.pone.0001082.
4. Hoffmann M, Kleine-Weber H, Schroeder S, et al. (2020) SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is blocked by a clinically proven protease inhibitor. Cell 181 (2): 271–280.e8. doi:10.1016/j.cell.2020.02.052.
5. Kuba K, Imai Y, Rao S, et al. (2005) A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 11 (8): 875–879. doi:10.1038/nm1267.
6. Zheng YY, Ma YT, Zhang JY, et al. (2020) COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 17 (5): 259–260. doi:10.1038/s41569-020-0360-5.
7. Guo J, Huang Z, Lin L, et al. (2020) Coronavirus Disease 2019 (COVID-19) and cardiovascular disease: A viewpoint on the potential influence of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers on onset and severity of severe acute respiratory syndrome coronavirus 2 infection. J. Am. Heart Assoc. 9 (7): e016219. doi:10.1161/JAHA.120.016219.
8. Patel VB, Zhong JC, Grant MB, Oudit GY (2016) Role of the ACE2/angiotensin 1-7 axis of the renin-angiotensin system in heart failure. Circ Res. 118 (8): 1313–1326. doi:10.1161/CIRCRESAHA.116.307708.
9. Clerkin KJ, Fried JA, Raikhelkar J, et al. (2020) Coronavirus disease 2019 (COVID-19) and cardiovascular disease. circulation. Circulation 141 (20):1 648‐1655. doi:10.1161/CIRCULATIONAHA.120.046941.
10. Chen J, Jiang Q, Xia X, et al. (2020) Individual variation of the SARS-CoV2 receptor ACE2 gene expression and regulation. Preprints 2020: 2020030191.
11. Jia HP, Look DC, Shi L, et al. (2005) ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J. Virol. 79 (23): 14614–14621. doi:10.1128/JVI.79.23.14614-14621.2005.
12. Leung JM, Yang CX, Tam A, et al. (2020) ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur. Respir. J. 55 (5): 2000688. doi:10.1183/13993003.00688-2020.
13. Gattinoni L, Coppola S, Cressoni M, et al. (2020) Covid-19 does not lead to a "typical" acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 10: 1164/rccm.202003-0817LE. doi:10.1164/rccm.202003-0817LE.
14. Cascella M, Rajnik M, Cuomo A, et al. (2020) Features, evaluation and treatment coronavirus (COVID-19). In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. Available from: https://www.ncbi.nlm.nih.gov/books/NBK554776/.
15. Arentz M, Yim E, Klaff L, et al. (2020) Characteristics and outcomes of 21 critically Ill patients with COVID-19 in Washington State. JAMA. e204326. doi:10.1001/jama.2020.4326.
16. Shi S, Qin M, Shen B, et al. (2020) Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020: e200950. doi:10.1001/jamacardio.2020.0950.
17. Guo T, Fan Y, Chen M, et al. (2019) Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020: e201017. doi:10.1001/jamacardio.2020.1017.
18. Liu W, Li H (2020) COVID-19: Attacks the 1-beta chain of hemoglobin and captures the porphyrin to inhibit human heme metabolism. ChemRxiv https://doi.org/10.26434/chemrxiv.11938173.v5.
19. Wang K, Chen Q, Zhou YS, et al. (2020) SARS-CoV-2 invades host cells via a novel route: CD147-spike protein. (preprint) bioRxiv 2020: 03.14.988345; doi: https://doi.org/10.1101/2020.03.14.988345.
20. Coste I, Gauchat JF, Wilson A, et al. (2001) Unavailability of CD147 leads to selective erythrocyte trapping in the spleen. Blood 97 (12): 3984–3988. doi:10.1182/blood.v97.12.3984.
21. Venkatesan B, Valente AJ, Prabhu SD, et al. (2010) EMMPRIN activates multiple transcription factors in cardiomyocytes, and induces interleukin-18 expression via Rac1-dependent PI3K/Akt/IKK/NF-kappaB andMKK7/JNK/AP-1 signaling. J. Mol. Cell Cardiol. 49 (4): 655–663. doi:10.1016/j.yjmcc.2010.05.007.
22. Bian H, Zheng ZH, Wei D, et al. (2020) Meplazumab treats COVID-19 pneumonia: an open-labelled, concurrent controlled add-on clinical trial. medRxiv 2020: 03.21.20040691; doi: https://doi.org/10.1101/2020.03.21.20040691.
23. Takashi Muramatsu (2016) Basigin (CD147), a multifunctional transmembrane glycoprotein with various binding partners. J. Biochem. 159 (5): 481–490. https://doi.org/10.1093/jb/mvv127.
24. Guindolet D, Gabison EE (2020) Role of CD147 (EMMPRIN/Basigin) in tissue remodeling. Anat. Rec. 303: 1584-1589. doi:10.1002/ar.24089.
25. Ge H, Yuan W, Liu J, et al. (2015) Functional relevance of protein glycosylation to the pro‐inflammatory effects of extracellular matrix metalloproteinase inducer (EMMPRIN) on monocytes/macrophages. PloS One 10: e0117463.
26. Pennings GJ, Kritharides L (2014) CD147 in cardiovascular disease and thrombosis. Semin. Thromb. Hemost. 40 (7): 747–755. doi:10.1055/s-0034-1390001.
27. Crosnier C, Bustamante L, Bartholdson S, et al. (2011) Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum. Nature 480: 534–537. doi: 10.1038/nature106.
28. Poole RC, Sansom CE, Halestrap AP (1996) Studies of the membrane topology of the rat erythrocyte H+/lactate cotransporter (MCT1). Biochem. J. 320: 817–824.
29. Wilson MC, Meredith D, Halestrap AP (2002) Fluorescence resonance energy transfer studies on the interaction between the lactate transporter MCT1 and CD147 provide information on the topology and stoichiometry of the complex in situ. J. Biol. Chem. 277 (5): 3666–3672. doi:10.1074/jbc.M109658200.
30. Sun S, Li H, Chen J, Qian Q (2017) Lactic acid: No longer an inert and end-product of glycolysis. Physiology (Bethesda) 32 (6): 453–463. doi:10.1152/physiol.00016.2017.
31. Tilton WM, Seaman C, Carriero D, Piomelli S (1991) Regulation of glycolysis in the erythrocyte: role of the lactate/pyruvate and NAD/NADH ratios. J. Lab. Clin. Med. 118 (2): 146–152.
32. Pivkin IV, Peng Z, Karniadakis GE, et al. (2017) Biomechanics of red blood cells in human spleen and consequences for physiology and disease. Proc. Natl. Acad. Sci.113 (28): 7804–7809. doi:10.1073/pnas.1606751113.
33. Li L, Duan M, Chen W, et al. (2017) The spleen in liver cirrhosis: revisiting an old enemy with novel targets. J. Transl. Med. 15 (1): 111. doi:10.1186/s12967-017-1214-8.
34. Li Y, Wu J, Xu L, et al. (2017) Regulation of leukocyte recruitment to the spleen and peritoneal cavity during pristane-induced inflammation. J. Immunol. Res. 2017: 9891348. doi:10.1155/2017/9891348
35. Mitra A, Dwyre DM, Schivo M, et al. (2020) Leukoerythroblastic reaction in a patient with COVID‐19 infection. Am. J. Hematol. 10.1002/ajh.25793. doi:10.1002/ajh.25793.
36. Arber N, Berliner S, Pras E, et al. (1991) Heterotypic leukocyte aggregation in the peripheral blood of patients with leukemia, inflammation and stress. Nouv. Rev. Fr. Hematol. 33 (3): 251–255.
37. Yee C, Main NM, Terry A, et al. (2019) CD147 mediates intrahepatic leukocyte aggregation and determines the extent of liver injury. PLoS One 14 (7): e0215557. doi:10.1371/journal.pone.0215557.
38. Zhang C, Shi L, Wang FS (2020) Liver injury in COVID-19: management and challenges. Lancet Gastroenterol. Hepatol. 5 (5): 428–430. doi:10.1016/S2468-1253(20)30057-1.
39. Tan L, Wang Q, Zhang D, et al. (2020) Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther. 5: 33. doi:10.1038/s41392-020-0148-4.
40. Ruan Q, Yang K, Wang W, et al. (2020) Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 46 (5): 846‐848. doi:10.1007/s00134-020-05991-x.
41. Zheng M, Gao Y, Wang G, et al. (2020) Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol. Immunol. 17 (5): 533‐535. doi:10.1038/s41423-020-0402-2.
42. Qin C, Zhou L, Hu Z, et al. (2020) Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect. Dis. ciaa248. doi:10.1093/cid/ciaa248.
43. Wang, X., Xu, W., Hu, G. et al. (2020) SARS-CoV-2 infects T lymphocytes through its spike protein-mediated membrane fusion. Cell Mol. Immunol. 1‐3. doi:10.1038/s41423-020-0424-9.
44. Lv M, Miao J, Zhao P, et al. (2018) CD147-mediated chemotaxis of CD4(+)CD161(+) T cells may contribute to local inflammation in rheumatoid arthritis. Clin. Rheumatol. 37 (1): 59–66.
45. Qu R, Ling Y, Zhang YH, et al. (2020) Platelet‐to‐lymphocyte ratio is associated with prognosis in patients with coronavirus disease‐19. J. Med. Virol. 10.1002/jmv.25767. doi:10.1002/jmv.25767.
46. Semeraro N, Ammollo CT, Semeraro F, et al. (2015) Coagulopathy of acute sepsis. Semin Thromb Hemost. 41 (6):650–658. doi:10.1055/s-0035-1556730.
47. Lippi G, Plebani M, Henry BM (2020) Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A meta-analysis. Clin. Chim. Acta. 506: 145–148. doi:10.1016/j.cca.2020.03.022.
48. Yang X, Yang Q, Wang Y, et al. (2020) Thrombocytopenia and its association with mortality in patients with COVID‐19. J. Thromb. Haemost. 18: 1469–1472. https://doi.org/10.1111/jth.14848
49. Gralnick HR, Williams SB, McKeown LP, et al. (1991) Platelet von Willebrand factor. Mayo Clin. Proc. 66 (6): 634‐640. doi:10.1016/s0025-6196(12)60524-2.
50. Turner NA, Moake JL (2015) Factor VIII is synthesized in human endothelial cells, packaged in weibel-palade bodies and secreted bound to ULVWF strings. PLoS ONE 10 (10): e0140740. https://doi.org/10.1371/journal.pone.0140740.
51. Panigada, M, BottinoN, Tagliabue P, et al. (2020) Hypercoagulability of COVID‐19 patients in intensive care unit. a report of thromboelastography findings and other parameters of hemostasis. Thromb. Haemost.10.1111/jth.14850. doi:10.1111/jth.14850.
52. Zachariah U, Nair SC, Goel A, et al. (2020) Targeting raised von Willebrand factor levels and macrophage activation in severe COVID-19: Consider low volume plasma exchange and low dose steroid. Thromb. Res. 192: 2. doi:10.1016/j.thromres.2020.05.001.
53. Sadler JE (2008) Von Willebrand factor, ADAMTS13, and thrombotic thrombocytopenic purpura. Blood 112 (1): 11–18. doi:10.1182/blood-2008-02-078170.
54. Dai L, Trillo-Tinoco J, Chen Y, et al. (2016) CD147 and downstream ADAMTSs promote the tumorigenicity of Kaposi's sarcoma-associated herpesvirus infected endothelial cells. Oncotarget 7 (4): 3806–3818. doi:10.18632/oncotarget.6584.
55. Schmidt R, Bültmann A, Fischel S, et al. (2008) Extracellular matrix metalloproteinase inducer (CD147) is a novel receptor on platelets, activates platelets, and augments nuclear factor kappaB-dependent inflammation in monocytes. Circ. Res. 102: 302–309. doi: 10.1161/CIRCRESAHA.107.157990.
56. Pennings GJ, Yong AS, Kritharides L (2010) Expression of EMMPRIN (CD147) on circulating platelets in vivo. J. Thromb. Haemost. 8 (3): 472–481. doi:10.1111/j.1538-7836.2009.03716.x.
57. Seizer P, Ungern-Sternberg SN, Schönberger T, et al. (2015) Extracellular cyclophilin A activates platelets via EMMPRIN (CD147) and PI3K/Akt signaling, which promotes platelet adhesion and thrombus formation in vitro and in vivo. Arterioscler. Thromb. Vasc. Biol. 35 (3): 655–663. doi:10.1161/ATVBAHA.114.305112.
58. Zhou F, Yu T, Du R, et al. (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395 (10229): 1054–1062. https://doi.org/10.1016/S0140-6736(20)30566-3.
59. Liu XY, Li Z, Liu S, et al. (2020) Therapeutic effects of dipyridamole on COVID-19 patients with coagulation dysfunction. medRxiv 2020: 02.27.20027557; doi: https://doi.org/10.1101/2020.02.27.20027557.
60. Helms CC, Marvel M, Zhao W, et al. (2013) Mechanisms of hemolysis-associated platelet activation. J. Thromb. Haemost. 11 (12): 2148–2154. doi:10.1111/jth.12422.
61. Schanze N, Bode C, Duerschmied D (2019) Platelet contributions to myocardial ischemia/reperfusion injury. Front Immunol. 10: 1260. doi:10.3389/fimmu.2019.01260.
62. Schmidt R, Bültmann A, Fischel S, et al. (2008) Extracellular matrix metalloproteinase inducer (CD147) is a novel receptor on platelets, activates platelets, and augments nuclear factor kappaB-dependent inflammation in monocytes. Circ. Res. 102 (3): 302–309. doi:10.1161/CIRCRESAHA.107.157990.
63. Suzuki K, Satoh K, Ikeda S, et al. (2016) Basigin promotes cardiac fibrosis and failure in response to chronic pressure overload in mice. Arterioscler. Thromb. Vasc. Biol. 36 (4): 636–646. doi:10.1161/ATVBAHA.115.306686.
64. Qu R, Ling Y, Zhang YHZ, et al. (2020) Platelet‐to‐lymphocyte ratio is associated with prognosis in patients with coronavirus disease‐19. J. Med. Virol. doi:10.1002/jmv.25767.
65. Hao Zhou H, Wanxin Chen W, Ziping Li Z, et al. (2020) Delayed-phase thrombocytopenia in patients of coronavirus disease 2019 (COVID-19). medRxiv doi: https://doi.org/10.1101/2020.04.11.20059170.
66. Karsten E, Breen E, Herbert BR, (2018) Red blood cells are dynamic reservoirs of cytokines. Sci. Rep. 8 (1): 3101. doi:10.1038/s41598-018-21387-w.
67. Beaulieu LM, Lin E, Mick E, et al. (2014) Interleukin 1 receptor 1 and interleukin 1β regulate megakaryocyte maturation, platelet activation, and transcript profile during inflammation in mice and humans. Arterioscler. Thromb. Vasc. Biol. 34 (3): 552‐564. doi:10.1161/ATVBAHA.113.302700.
68. Erdei J, Tóth A, Balogh E, et al. (2018) Induction of NLRP3 inflammasome activation by heme in human endothelial cells. Oxid. Med. Cell Longev. 2018: 4310816. doi:10.1155/2018/4310816.
69. Nyakundi BB, Tóth A, Balogh E, et al. (2019) Oxidized hemoglobin forms contribute to NLRP3 inflammasome-driven IL-1β production upon intravascular hemolysis. Biochim. Biophys. Acta Mol. Basis Dis. 1865 (2): 464‐475. doi:10.1016/j.bbadis.2018.10.030.
70. Silveira AAA, Mahon OR, Cunningham CC, et al. (2019) S100A8 acts as an autocrine priming signal for heme-induced human Mϕ pro-inflammatory responses in hemolytic inflammation. J. Leukoc. Biol. 106 (1): 35‐43. doi:10.1002/JLB.3MIA1118-418RR.
71. Dutra FF, Alves LS, Rodrigues D, et al. (2014) Hemolysis-induced lethality involves inflammasome activation by heme. Proc. Natl. Acad. Sci. 111 (39): E4110‐E4118. doi:10.1073/pnas.1405023111.
72. Qu R, Ling Y, Zhang YH, et al. (2020) Platelet-to-lymphocyte ratio is associated with prognosis in patients with coronavirus disease-19. J. Med. Virol. 10.1002/jmv.25767. doi:10.1002/jmv.25767.
73. Chapin JC, Hajjar KA (2015) Fibrinolysis and the control of blood coagulation. Blood Rev. 29 (1): 17‐24. doi:10.1016/j.blre.2014.09.003.
74. Wright FL, Vogler TO, Moore EE, et al. (2020) Fibrinolysis shutdown correlates to thromboembolic events in severe COVID-19 infection. J. Am. Coll Surg. S1072-7515(20) 30400-2. doi:10.1016/j.jamcollsurg.2020.05.007.
75. Da Q, Teruya M, Guchhait P, et al. (2015) Free hemoglobin increases von Willebrand factor-mediated platelet adhesion in vitro: implications for circulatory devices. Blood 126 (20): 2338‐2341. doi:10.1182/blood-2015-05-648030.
76. Poon TC, Pang RT, Chan KC, et al. (2012) Proteomic analysis reveals platelet factor 4 and beta-thromboglobulin as prognostic markers in severe acute respiratory syndrome. Electrophoresis 33 (12): 1894‐1900. doi:10.1002/elps.201200002.
77. Lefrançais E, Ortiz-Muñoz G, Caudrillier A, et al. (2017) The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature 544 (7648): 105‐109. doi:10.1038/nature21706.
78. Nieswandt B, Stritt S (2015) Megakaryocyte rupture for acute platelet needs. J. Cell Biol. 209 (3): 327‐328. doi:10.1083/jcb.201504026.
79. Xu P, Zhou Q, Xu J (2020) Mechanism of thrombocytopenia in COVID-19 patients. Ann. Hematol. 99 (6): 1205‐1208. doi:10.1007/s00277-020-04019-0.
80. Foda HD, Rollo EE, Drews M, et al. (2001) Ventilator-induced lung injury upregulates and activates gelatinases and EMMPRIN: attenuation by the synthetic matrix metalloproteinase inhibitor, Prinomastat (AG3340). Am. J. Respir. Cell Mol. Biol. 25 (6): 717‐724. doi:10.1165/ajrcmb.25.6.4558f.
81. Wang C, Jin R, Zhu X, et al. (2015) Function of CD147 in atherosclerosis and atherothrombosis. J. Cardiovasc. Transl. Res. 8 (1): 59‐66. doi:10.1007/s12265-015-9608-6.
82. Lu G, Wang Q, Gao GF (2015) Bat-to-human: spike features determining 'host jump' of coronaviruses SARS-CoV, MERS-CoV, and beyond. Trends Microbiol. 23 (8): 468–478. doi:10.1016/j.tim.2015.06.003.
83. Reiter CD, Wang X, Tanus-Santos JE, et al. (2002) Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat. Med. 8 (12): 1383–1389. doi:10.1038/nm1202-799.
84. Kuhn V, Diederich L, Keller TCS 4th, et al. (2017) Red blood cell function and dysfunction: redox regulation, nitric oxide metabolism, anemia. Antioxid. Redox Signal. 26 (13): 718–742. doi:10.1089/ars.2016.6954.
85. Franco R, Navarro G, Martínez-Pinilla E (2019) Antioxidant defense mechanisms in erythrocytes and in the central nervous system. Antioxidants (Basel) 8 (2):46. doi:10.3390/antiox8020046
86. Schaer DJ, Vinchi F, Ingoglia G (2014) Haptoglobin, hemopexin, and related defense pathways-basic science, clinical perspectives, and drug development. Front Physiol. 5: 415. doi:10.3389/fphys.2014.00415
87. Kassa T, Jana S, Meng F (2016) Differential heme release from various hemoglobin redox states and the upregulation of cellular heme oxygenase-1. FEBS Open Bio. 6 (9):876–884. doi:10.1002/2211-5463.12103.
88. Sadrzadeh SM, Graf E, Panter SS, et al. (1984) Hemoglobin. A biologic fenton reagent. J. Biol. Chem. 259 (23): 14354‐14356.
89. Brabec V, Cermák J, Sebestík V (1990) Serum ferritin in patients with various haemolytic disorders. Folia Haematol. Int. Mag. Klin. Morphol. Blutforsch. 117 (2): 219–227.
90. Youssef L.A., Spitalnik S.L. (2019) Ferroptosis in hemolytic disorders. In: Tang D. (eds) Ferroptosis in Health and Disease. Springer, Cham.
91. Lippi G, Mattiuzzi C (2020) Hemoglobin value may be decreased in patients with severe coronavirus disease 2019 Hematol. Transfu.s Cell Ther. S2531-1379(20)30029-8. doi:10.1016/j.htct.2020.03.001.
92. Schaer DJ, Buehler PW, Alayash AI (2013) Hemolysis and free hemoglobin revisited: exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. Blood 121 (8): 1276–1284. doi:10.1182/blood-2012-11-451229.
93. Zhou Z, Yee DL, Guchhait P (2012) Molecular link between intravascular hemolysis and vascular occlusion in sickle cell disease. Curr. Vasc. Pharmacol. 10 (6): 756–761.
94. Goldhaber SZ, Bounameaux H (2012) Pulmonary embolism and deep vein thrombosis. Lancet 379 (9828):1835-46.
95. Tapson VF, Platt DM, Xia F, et al. (2016) Monitoring for Pulmonary Hypertension Following Pulmonary Embolism: The INFORM Study. Am. J. Med. 129 (9): 978–985.e2. doi:10.1016/j.amjmed.2016.03.006.
96. Ataga KI (2009) Hypercoagulability and thrombotic complications in hemolytic anemias. Haematologica 94 (11): 1481–1484. doi:10.3324/haematol.2009.013672.
97. Cui S, Chen S, Li X, et al. (2020) Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J. Thromb. Haemost. 18 (6): 1421‐1424. doi:10.1111/jth.14830.
98. Huerta C, et al. (2008) Risk of myocardial infarction and overall mortality in survivors of venous thromboembolism. Thrombosis J. 6: 10. https://doi.org/10.1186/1477-9560-6-10.
99. Han H, Yang L, Liu R, et al. (2020) Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clinical chemistry and laboratory medicine. DOI: 10.1515/cclm-2020-0188.
100. Casey K, Iteen A, Nicolini R, et al. (2020) COVID-19 pneumonia with hemoptysis: Acute segmental pulmonary emboli associated with novel coronavirus infection. Am. J. Emerg. Med. S0735-6757(20)30239-4. doi:10.1016/j.ajem.2020.04.011.
101. Huet Y, Lemaire F, Brun-Buisson C, et al. (1985) Hypoxemia in acute pulmonary embolism. Chest 88 (6): 829–836. doi:10.1378/chest.88.6.829.
102. Mehta C, Mehta Y (2016) Management of refractory hypoxemia. Ann. Card. Anaesth. 19 (1): 89–96. doi:10.4103/0971-9784.173030.
103. Aoki N, Yanagisawa A, Shimoyama K, et al. (1998) Clinical significance of hypoxemia without congestive heart failure in patients presenting with acute myocardial infarction. Cardiology 89 (1): 40–45. doi:10.1159/000006742.
104. Carreau A, El Hafny-Rahbi B, Matejuk A, et al. (2011) Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J. Cell Mol. Med. 15 (6): 1239–1253. doi:10.1111/j.1582-4934.2011.01258.x.
105. Haase VH (2013) Regulation of erythropoiesis by hypoxia-inducible factors. Blood Rev. 27 (1): 41–53. doi:10.1016/j.blre.2012.12.003.
106. Spinello I, Saulle E, Quaranta MT, et al. (2019) The small-molecule compound AC-73 targeting CD147 inhibits leukemic cell proliferation, induces autophagy and increases the chemotherapeutic sensitivity of acute myeloid leukemia cells. Haematologica 104 (5): 973–985. doi:10.3324/haematol.2018.199661.
107. Amati E, Perbellini O, Rotta G, et al. (2018) High-throughput immunophenotypic characterization of bone marrow- and cord blood-derived mesenchymal stromal cells reveals common and differentially expressed markers: identification of angiotensin-converting enzyme (CD143) as a marker differentially expressed between adult and perinatal tissue sources. Stem Cell Res. Ther. 9 (1): 10, https://doi.org/10.1186/s13287-017-0755-3.
108. Ulrich H, Pillat MM (2020) CD147 as a target for COVID-19 treatment: suggested effects of azithromycin and stem cell engagement. Stem Cell Rev. Rep. 16 (3): 434‐440. doi:10.1007/s12015-020-09976-7.
109. Falanga V (2012) Stem cells in tissue repair and regeneration. J. Invest. Dermatol. 132 (6): 1538‐1541. doi:10.1038/jid.2012.77.
110. Bortner CD, Gomez-Angelats M, Cidlowski JA (2001) Plasma membrane depolarization without repolarization is an early molecular event in anti-Fas-induced apoptosis. J. Biol. Chem. 276 (6): 4304–4314. doi:10.1074/jbc.M005171200.
111. Varga Z, Flammer AJ, Steige P, et al. (2020) Endothelial cell infection and endotheliitis in COVID-19. Lancet 395 (10234): 1417‐1418. doi:10.1016/S0140-6736(20)30937-5.
112. Chan JF, Kok KH, Zhu Z, et al. (2020) Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 9 (1): 221–236. doi:10.1080/22221751.2020.1719902.
113. Schoeman D, Fielding BC (2019) Coronavirus envelope protein: current knowledge. Virol. J. 16 (1): 69. doi:10.1186/s12985-019-1182-0.
114. Lu W, Zheng BJ, Xu K, et al. (2006) Severe acute respiratory syndrome-associated coronavirus 3a protein forms an ion channel and modulates virus release. Proc. Natl. Acad. Sci. 103 (33): 12540–12545. doi:10.1073/pnas.0605402103.
115. Verdiá-Báguena C, Nieto-Torres JL, Alcaraz A, et al. (2010) Coronavirus E protein forms ion channels with functionally and structurally-involved membrane lipids. Virology 432 (2): 485‐494. doi:10.1016/j.virol.2012.07.005.
116. Liao Y, Tam JP, Liu DX (2006) Viroporin activity of SARS-CoV E protein. Adv. Exp. Med. Biol. 581: 199‐202. doi:10.1007/978-0-387-33012-9_34.
117. Nieto-Torres JL, Verdiá-Báguena C, Jimenez-Guardeño JM, et al. (2015) Severe acute respiratory syndrome coronavirus E protein transports calcium ions and activates the NLRP3 inflammasome. Virology 485: 330–339. doi:10.1016/j.virol.2015.08.010.
118. Castaño-Rodriguez C, Honrubia JM, Gutiérrez-Álvarez J, et al. (2018) Role of Severe Acute Respiratory Syndrome Coronavirus Viroporins E, 3a, and 8a in Replication and Pathogenesis. mBio. 9 (3): e02325-17. doi:10.1128/mBio.02325-17.
119. Latz E, Xiao TS, Stutz A (2013) Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 13 (6): 397–411. doi:10.1038/nri3452.
120. Murakami T, Ockinger J, Yu J, et al. (2012) Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc. Natl. Acad. Sci. 109 (28): 11282‐11287. doi:10.1073/pnas.1117765109.
121. Nieto-Torres JL, DeDiego ML, Verdiá-Báguena C, et al. (2014) Severe acute respiratory syndrome coronavirus envelope protein ion channel activity promotes virus fitness and pathogenesis. PLoS Pathog. 10 (5): e1004077. doi:10.1371/journal.ppat.1004077.
122. Alam I, Kamau A, Kulmanov M, et al. (2020) Functional pangenome analysis suggests inhibition of the protein E as a readily available therapy for COVID-2019. bioRxiv 02.17.952895. doi: https://doi.org/10.1101/2020.02.17.952895.
123. Gupta MK, Vemula S, Donde R, et al. (2020). In-silico approaches to detect inhibitors of the human severe acute respiratory syndrome coronavirus envelope protein ion channel J. Biomol. Struct. Dyn. 1‐11 DOI: 10.1080/07391102.2020.1751300.
124. Chen IY, Moriyama M, Chang MF, Ichinohe T (2019) Severe acute respiratory syndrome coronavirus viroporin 3a activates the NLRP3 inflammasome. Front Microbiol. 10: 50. doi:10.3389/fmicb.2019.00050.
125. Issa E, Merhi G, Panossian B, et al. (2020) SARS-CoV-2 and ORF3a: Nonsynonymous mutations, functional domains, and viral pathogenesis. mSystems 5 (3): e00266-20. DOI: 10.1128/mSystems.00266-20.
126. Chan CM, Tsoi H, Chan WM, et al. (2009) The ion channel activity of the SARS-coronavirus 3a protein is linked to its pro-apoptotic function. Int. J. Biochem. Cell Biol. 41 (11): 2232‐2239. doi:10.1016/j.biocel.2009.04.019.
127. Chen D Jr., Li X, Song Q Sr., et al. (2020) Hypokalemia and clinical implications in patients with coronavirus disease 2019 (COVID-19) medRxiv 02.27.20028530. doi: https://doi.org/10.1101/2020.02.27.20028530.
128. Yaron JR, Gangaraju S, Rao MY, et al. (2015) K(+) regulates Ca(2+) to drive inflammasome signaling: dynamic visualization of ion flux in live cells. Cell Death Dis. 6 (10): e1954. Published 2015 Oct 29. doi:10.1038/cddis.2015.277.
129. Minakshi R, Padhan K, Rehman S, et al. (2014) The SARS Coronavirus 3a protein binds calcium in its cytoplasmic domain. Virus Res. 191: 180‐183. doi:10.1016/j.virusres.2014.08.001.
130. Caulier A, Rapetti‐Mauss R, Guizouarn H, et al. (2018) Primary red cell hydration disorders: Pathogenesis and diagnosis. Int. J. Lab. Hem. 40 (Suppl. 1): 68‐73.
131. George Taiaroa, Daniel Rawlinson, Leo Featherstone, et al. (2020) Direct RNA sequencing and early evolution of SARS-CoV-2. bioRxiv 03.05.976167. doi: https://doi.org/10.1101/2020.03.05.976167.
132. Liao Y, Lescar J, Tam JP, et al. (2004) Expression of SARS-coronavirus envelope protein in Escherichia coli cells alters membrane permeability. Biochem. Biophys. Res. Commun. 325 (1): 374–380. doi:10.1016/j.bbrc.2004.10.050.
133. Tiruppathi C, et al. (2002) Role of Ca2+ signaling in the regulation of endothelial permeability. Vascul. Pharmacol. 39 (4-5): 173–185. doi:10.1016/s1537-1891(03)00007-7.
134. He P, Curry FE (1991) Depolarization modulates endothelial cell calcium influx and microvessel permeability. Am. J. Physiol. 261 (4 Pt 2): H1246–H1254. doi:10.1152/ajpheart.1991.261.4.H1246.
135. Hertz L, Huisjes R, Llaudet-Planas E, et al. (2017) Is increased intracellular calcium in red blood cells a common component in the molecular mechanism causing anemia? Front Physiol. 8: 673. doi:10.3389/fphys.2017.00673.
136. Chaves-Moreira D, Souza FN, Fogaça RT, et al. (2011) The relationship between calcium and the metabolism of plasma membrane phospholipids in hemolysis induced by brown spider venom phospholipase-D toxin. J. Cell Biochem. 112 (9): 2529–2540. doi:10.1002/jcb.23177.
137. Smeets MW, Bierings R, Meems H, et al. (2017) Platelet-independent adhesion of calcium-loaded erythrocytes to von Willebrand factor PLoS One 12 (3): e0173077. doi:10.1371/journal.pone.0173077.
138. Ahn YS, Jy W, Harrington WJ, et al. (1987) Increased platelet calcium in thrombosis and related disorders and its correction by nifedipine. Thromb. Res. 45 (2): 135–143. doi:10.1016/0049-3848(87)90167-8.
139. Lissoni P, Mandala M, Rossini F, et al. (1999) Thrombopoietic property of the pineal hormone melatonin. Hematology 4: 4, 335-343, DOI: 10.1080/10245332.1999.11746457.
140. Squecco R, Tani A, Zecchi-Orlandini S, et al. (2015) Melatonin affects voltage-dependent calcium and potassium currents in MCF-7 cell line cultured either in growth or differentiation medium. Eur. J. Pharmacol. 758: 40–52. doi:10.1016/j.ejphar.2015.03.068.
141. León J, Acuña-Castroviejo D, Escames G, Tan DX, Reiter RJ. (2005) Melatonin mitigates mitochondrial malfunction. J. Pineal Res. 38 (1): 1–9. doi:10.1111/j.1600-079X.2004.00181.x.
142. Reiter RJ, Rosales-Corral S, Tan DX, et al. (2017) Melatonin as a mitochondria-targeted antioxidant: one of evolution's best ideas. Cell Mol. Life Sci. 74 (21): 3863–3881. doi:10.1007/s00018-017-2609-7.
143. Suofu Y, Li W, Jean-Alphonse FG, et al. (2017) Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc. Natl. Acad. Sci. 114 (38): E7997–E8006. doi:10.1073/pnas.1705768114.
144. Zhao D, Yu Y, Shen Y, et al. (2019) Melatonin Synthesis and Function: Evolutionary History in Animals and Plants. Front Endocrinol. (Lausanne) 10: 249. doi:10.3389/fendo.2019.00249.
145. Hardeland R, Cardinali DP, Srinivasan V (2011) Melatonin--a pleiotropic, orchestrating regulator molecule. Prog. Neurobiol. 93 (3): 350–384. doi:10.1016/j.pneurobio.2010.12.004.
146. Reiter RJ (2000) Melatonin: Lowering the high price of free radicals. News Physiol. Sci. 15: 246–250. doi:10.1152/physiologyonline.2000.15.5.246.
147. Reiter RJ, Tan DX, Terron MP, et al. (2007) Melatonin and its metabolites: new findings regarding their production and their radical scavenging actions. Acta Biochim. Pol. 54 (1): 1–9.
148. Tan DX, Manchester LC, Esteban-Zubero E, et al. (2015) Melatonin as a potent and inducible endogenous antioxidant: synthesis and metabolism. Molecules 20 (10): 18886–18906. doi:10.3390/molecules201018886.
149. Jou M‐J, Peng T‐I, Reiter RJ (2019) Protective stabilization of mitochondrial permeability transition and mitochondrial oxidation during mitochondrial Ca2+ stress by melatonin's cascade metabolites C3‐OHM and AFMK in RBA1 astrocytes. J. Pineal Res. 66: e12538. https://doi.org/10.1111/jpi.12538.
150. Reddy VS, Prabhu SD, Mummidi S, et al. (2010) Interleukin-18 induces EMMPRIN expression in primary cardiomyocytes via JNK/Sp1 signaling and MMP-9 in part via EMMPRIN and through AP-1 and NF-kappaB activation. Am. J. Physiol. Heart Circ. Physiol. 299 (4): H1242–H1254. doi:10.1152/ajpheart.00451.2010.
151. Han P, Trinidad BJ, Shi J (2015) Hypocalcemia-induced seizure: demystifying the calcium paradox. ASN Neuro. 7 (2): 1759091415578050. doi:10.1177/1759091415578050.
152. Yarmohammadi H, Uy-Evanado A, Reinier K, et al. (2017) Serum calcium and risk of sudden cardiac arrest in the general population. Mayo Clin Proc. 92 (10): 1479–1485. doi:10.1016/j.mayocp.2017.05.028.
153. Sun JK, Zhang WH, Lei Zou L, et al. (2020) Serum calcium as a biomarker of clinical severity and prognosis in patients with coronavirus disease 2019: a retrospective cross-sectional study. Crit. Care Emerg. Med. doi:10.21203/rs.3.rs-17575/v1.
154. Spinale FG, Coker ML, Heung LJ, et al. (2000) A matrix metalloproteinase induction/activation system exists in the human left ventricular myocardium and is upregulated in heart failure. Circulation 102 (16):1944–1949. doi:10.1161/01.cir.102.16.1944.
155. Cao M, Yuan W, Peng M, et al. (2019) Role of CyPA in cardiac hypertrophy and remodeling. Biosci Rep. 39 (12): BSR20193190. doi:10.1042/BSR20193190.
156. Su H, Li J, Chen T, et al. (2016) Melatonin attenuates angiotensin II-induced cardiomyocyte hypertrophy through the CD147-CyPA signaling pathway. Mol. Cell Biochem. 422 (1-2): 85–95. doi:10.1007/s11010-016-2808-9.
157. Pennings GJ, Yong ASC, Wong C, et al. (2014) Circulating levels of soluble EMMPRIN (CD147) correlate with levels of soluble glycoprotein VI in human plasma. Platelets 25: 8, 639-642, DOI: 10.3109/09537104.2013.852660.
158. Satoh K, Satoh T, Kikuchi N, et al. (2014) Basigin mediates pulmonary hypertension by promoting inflammation and vascular smooth muscle cell proliferation. Circ. Res. 115 (8): 738–750. doi:10.1161/CIRCRESAHA.115.304563.
159. Garcia-Dorado D, Ruiz-Meana M, Inserte J, et al. (2012) Calcium-mediated cell death during myocardial reperfusion. Cardiovasc. Res. 94 (2): 168-180. DOI: 10.1093/cvr/cvs116.
160. Hu S, Zhu P, Zhou H, Zhang Y, et al. (2018) Melatonin-induced protective effects on cardiomyocytes against reperfusion injury partly through modulation of IP3R and SERCA2a via activation of ERK1. Arquivos Brasileiros de Cardiologia 110 (1): 44-51. DOI: 10.5935/abc.20180008.
161. Davidson SM, Duchen MR (2007) Endothelial mitochondria contributing to vascular function and disease. Circ. Res. 100 (8): 1128–1141. Doi: 10.1161/01.RES.0000261970.18328.1d.
162. Zhu H, Jin Q, Li Y, et al. (2018) Melatonin protected cardiac microvascular endothelial cells against oxidative stress injury via suppression of IP3R-[Ca2+]c/VDAC-[Ca2+]m axis by activation of MAPK/ERK signaling pathway. Cell Stress Chaperones 23 (1): 101–113. doi:10.1007/s12192-017-0827-4.
163. Imai Y, Kuba K, Rao S, et al. (2005) Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 436 (7047): 112–116. doi:10.1038/nature03712.
164. Rabelo LA, Todiras M, Nunes-Souza V, et al. (2016) Genetic deletion of ACE2 induces vascular dysfunction in C57BL/6 mice: role of nitric oxide imbalance and oxidative stress. PLoS One, 11 (4): e0150255. doi:10.1371/journal.pone.0150255.
165. Ferrini MG, Vernet D, Magee TR, et al. (2002) Antifibrotic role of inducible nitric oxide synthase. Nitric Oxide 6 (3): 283-294. doi: 10.1006/niox.2001.0421.
166. Jones SP, Bolli R (2006) The ubiquitous role of nitric oxide in cardioprotection. J. Mo.l Cell Cardiol. 40 (1): 16–23. doi:10.1016/j.yjmcc.2005.09.011.
167. Simko F (2012) Chronobiology of blood pressure: emerging implications of melatonin. Eur. J. Clin. Invest. 42 (11): 1252–1254. doi:10.1111/j.1365-2362.2012.02705.x.
168. Pechanova O, Paulis L, Simko F (2014) Peripheral and central effects of melatonin on blood pressure regulation. Int. J. Mol. Sci. 15 (10): 17920–17937. doi:10.3390/ijms151017920.
169. Koh PO (2008) Melatonin regulates nitric oxide synthase expression in ischemic brain injury. J. Vet. Med. Sci. 70 (7): 747–750. doi:10.1292/jvms.70.747.
170. Simko F, Baka T, Krajcirovicova K, et al. (2018) Effect of melatonin on the renin-angiotensin-aldosterone system in l-NAME-induced hypertension. Molecules 23 (2): 265. Published 2018 Jan 29. doi:10.3390/molecules23020265.
171. Kosonen O, Kankaanranta H, Uotila J, et al. (2000) Inhibition by nitric oxide-releasing compounds of E-selectin expression in and neutrophil adhesion to human endothelial cells. Eur. J. Pharmacol. 394 (1): 149–156. doi:10.1016/s0014-2999(00)00141-2.
172. Sonar SA, Lal G (2019) The iNOS activity during an immune response controls the cns pathology in experimental autoimmune encephalomyelitis. Front Immunol. 10: 710. doi:10.3389/fimmu.2019.00710.
173. Donnelly LE, Barnes PJ (2002) Expression and regulation of inducible nitric oxide synthase from human primary airway epithelial cells. Am. J. Respir Cell Mol. Biol. 26 (1): 144‐151. doi:10.1165/ajrcmb.26.1.4477.
174. Zamora R, Vodovotz Y, Billiar TR (2000) Inducible nitric oxide synthase and inflammatory diseases. Mol. Med. 6 (5): 347‐373.
175. Prado CM, et al. (2019) iNOS inhibition reduces lung mechanical alterations and remodeling induced by particulate matter in mice. Pulm. Med. 2019: 4781528. doi:10.1155/2019/4781528.
176. Hardeland R (2020) Melatonin and inflammation—Story of a double‐edged blade. J. Pineal Res. 65: e12525. https://doi.org/10.1111/jpi.12525.
177. Wu, G., Peng, C., Liao, W. et al. (2020) Melatonin receptor agonist protects against acute lung injury induced by ventilator through up-regulation of IL-10 production. Respir. Res. 21 (1): 65. https://doi.org/10.1186/s12931-020-1325-2.
178. Barnes BJ, Adrover JM, Baxter-Stoltzfus A, et al. (2020) Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J. Exp. Med. 217 (6): e20200652. doi:10.1084/jem.20200652.
179. Ma Y, Yang X, Chatterjee V, et al. (2019) Role of neutrophil extracellular traps and vesicles in regulating vascular endothelial permeability. Front Immunol. 10: 1037. doi:10.3389/fimmu.2019.01037.
180. Hernández-Reséndiz S, Muñoz-Vega M, Contreras WE, et al. (2018) Responses of endothelial cells towards ischemic conditioning following acute myocardial infarction. Cond Med. 1(5):247–258.
181. Lotufo CM, Yamashita CE, Farsky SH, et al. (2006) Melatonin effect on endothelial cells reduces vascular permeability increase induced by leukotriene B4. Eur. J. Pharmacol. 534 (1-3): 258–263. doi:10.1016/j.ejphar.2006.01.050.
182. Wirtz PH, Spillmann M, Bärtschi C, et al. (2008) Oral melatonin reduces blood coagulation activity: a placebo-controlled study in healthy young men. J. Pineal Res. 44 (2): 127–133. doi:10.1111/j.1600-079X.2007.00499.x.
183. Barcellini W, Fattizzo B (2015) Clinical applications of hemolytic markers in the differential diagnosis and management of hemolytic anemia. Dis. Markers. 2015: 635670. doi:10.1155/2015/635670
184. Schaer CA, Deuel JW, Schildknecht D, et al. (2016) Haptoglobin preserves vascular nitric oxide signaling during hemolysis. Am. J. Respir. Crit. Care Med. 193 (10): 1111–1122. doi:10.1164/rccm.201510-2058OC.
185. Reiter CD, Wang X, et al. (2002) Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat. Med. 8 (12): 1383–1389. doi:10.1038/nm1202-799.
186. Helms CC, Gladwin MT, Kim-Shapiro DB (2018) Erythrocytes and vascular function: Oxygen and nitric oxide. Front Physiol. 9: 125. doi:10.3389/fphys.2018.00125.
187. Loscalzo J (2001) Nitric oxide insufficiency, platelet activation, and arterial thrombosis. Circ. Res. 88 (8): 756–762. doi:10.1161/hh0801.089861.
188. Nitric oxide gas inhalation for severe acute respiratory syndrome in COVID-19. (NOSARSCOVID) ClinicalTrials.gov Identifier: NCT04290871 https://clinicaltrials.gov/ct2/show/NCT04290871.
189. Holcomb C, Erdmann W, Corssen G (1976) The significance of diffusion hypoxemia. South Med. J. 69 (10): 1282–1284.
190. Yaron Ogen (2020) Assessing nitrogen dioxide (NO2) levels as a contributing factor to coronavirus (COVID-19) fatality. Sci. Total Environ. 726: 138605. doi:10.1016/j.scitotenv.2020.138605.
191. Raut MS, Maheshwari A (2017) Inhaled nitric oxide, methemoglobinemia, and route of delivery. Saudi. J. Anaesth. 11 (3): 364. doi:10.4103/sja.SJA_82_17.
192. Kon K, Maeda N, Shiga T (1977) Effect of nitric oxide on the oxygen transport of human erythrocytes. J. Toxicol. Environ. Health 2 (5): 1109–1113. doi:10.1080/15287397709529508.
193. Oda H, et al. (1980) Reaction of hemoglobin with nitric oxide and nitrogen dioxide in mice. J. Toxicol. Environ. Health 6 (3): 673–678. doi:10.1080/15287398009529884.
194. Stepuro TL, Zinchuk VV (2006) Nitric oxide effect on the hemoglobin-oxygen affinity. J Physiol Pharmacol. 57 (1): 29–38.
195. Dei Zotti F, Lobysheva II, Balligand J-L (2018) Nitrosyl-hemoglobin formation in rodent and human venous erythrocytes reflects NO formation from the vasculature in vivo. PLoS ONE 13 (7): e0200352.
196. Shao G, Zhang S, Nie J, et al. (2017) Effects of melatonin on mechanisms involved in hypertension using human umbilical vein endothelial cells. J. Toxicol. Environ. Health A. 80 (23-24): 1342–1348. doi:10.1080/15287394.2017.1384171.
197. Klimentova J, Cebova M, Barta A, et al. (2016) Effect of melatonin on blood pressure and nitric oxide generation in rats with metabolic syndrome. Physiol. Res. 65 (Suppl 3): S373–S380. doi:10.33549/physiolres.933436.
198. Huang CC, Lai CJ, Tsai MH, et al. (2015) Effects of melatonin on the nitric oxide system and protein nitration in the hypobaric hypoxic rat hippocampus. BMC Neurosci. 16: 61. doi:10.1186/s12868-015-0199-6.
199. Klok FA, Kruip MJHA, van der Meer NJM, et al. (2020) Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. S0049-3848(20)30120-1. doi:10.1016/j.thromres.2020.04.013.
200. Hung MW, Yeung HM, Lau CF, et al. (2017) Melatonin attenuates pulmonary hypertension in chronically hypoxic rats. Int. J. Mo.l Sci. 18 (6): 1125. doi:10.3390/ijms18061125.
201. Jensen FB (2009) The role of nitrite in nitric oxide homeostasis: a comparative perspective. Biochim. Biophys. Acta. 1787 (7): 841–848. doi:10.1016/j.bbabio.2009.02.010.
202. Grygorczyk R, Orlov SN (2017) Effects of hypoxia on erythrocyte membrane properties-implications for intravascular hemolysis and purinergic control of blood flow. Front Physiol. 8: 1110. Published 2017 Dec 22. doi:10.3389/fphys.2017.01110.
203. Kanias T, Acker JP (2010) Biopreservation of red blood cells – the struggle with hemoglobin oxidation. FEBS J. 277 (2): 343–356. doi:10.1111/j.1742-4658.2009.07472.x.
204. Nagababu E, Mohanty JG, Bhamidipaty S, et al. (2010) Role of the membrane in the formation of heme degradation products in red blood cells. Life Sci. 86 (3-4): 133–138. doi:10.1016/j.lfs.2009.11.015.
205. Chiu D, Lubin B (1989) Oxidative hemoglobin denaturation and RBC destruction: the effect of heme on red cell membranes. Semin. Hematol. 26 (2): 128–135.
206. Tesoriere L, D'Arpa D, Conti S, et al. (1999), Melatonin protects human red blood cells from oxidative hemolysis: New insights into the radical‐scavenging activity. J. Pineal Res. 27: 95-105. doi:10.1111/j.1600-079X.1999.tb00602.x.
207. Maitra D, Abdulhamid I, Diamond MP, et al. (2012) Melatonin attenuates hypochlorous acid-mediated heme destruction, free iron release, and protein aggregation in hemoglobin. J. Pineal Res. 53 (2): 198–205. doi:10.1111/j.1600-079X.2012.00988.x.
208. Tesoriere L, et al. (2001) Reaction of melatonin with hemoglobin-derived oxoferryl radicals and inhibition of the hydroperoxide-induced hemoglobin denaturation in red blood cells. J. Pineal Res. 1 (2): 114–119. doi:10.1034/j.1600-079x.2001.310204.x.
209. Reeder BJ, Wilson MT (2005) Hemoglobin and myoglobin associated oxidative stress: from molecular mechanisms to disease States. Curr. Med. Chem. 12 (23): 2741–2751. doi:10.2174/092986705774463021.
210. Borgese N, Pietrini G, Gaetani S (1987) Concentration of NADH-cytochrome b5 reductase in erythrocytes of normal and methemoglobinemic individuals measured with a quantitative radioimmunoblotting assay. J. Clin. Invest. 80 (5): 1296‐1302. doi:10.1172/JCI113205.
211. Choury D, Leroux A, Kaplan JC (1987) Membrane-bound cytochrome b5 reductase (methemoglobin reductase) in human erythrocytes. Study in normal and methemoglobinemic subjects. J. Clin. Invest. 67 (1): 149‐155. doi:10.1172/JCI110007.
212. Bulbarelli A, Valentini A, DeSilvestris M, et al. (1998) An erythroid-specific transcript generates the soluble form of NADH-cytochrome b5 reductase in humans. Blood 92 (1): 310‐319.
213. Percy MJ, Lappin TR (2008) Recessive congenital methaemoglobinaemia: cytochrome b5 reductase deficiency. Br. J. Haematol. 141: 298-308. doi:10.1111/j.1365-2141.2008.07017.x.
214. Martin-Montalvo A, Sun Y, Diaz-Ruiz A, et al. (2016) Cytochrome b5 reductase and the control of lipid metabolism and healthspan. NPJ Aging Mech. Dis. 2: 16006. https://doi.org/10.1038/npjamd.2016.6.
215. Martinez GR, Almeida EA, Klitzke CF, et al. (2005) Measurement of melatonin and its metabolites: importance for the evaluation of their biological roles. Endocrine. 27 (2): 111‐118. doi:10.1385/endo:27:2:111.
216. Tan DX, Manchester LC, Sainz RM, et al. (2005) Interactions between melatonin and nicotinamide nucleotide: NADH preservation in cells and in cell-free systems by melatonin. J. Pineal Res. 39 (2): 185‐194. doi:10.1111/j.1600-079X.2005.00234.x.
217. Reiter RJ, Tan DX, Terron MP, et al. (2007) Melatonin and its metabolites: new findings regarding their production and their radical scavenging actions. Acta Biochim. Pol. 54 (1): 1‐9.
218. Ross D, Siegel D (2017) Functions of NQO1 in cellular protection and CoQ10 metabolism and its potential role as a redox sensitive molecular switch. Front Physiol. 8: 595. doi:10.3389/fphys.2017.00595.
219. Yan C, Shieh B, David Siegel D, et al. (2007) Overexpression of NQO1 protects Jurkat leukemia cells from apoptosis. Cancer Res. 67 (9 Supplement): 4862.
220. Graves PR, Kwiek JJ, Fadden P, et al. (2002) Discovery of novel targets of quinoline drugs in the human purine binding proteome. Mol. Pharmacol. 62 (6): 1364‐1372. doi:10.1124/mol.62.6.1364.
221. Vasiliou V, Ross D, Nebert DW (2006) Update of the NAD(P)H:quinone oxidoreductase (NQO) gene family. Hum Genomics. 2 (5): 329–335. doi:10.1186/1479-7364-2-5-329.
222. Mailliet F, Ferry G, Vella F, et al. (2005) Characterization of the melatoninergic MT3 binding site on the NRH:quinone oxidoreductase 2 enzyme. Biochem. Pharmacol. 71 (1-2): 74–88. doi:10.1016/j.bcp.2005.09.030.
223. Calamini B, Santarsiero BD, Boutin JA, et al. (2008) Kinetic, thermodynamic and X-ray structural insights into the interaction of melatonin and analogues with quinone reductase 2. Biochem. J. 413 (1): 81–91. doi:10.1042/BJ20071373.
224. Boutin JA, Ferry G, (2018) Is there sufficient evidence that the melatonin binding site MT3 is quinone reductase 2? J. Pharmacol. Exp. Ther. 368 (1): 59‐65. doi:10.1124/jpet.118.253260.
225. Wu K, Knox R, Sun XZ, et al. (1997) Catalytic properties of NAD(P)H:quinone oxidoreductase-2 (NQO2), a dihydronicotinamide riboside dependent oxidoreductase. Arch. Biochem. Biophys. 347 (2): 221‐228. doi:10.1006/abbi.1997.0344.
226. Reybier K, Perio P, Ferry G, et al. (2011) Insights into the redox cycle of human quinone reductase 2. Free Radic. Res. 45 (10): 1184‐1195. doi:10.3109/10715762.2011.605788.
227. Chen D, Li X, Liu X, et al. (2017) NQO2 inhibition relieves reactive oxygen species effects on mouse oocyte meiotic maturation and embryo development. Biol. Reprod. 97 (4): 598‐611. doi:10.1093/biolre/iox098.
228. Cassagnes LE, Rakotoarivelo N, Sirigu S, et al. (2017) Role of quinone reductase 2 in the antimalarial properties of indolone-type derivatives. Molecules 22 (2): 210. doi:10.3390/molecules22020210.
229. Tan DX, Manchester LC, Terron MP, et al. (2007) Melatonin as a naturally occurring co-substrate of quinone reductase-2, the putative MT3 melatonin membrane receptor: hypothesis and significance. J. Pineal Res. 43 (4): 317‐320. doi:10.1111/j.1600-079X.2007.00513.x.
230. Boutin JA, Marcheteau E, Hennig P, et al. (2008) MT3/QR2 melatonin binding site does not use melatonin as a substrate or a co-substrate. J. Pineal Res 45 (4): 524-531. DOI: 10.1111/j.1600-079x.2008.00631.x.
231. Reithmeier RA, Casey JR, Kalli AC, et al. (2016) Band 3, the human red cell chloride/bicarbonate anion exchanger (AE1, SLC4A1), in a structural context. Biochim. Biophys. Acta. 1858 (7 Pt A): 1507–1532. doi:10.1016/j.bbamem.2016.03.030.
232. Hamasaki N (1999) The role of band 3 protein in oxygen delivery by red blood cells. Indian J. Clin. Biochem. 14 (1): 49–58. doi:10.1007/BF02869151.
233. Wang, Da Neng (1994), Band 3 protein: Structure, flexibility and function, FEBS Lett. 346 (1): 26‐31. doi:10.1016/0014-5793(94)00468-4.
234. Ke X, Fei F, Chen Y, et al. (2012) Hypoxia upregulates CD147 through a combined effect of HIF-1α and Sp1 to promote glycolysis and tumor progression in epithelial solid tumors. Carcinogenesis 33 (8): 1598–1607. doi:10.1093/carcin/bgs196.
235. Zipser Y, Piade A, Barbul A, et al. (2002) Ca2+ promotes erythrocyte band 3 tyrosine phosphorylation via dissociation of phosphotyrosine phosphatase from band 3. Biochem. J. 368 (Pt 1): 137–144. doi:10.1042/BJ20020359.
236. Pantaleo A, Ferru E, Pau MC, et al. (2016) Band 3 erythrocyte membrane protein acts as redox stress sensor leading to its phosphorylation by p (72) syk. Oxid. Med. Cell Longev. 2016: 6051093. doi:10.1155/2016/6051093.
237. Shimo H, Arjunan SN, Machiyama H, et al. (2015) Particle simulation of oxidation induced band 3 clustering in human erythrocytes. PLoS Comput. Biol. 11 (6): e1004210. doi:10.1371/journal.pcbi.1004210.
238. Morabito R, Remigante A, Marino A. (2019) Melatonin protects band 3 protein in human erythrocytes against H2O2-induced oxidative stress. Molecules 24 (15): 2741. doi:10.3390/molecules24152741.
239. Huisjes R, Bogdanova A, van Solinge WW, et al. (2018) Squeezing for life - properties of red blood cell deformability. Front Physiol. 9: 656. doi:10.3389/fphys.2018.00656.
240. Richardson SL, Swietach P (2016) Red blood cell thickness is evolutionarily constrained by slow, hemoglobin-restricted diffusion in cytoplasm. Sci. Rep. 6: 36018. doi:10.1038/srep36018.
241. Jeney V, Balla J, Yachie A et al. (2002) Pro-oxidant and cytotoxic effects of circulating heme. Blood 100 (3): 879–887. doi:10.1182/blood.v100.3.879.
242. Balla G, Jacob HS, Eaton JW, et al. (1991) Hemin: a possible physiological mediator of low density lipoprotein oxidation and endothelial injury. Arterioscler. Thromb. 11 (6): 1700‐1711. doi:10.1161/01.atv.11.6.1700.
243. Rother RP, Bell L, Hillmen P, et al. (2005) The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA 293 (13): 1653‐1662, doi:10.1001/jama.293.13.1653.
244. Immenschuh S, Vijayan V, Janciauskiene S, et al. (2017) Heme as a target for therapeutic interventions. Front Pharmacol. 8: 146. doi:10.3389/fphar.2017.00146
245. Higdon AN, Benavides GA, Chacko BK, et al. (2012) Hemin causes mitochondrial dysfunction in endothelial cells through promoting lipid peroxidation: the protective role of autophagy. Am. J. Physiol. Heart Circ. Physiol. 302 (7): H1394‐H1409. doi:10.1152/ajpheart.00584.2011.
246. Frimat M, Boudhabhay I, Roumenina LT (2019) Hemolysis derived products toxicity and endothelium: model of the second hit. Toxins (Basel). 11 (11): 660 doi:10.3390/toxins11110660.
247. Riphagen S, Gomez X, Gonzalez-Martinez C, et al. (2020) Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 395 (10237): 1607‐1608. doi:10.1016/S0140-6736(20)31094-1.
248. Nakatani K, Takeshita S, Tsujimoto H, et al. (2003) Circulating endothelial cells in Kawasaki disease. Clin. Exp. Immunol. 131 (3): 536‐540. doi:10.1046/j.1365-2249.2003.02091.x.
249. Ishikawa T, Seki K (2018) The association between oxidative stress and endothelial dysfunction in early childhood patients with Kawasaki disease. BMC Cardiovasc. Disord. 18 (1): 30. doi:10.1186/s12872-018-0765-9.
250. Ueno K, Ninomiya Y, Hazeki D, et al. (2017) Disruption of endothelial cell homeostasis plays a key role in the early pathogenesis of coronary artery abnormalities in Kawasaki disease. Sci. Rep. 7, 43719. https://doi.org/10.1038/srep43719.
251. Dhillon R, Clarkson P, Donald AE, et al. (1996) Endothelial dysfunction late after Kawasaki disease. Circulation 94 (9): 2103‐2106. doi:10.1161/01.cir.94.9.2103.
252. Paul, S, Naaz, S, Ghosh, A, et al. (2018) Melatonin chelates iron and binds directly with phenylhydrazine to provide protection against phenylhydrazine induced oxidative damage in red blood cells along with its antioxidant mechanisms: an in vitro study. Melatonin Res. 1 (1): 1-20. DOI:https://doi.org/https://doi.org/10.32794/mr11250001.
253. Elkader MAE, Aly H (2015) Protective effect of melatonin against iron overload-induced toxicity in rats. Int. J. Pharmac. Pharmaceutic. Sci. 7: (9): 116-121. https://innovareacademics.in/journals/index.php/ijpps/article/view/7317.
254. Arnould T, Michiels C, Alexandre I, et al. (1992) Effect of hypoxia upon intracellular calcium concentration of human endothelial cells. J. Cell Physiol. 152 (1): 215–221. doi:10.1002/jcp.1041520127.
255. Yeung HM, Hung MW, Fung ML (2008) Melatonin ameliorates calcium homeostasis in myocardial and ischemia-reperfusion injury in chronically hypoxic rats. J. Pineal Res. 45 (4): 373–382. doi:10.1111/j.1600-079X.2008.00601.x.
256. Yugo Tabata, Daisuke Yoshino, Kiyoe Funamoto, et al. (2019) Migration of vascular endothelial cells in monolayers under hypoxic exposure. Integr. Biol. (Camb). 11 (1): 26‐35. doi:10.1093/intbio/zyz002.
257. Yang L, Zheng J, Xu R, et al. (2014) Melatonin suppresses hypoxia-induced migration of HUVECs via inhibition of ERK/Rac1 activation. Int. J. Mol. Sci. 15 (8): 14102‐14121. doi:10.3390/ijms150814102.
258. Cheng J, Yang HL, Gu CJ, et al. (2019) Melatonin restricts the viability and angiogenesis of vascular endothelial cells by suppressing HIF-1α/ROS/VEGF. Int. J. Mol. Med. 43 (2): 945–955. doi:10.3892/ijmm.2018.4021.
259. Favero G, Franceschetti L, Bonomini F, et al. (2017) Melatonin as an anti-inflammatory agent modulating inflammasome activation. Int. J. Endocrinol. 2017: 1835195. doi:10.1155/2017/1835195 1835195.
260. Bonomini F, Dos Santos M, Veronese FV, et al. (2019) NLRP3 inflammasome modulation by melatonin supplementation in chronic pristane-induced lupus nephritis. Int. J. Mol. Sci. 20 (14): 3466. doi:10.3390/ijms20143466.
261. Reiter RJ, Sharma R, Ma Q, et al. (2020) Melatonin inhibits COVID-19-induced cytokine storm by reversing aerobic glycolysis in immune cells: A mechanistic analysis Med. Drug Discov. 6: 100044. doi:10.1016/j.medidd.2020.100044.
262. Hevia D, González-Menéndez P, Quiros-González I, et al. (2015) Melatonin uptake through glucose transporters: a new target for melatonin inhibition of cancer. J. Pineal Res. 58 (2): 234–250. doi:10.1111/jpi.12210.
263. Berg JM, Tymoczko JL, Stryer L (2002) The pentose phosphate pathway generates NADPH and synthesizes five-carbon sugars. biochemistry 5th edition. New York: W H Freeman; 20.3 Available from: https://www.ncbi.nlm.nih.gov/books/NBK22416/.
264. Prchal JT, Gregg XT (2005) Red cell enzymes. Hematol. Am. Soc. Hematol. Educ. Program. 2005 (1): 19–23. doi: https://doi.org/10.1182/asheducation-2005.1.19.
265. Doshi B, Kamdar A, Smith-Whitley K, et al. (2016) Incidence of hemolytic events after exposure to triggering medications in pediatric patients with G6PD deficiency. Blood 128 (22): 4810. doi: https://doi.org/10.1182/blood.V128.22.4810.4810.
266. Francis RO, Jhang JS, Pham HP, et al. (2013) Glucose-6-phosphate dehydrogenase deficiency in transfusion medicine: the unknown risks. Vox Sang. 105 (4): 271–282. doi:10.1111/vox.12068.
267. Horowitz RI, Freeman PR, Bruzzese J (2020) Efficacy of glutathione therapy in relieving dyspnea associated with COVID-19 pneumonia: A report of 2 cases, Respir. Med. Case Rep. 30: 101063. doi:10.1016/j.rmcr.2020.101063.
268. Rovira A, Angioletti MD, Camacho-Vanegas O, et al. (2000) Stable in vivo expression of glucose-6-phosphate dehydrogenase (G6PD) and rescue of G6PD deficiency in stem cells by gene transfer. Blood 96 (13): 4111–4117. doi: https://doi.org/10.1182/blood.V96.13.4111.
269. Ma F, Ebihara Y, Umeda K, et al. (2008) Generation of functional erythrocytes from human embryonic stem cell-derived definitive hematopoiesis. Proc. Natl. Aca. Sci. 105 (35): 13087-13092. DOI: 10.1073/pnas.0802220105
270. Qiu C, Olivier EN, Velho M, et al. (2008) Globin switches in yolk sac-like primitive and fetal-like definitive red blood cells produced from human embryonic stem cells. Blood 111 (4): 2400-2408. DOI: 10.1182/blood-2007-07-10208.
271. Paglialunga F, Fico A, Iaccarino I, et al. (2004) G6PD is indispensable for erythropoiesis after the embryonic-adult hemoglobin switch. Blood 104 (10): 3148–3152. doi:10.1182/blood-2004-03-0835.
272. Ciftçi M, Bilici D, Küfrevioğlu OI (2001) Effects of melatonin on enzyme activities of glucose-6-phosphate dehydrogenase from human erythrocytes in vitro and from rat erythrocytes in vivo. Pharmacol. Res. 44 (1): 7–11. doi:10.1006/phrs.2001.0837.
273. El-Missiry MA (2000) Prophylactic effect of melatonin on lead-induced inhibition of heme biosynthesis and deterioration of antioxidant systems in male rats. J. Biochem. Mol. Toxicol. 14 (1): 57–62. doi:10.1002/(sici)1099-0461(2000)14:1<57::aid-jbt8>3.0.co;2-b.
274. Hara T, Mukai H, Nakashima T, et al. (2015) Factors contributing to erythropoietin hyporesponsiveness in patients on long-term continuous ambulatory peritoneal dialysis: A cross-sectional study. Nephron Extra. 5 (3): 79–86. doi:10.1159/000441154.
275. HF, Allami HCA (2019) Melatonin improves erythropoietin hyporesponsiveness via suppression of inflammation. Rev. Recent Clin. Trials. 14 (3): 203–208. doi:10.2174/1574887114666190528120357.
276. Walsh AC, Michaud SG, Malossi JA, et al. (1995) Glutathione depletion in human T lymphocytes: analysis of activation-associated gene expression and the stress response. Toxicol. Appl. Pharmacol. 133 (2): 249–261. doi:10.1006/taap.1995.1149.
277. Puskas F, Gergely P Jr, Banki K, et al. (2000) Stimulation of the pentose phosphate pathway and glutathione levels by dehydroascorbate, the oxidized form of vitamin C. FASEB J. 14 (10): 1352–1361. doi:10.1096/fj.14.10.1352.
278. Chen G, Wang SH, Converse CA (1994) Glutathione increases interleukin-2 production in human lymphocytes. Int. J. Immunopharmacol. 16 (9): 755‐760. doi:10.1016/0192-0561(94)90095-7.
279. Carrillo-Vico A, Calvo JR, Abreu P, et al. (2004) Evidence of melatonin synthesis by human lymphocytes and its physiological significance: possible role as intracrine, autocrine, and/or paracrine substance. FASEB J. 18 (3): 537–539. doi:10.1096/fj.03-0694fje.
280. Waters RS, Perry JSA, Han S, et al. (2018) The effects of interleukin-2 on immune response regulation. Math. Med. Biol. 35 (1): 79‐119. doi:10.1093/imammb/dqw021.
281. McKinstry KK, Alam F, Flores-Malavet V, et al. (2019) Memory CD4 T cell-derived IL-2 synergizes with viral infection to exacerbate lung inflammation. PLoS Pathog. 15 (8): e1007989. doi:10.1371/journal.ppat.1007989.
282. McKinstry KK, Strutt TM, Bautista B, et al. (2014) Effector CD4 T-cell transition to memory requires late cognate interactions that induce autocrine IL-2. Nat. Commun. 5: 5377. doi:10.1038/ncomms6377.
283. Abbas AK, Murphy KM, Sher A (1996) Functional diversity of helper T lymphocytes. Nature 383 (6603): 787‐793. doi:10.1038/383787a0.
284. Hardeland R (2018) Melatonin and inflammation—Story of a double‐edged blade. J. Pineal Res. 65: e12525. https://doi.org/10.1111/jpi.12525.
285. Carrillo-Vico A, Lardone PJ, José M, et al. (2005) Human lymphocyte-synthesized melatonin is involved in the regulation of the interleukin-2/interleukin-2 receptor system, J Clin Endocrinol Metab. 90 (2): 992‐1000. doi:10.1210/jc.2004-1429.
286. Al Amir Dache Z, et al. (2020) Blood contains circulating cell-free respiratory competent mitochondria. FASEB J. 34 (3): 3616–3630. doi:10.1096/fj.201901917RR.
287. NaveenKumar SK, et al. (2017) Cell-free methemoglobin drives platelets to apoptosis via mitochondrial ROS-mediated activation of JNK and p38 MAP kinase. Biochem. Biophys. Res. Commun. 491 (1): 183–191. doi:10.1016/j.bbrc.2017.07.073.
288. Hofmann J, Bosia A, Arese P, et al. (1981) Glucose-6-phosphate dehydrogenase deficiency in human platelets and its effect on platelet aggregation. Acta Biol. Med. Ger. 40 (12): 1707–1714.
289. Yang M, Zhou M, Ye JY, et al. (2008) The effect and underlying mechanism of melatonin on platelet formation and survival in a thrombocytopenic model. Blood 112 (11): 1241. doi: https://doi.org/10.1182/blood.V112.11.1241.1241.
290. Lissoni P, Mandala M, Rossini F (1999) Thrombopoietic property of the pineal hormone melatonin. Hematology 4: 4, 335-343. DOI: 10.1080/1.
291. Tanaka Y, Sato Y, Sasaki T (2013) Suppression of coronavirus replication by cyclophilin inhibitors. Viruses 5 (5):1250‐1260. doi:10.3390/v5051250.
292. Zhou D, Mei Q, Li J, He H (2012) Cyclophilin A and viral infections. Biochem. Biophys. Res. Commun. 424 (4): 647‐650. doi:10.1016/j.bbrc.2012.07.024.
293. Dawar FU, Tu J, Khattak MN, et al. (2017) Cyclophilin A: A key factor in virus replication and potential target for anti-viral therapy. Curr. Issues Mol. Biol. 21: 1‐20. doi:10.21775/cimb.021.001.
294. Hardeland, R. (2019) Melatonin and chromatin. Melatonin Res. 2 (1): 67-93. DOI:https://doi.org/https://doi.org/10.32794/mr11250012.
295. Tan DX, Hardeland R (2020) Potential utility of melatonin in deadly infectious diseases related to the overreaction of innate immune response and destructive inflammation: focus on COVID-19. Melatonin Res. 3 (1): 120-143. doi:https://doi.org/https://doi.org/10.32794/mr11250052.
296. Anderson G, Reiter RJ (2020) Melatonin: Roles in influenza, Covid‐19, and other viral infections. Rev Med Virol. 30 (3): e2109. doi:10.1002/rmv.2109.
297. Reiter RJ, Abreu-Gonzalez P, Marik PE, et al. (2020) Therapeutic algorithm for use of melatonin in patients with COVID-19. Front. Med. 7: 226. doi: 10.3389/fmed.2020.00226.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


