ER stress and autophagy induced by SARS-CoV-2: The targets for melatonin treatment
Melatonin as treatment against COVID19
Abstract
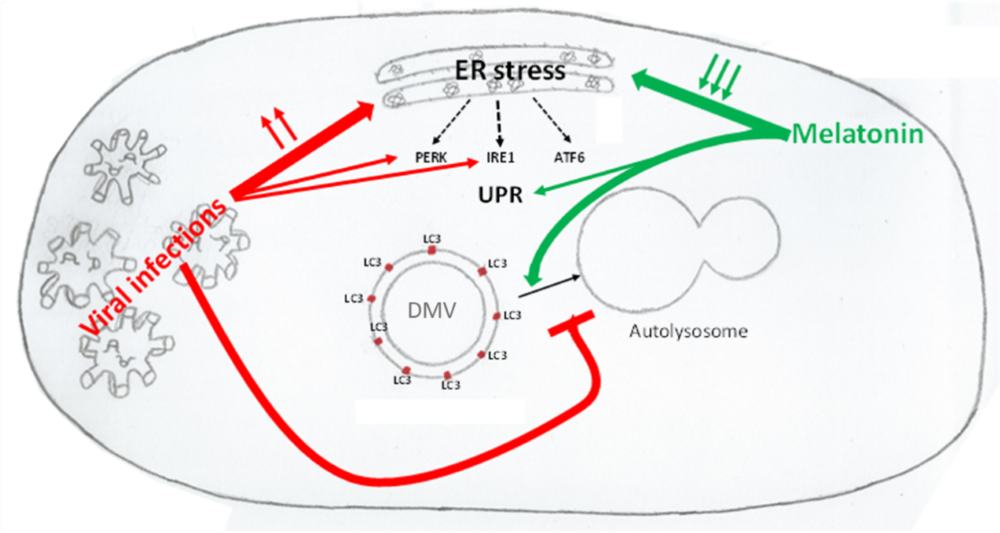
Coronavirus disease 19 (COVID-19) is a viral disease caused by the new coronavirus SARS-CoV-2. Like other coronaviral infections, SARS-CoV-2 causes oxidative and ER stress triggering cellular response pathways, mainly PERK and IRE1 branches of the UPR. This excessive oxidative stress and the increasing of unfolded and misfolded proteins induce autophagy. Once this process is triggered, the blockage of the fusion of autophagosomes and lysosomes induced by virus leads to an incomplete autophagy. Double-membraned vesicles, which create a membranous support for viral RNA replication complexes, are formed. Melatonin is a pleiotropic molecule, which reduces oxidative and ER stress, regulates immune system, and modulates autophagy pathway. Thus, melatonin reinforces UPR and unlocks autophagy blockage, allowing autophagosomes to bind to lysosomes, completing the process of autophagy and decreasing viral replication capacity. Based on these activities of melatonin the recommendation of melatonin for patients with COVID-19 should be seriously considered, especially in elderlies and patients with different comorbidities, which are the highest risk population for serious cases.
References
2. Hardeland R, Poeggeler B, Srinivasan V, Trakht I, Pandi-Perumal SR, Cardinali DP (2008) Melatonergic drugs in clinical practice. Arzneimittelforschung 58 (1): 1‐10. DOI:10.1055/s-0031-1296459.
3. Hardeland R, Coto-Montes A, Poeggeler B (2003) Circadian rhythms, oxidative stress, and antioxidative defense mechanisms. Chronobiol. Int. 20 (6): 921‐962. DOI:10.1081/cbi-120025245.
4. Bonnefont-Rousselot D, Collin F (2010) Melatonin: action as antioxidant and potential applications in human disease and aging. Toxicology 278 (1): 55‐67. DOI:10.1016/j.tox.2010.04.008.
5. Wu H, Liu J, Yin Y, Zhang D, Xia P, Zhu G. (2019) Therapeutic opportunities in colorectal cancer: focus on melatonin antioncogenic action. Biomed. Res. Int. 9740568: DOI:10.1155/2019/9740568.
6. Lim Y, Cho H, Kim EK (2016) Brain metabolism as a modulator of autophagy in neurodegeneration. Brain Res. 1649 (Pt B): 158‐165. DOI:10.1016/j.brainres.2016.02.049.
7. Yang L, Liu X, Song L, Su G, Di A, Bai Ch, Wei Z, Li G (2019) Inhibiting repressive epigenetic modification promotes telomere rejuvenation in somatic cell reprogramming. FASEB J. 33 (12): 13982‐13997. DOI:10.1096/fj.201901486RR.
8. Junaid A, Tang H, van Reeuwijk A, Abouleila Y, Wuelfroth P, van Duinen V, Stam W, van Zonneveld AJ, Hankemeier T, Mashaghi A (2020) Ebola hemorrhagic shock syndrome-on-a-chip. iScience 23 (1): 100765. DOI:10.1016/j.isci.2019.100765.
9. Crespo I, Miguel BS, Laliena A, Alvarez M, Culebras JM, Gonzalez-Galleo J, Tuñón MJ (2010) Melatonin prevents the decreased activity of antioxidant enzymes and activates nuclear erythroid 2-related factor 2 signaling in an animal model of fulminant hepatic failure of viral origin. J. Pineal Res. 49 (2): 193–200. DOI:10.1111/j.1600-079X.2010.00787.x.
10. Crespo I, San-Miguel B, Sánchez DI, Gonzalez-Fernandez B, Alvarez M, Gonzalez-Gallego J, Tuñón MJ (2016) Melatonin inhibits the sphingosine kinase 1/sphingosine-1-phosphate signaling pathway in rabbits with fulminant hepatitis of viral origin. J. Pineal Res. 61 (2): 168–176. DOI:10.1111/jpi.12335.
11. Boga JA, Caballero B, Potes Y, Perez-Martinez Z, Reiter RJ, Vega-Naredo I, Coto-Montes A (2019) Therapeutic potential of melatonin related to its role as an autophagy regulator: A review. J. Pineal Res. 66 (1): e12534. DOI:10.1111/jpi.12534.
12. Moustafa-Farag M, Almoneafy A, Mahmoud A, Elkelish A, Arnao MB, Li L, Ai S (2019) Melatonin and its protective role against biotic stress impacts on plants. Biomolecules 10 (1): 54. DOI:10.3390/biom10010054.
13. Lu R, Liu Z, Shao Y, Sun F, Zhang Y, Cui J, Zhou Y, Shen W, Zhou T (2019) Melatonin is responsible for rice resistance to rice stripe virus infection through a nitric oxide-dependent pathway. Virol. J. 16 (1): 141 DOI:10.1186/s12985-019-1228-3.
14. Wang M, Zhang S, Ding F (2020) Melatonin mitigates chilling-induced oxidative stress and photosynthesis inhibition in tomato plants. Antioxidants (Basel) 9 (3): 218. DOI:10.3390/antiox9030218.
15. Jose RJ, Manuel A. COVID-19 (202) Cytokine storm: the interplay between inflammation and coagulation. Lancet Respir. Med. S2213-2600 (20): 30216-2. DOI: 10.1016/S2213-2600(20)30216-2.
16. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW; and the Northwell COVID-19 Research Consortium, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP (2020) Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA DOI: 10.1001/jama.2020.6775.
17. Ribeiro AR, Howlett SE, Fernandes A. (2020) Frailty-A promising concept to evaluate disease vulnerability. Mech. Ageing Dev. 187: 111217. DOI:10.1016/j.mad.2020.111217.
18. Clohisey S, Baillie JK (2019) Host susceptibility to severe influenza A virus infection. Crit. Care 23 (1): 303. DOI:10.1186/s13054-019-2566-7.
19. Butterfield TR, Landay AL, Anzinger JJ (2020) Dysfunctional immunometabolism in HIV infection: Contributing factors and implications for age-related comorbid diseases. Curr. HIV/AIDS Rep. 17 (2): 125–137. DOI:10.1007/s11904-020-00484-4.
20. Dessie ZG, Zewotir T, Mwambi H, North D (2020) Modeling viral suppression, viral rebound and state-specific duration of HIV patients with CD4 count adjustment: parametric multistate frailty model approach. Infect. Dis. Ther. DOI:10.1007/s40121-020-00296-4.
21. Tan H, Wu WZ, Zhao Q, Hong ZC, Feng JY, Zeng H (2020) HIV/AIDS related frailty syndrome in the elderly and related research progress. Zhonghua Liu Xing Bing Xue Za Zhi 41 (1): 127–130. DOI: 10.3760/cma.j.issn.0254-6450.2020.01.023.
22. de Gonzalo-Calvo D, Neitzert K, Fernández M, Vega-Naredo I, Caballero B, García-Macía M, Suárez FM, Rodríguez-Colunga MJ, Solano JJ, Coto-Montes A (2010) Differential inflammatory responses in aging and disease: TNF-alpha and IL-6 as possible biomarkers. Free Radic. Biol. Med. 49 (5): 733–737. DOI:10.1016/j.freeradbiomed.2010.05.019.
23. de Gonzalo-Calvo D, de Luxán-Delgado B, Rodríguez-González S, García-Macia M, Suárez FM, Solano JJ, Rodríguez-Colunga MJ, Coto-Montes A (2012) Interleukin 6, soluble tumor necrosis factor receptor I and red blood cell distribution width as biological markers of functional dependence in an elderly population: a translational approach. Cytokine 58 (2): 193–198. DOI:10.1016/j.cyto.2012.01.005.
24. Potes Y, de Luxán-Delgado B, Rodriguez-González S, Rodrigues Moreira Guimarães M, Solano JJ, Fernandez-Fernandez M, Bermúdez M, Boga JA, Vega-Naredo I, Coto-Montes A (2017) Overweight in elderly people induces impaired autophagy in skeletal muscle. Free Radic. Biol. Med. 110: 31–41. DOI:10.1016/j.freeradbiomed.2017.05.018.
25. Michaud M, Balardy L, Moulis G, Gaudin C, Peyrot C, Vellas B, Cesari M, Nourhashemi F (2013) Proinflammatory cytokines, aging, and age-related diseases. J. Am. Med. Dir. Assoc. 14 (12): 877-882.
26. Lardone PJ, Alvarez-García O, Carrillo-Vico A, Vega-Naredo I, Caballero B, Guerrero JM, Coto-Montes A (2006) Inverse correlation between endogenous melatonin levels and oxidative damage in some tissues of SAM P8 mice. J. Pineal Res. 40 (2): 153–157. DOI:10.1111/j.1600-079X.2005.00289.x.
27. Hardeland R (2018) Melatonin and inflammation-story of a double-edged blade (2018) J. Pineal Res. 65 (4): e12525. DOI:10.1111/jpi.12525.
28. Hardeland R (2019) Aging, melatonin, and the pro- and anti-inflammatory networks. Int J. Mol. Sci. 20 (5): 1223. DOI:10.3390/ijms20051223.
29. Chuffa LG, Fioruci-Fontanelli BA, Mendes LO, Seiva FRF, Martínez M, Fávaro WJ, Domeniconi RF, Pinheiro PFF, Dos Santos LD, Martínez FE (2015) Melatonin attenuates the TLR4-mediated inflammatory response through MyD88- and TRIF-dependent signaling pathways in an in vivo model of ovarian cancer. BMC Cancer 15: 34. DOI:10.1186/s12885-015-1032-4.
30. Chuffa LGA, Reiter RJ, Lupi LA (2017) Melatonin as a promising agent to treat ovarian cancer: molecular mechanisms. Carcinogenesis 38 (10): 945‐952. DOI:10.1093/carcin/bgx054
31. Zarezadeh M, Khorshidi M, Emami M, Janmohammadi P, Kord-Varkaneh H, Mousavi SM, Mohammed SH, Saedisomeolia A, Alizadeh S (2019) Melatonin supplementation and pro-inflammatory mediators: a systematic review and meta-analysis of clinical trials. Eur. J. Nutr. DOI: 10.1007/s00394-019-02123-0.
32. Favero G, Franco C, Stacchiotti A, Rodella LF, Rezzani R. Sirtuin1 (2020) Role in the melatonin protective effects against obesity-related heart injury. Front. Physiol. 11: 103. DOI: 10.3389/fphys.2020.00103. eCollection 2020.
33. Zarezadeh M, Khorshidi M, Emami M, Janmohammadi P, Kord-Varkaneh H, Mouvasi SM, Mohammed SH, Saedisomeolia A, Alizadeh S (2019) Melatonin supplementation and pro-inflammatory mediators: a systematic review and meta-analysis of clinical trials Eur. J. Nutr. 10.1007/s00394-019-02123-0. DOI:10.1007/s00394-019-02123-0.
34. Zhang Y, He F, Chen Z, Su Q, Yan M, Zhang Q, Tan J, Qian L, Han Y (2019) Melatonin modulates IL-1β-induced extracellular matrix remodeling in human nucleus pulposus cells and attenuates rat intervertebral disc degeneration and inflammation. Aging (Albany NY) 11 (22): 10499‐10512. DOI:10.18632/aging.102472.
35. Bourne RS, Mills GH (2006) Melatonin: possible implications for the postoperative and critically ill patient. Intensive Care Med. 32 (3): 371–379.
36. Rinaldi S, Landucci F, De Gaudio AR (2009) Antioxidant therapy in critically septic patients. Curr. Drug Targets 10 (9): 872‐880. DOI:10.2174/138945009789108774.
37. Sato K, Meng F, Francis H, Wu N, Chen L, Kennedy L, Zhou T, Franchitto A, Onori P, Gaudio E, Glaser S, Alpini G (2020) Melatonin and circadian rhythms in liver diseases: Functional roles and potential therapies. J. Pineal Res. 68 (3): e12639. DOI:10.1111/jpi.12639
38. Imenshahidi M, Karimi G, Hosseinzadeh H. (2020) Effects of melatonin on cardiovascular risk factors and metabolic syndrome: a comprehensive review. Naunyn Schmiedebergs Arch. Pharmacol. 393 (4): 521–536. DOI:10.1007/s00210-020-01822-4.
39. Gorbalenya AE, Snijder EJ, Spaan WJM (2004) Severe acute respiratory syndrome coronavirus phylogeny: toward consensus. J. Virol. 78: 7863–7866. DOI: 10.1128/JVI.78.15.7863-7866.2004.
40. Chan JF, Kok KH, Zhu Z, Chu H, To KK, Yuan S, Yuen KY (2020) Genomic characterization of the novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 9 (1): 221-236.
41. Fehr A.R., Perlman S (2015) Coronaviruses: An overview of their replication and pathogenesis. In: Maier H., Bickerton E., Britton P. (eds) Coronaviruses. Methods in Molecular Biology, vol 1282. Humana Press, New York, NY.
42. Estébanez B, de Paz JA, Cuevas MJ, González-Gallego (2018) Endoplasmic reticulum unfolded protein response, aging and exercise: an update. Front. Physiol. 9:1744. DOI:10.3389/fphys.2018.01744.
43. Salminen A, Kaarniranta K, Kauppinen A (2020) ER stress activates immunosuppressive network: implications for aging and Alzheimer's disease J. Mol. Med. (Berl.) 10.1007/s00109-020-01904-z. DOI:10.1007/s00109-020-01904-z.
44. Zhao A, Zhang Z, Zhou Y, Li X, Li X, Li B, Ma B, Zhang Q (2020) β-Elemonic acid inhibits the growth of human Osteosarcoma through endoplasmic reticulum (ER) stress-mediated PERK/eIF2α/ATF4/CHOP activation and Wnt/β-catenin signal suppression Phytomedicine. 69: 153183. DOI:10.1016/j.phymed.2020.153183.
45. Uddin MJ, Dorotea D, Pak ES, Ha H (2020) Fyn kinase: A potential therapeutic target in acute kidney injury. Biomol. Ther. (Seoul) 28 (3): 213‐221. DOI:10.4062/biomolther.2019.214.
46. Kuss-Duerkop SK, Keestra-Gounder AM (2020) NOD1 and NOD2 activation by diverse stimuli: a possible role for sensing pathogen-induced ER stress. Infect. Immun. IAI.00898-19. DOI:10.1128/IAI.00898-19.
47. Sprooten J, Garg AD (2020) Type I interferons and endoplasmic reticulum stress in health and disease. Int. Rev. Cell. Mol. Biol. 350: 63–118. DOI: 10.1016/bs.ircmb.2019.10.004.
48. Gorbatyuk MS, Starr CR, Gorbatyuk OS (2020) Endoplasmic reticulum stress: New insights into the pathogenesis and treatment of retinal degenerative diseases Prog. Retin. Eye Res. 100860: DOI:10.1016/j.preteyeres.2020.100860.
49. Almanza A, Carlesso, A, Chintha C, Creedican S, Doultsinos D, Leuzzi B, Luıs A, McCarthy N, Montibeller L, More S, Papaioannou A, Püschel F, Sassano ML, Skoko J, Agostinis P, de Belleroche J, Eriksson LA, Fulda S, Gorman AM, Healy S, Kozlov A, Muñoz-Pinedo C, Rehm M, Chevet E, Samali A (2019) Endoplasmic reticulum stress signalling—from basic mechanisms to clinical applications. FEBS J. 286: 241–278.
50. Oka OB van Lith M, Rudolf J, Tungkum W, Pringle MA, Bulleid NJ (2019) ERp18 regulates activation of ATF6α during unfolded protein response. EMBO J. 38: e100990. DOI: 10.15252/embj.2018100990
51. Kolpikova EP, Tronco AR, Hartigh ABD, Jackson KJ, Iwawaki T, Fink SL (2020) IRE1α promotes Zika virus infection via XBP1. Viruses 12 (3): 278. DOI:10.3390/v12030278
52. Lee MK, Hyeon S, Ahn JH (2020) Loss of VCP/p97 induced by the human cytomegalovirus transmembrane protein pUL50 and its regulation by a small isoform of pUL50. J. Virol. JVI.00110-20. DOI:10.1128/JVI.00110-20.
53. Lewy TG, Offerdahl DK, Grabowski JM, Kellman E, Mlera L, Chiramel A, Bloom ME (2020) PERK-mediated unfolded protein response signaling restricts replication of the tick-borne flavivirus langat virus. Viruses 12 (3): 328. DOI:10.3390/v12030328.
54. Ríos-Ocampo WA, Navas MC, Buist-Homan M, Faber KN, Daemen T, Moshage H (2020) Hepatitis C virus proteins core and NS5A are highly sensitive to oxidative stress-induced degradation after eIF2α/ATF4 pathway activation. Viruses. 12 (4): E425. DOI:10.3390/v12040425.
55. Shinjo, S, Mizotani, Y, Tashiro, E, Imoto, M (2013) Comparative analysis of the expression patterns of UPR-target genes caused by UPR-inducing compounds. Biosci. Biotechnol. Biochem. 77: 729–735. DOI: 10.1271/bbb.120812.
56. Elfiky AA (2020) Ebola virus glycoprotein GP1-host cell-surface HSPA5 binding site prediction. Cell Stress Chaperones. 10.1007/s12192-020-01106-z. DOI:10.1007/s12192-020-01106-z
57. Prasad V, Suomalainen M, Jasiqi Y, Hemmi S, Hearing P, Burgert H-G, Greber UF (2020) The UPR sensor IRE1α and the adenovirus E3-19K glycoprotein sustain persistent and lytic infections. Nat. Commun. 11 (1): 1997. DOI:10.1038/s41467-020-15844-2.
58. Lim YX, Ng YL, Tam JP, Liu DX (2016) Human coronaviruses: a review of virus-host interactions. Diseases 4 (3): 26. DOI: 10.3390/diseases4030026.
59. De Diego ML, Nieto-Torres J.L., Jimenez-Guardeño J.M., Regla-Nava J.A., Álvarez E., Oliveros J.C., Zhao J., Fett C., Perlman S., Enjuanes L (2011) Severe acute respiratory syndrome coronavirus envelope protein regulates cell stress response and apoptosis. PloS Pathog. 7 (10): e1002315. DOI: 10.1371/journal.ppat.1002315. Epub 2011 Oct 20.
60. Lin CH, Nicol CJB, Chen Y-Ch, Chen S-J, Yen Ch-H, Huang R-N, Chiang M-Ch (2018) Rosiglitazone rescues human neural stem cells from amyloid-beta induced ER stress via PPARγ dependent signaling. Exp. Cell Res. 370 (2): 312‐321. DOI:10.1016/j.yexcr.2018.06.033.
61. de Luxán-Delgado B, Potes Y, Rubio-González A, Caballero B, Solano JJ, Fernández-Fernández M, Bermúdez M, Rodrigues Moreira Guimarães M, Vega-Naredo I, Boga JA, Coto-Montes A (2016) Melatonin reduces endoplasmic reticulum stress and autophagy in liver of leptin-deficient mice. J. Pineal Res. 61 (1): 108‐123. DOI:10.1111/jpi.12333.
62. Zhang J, Wang L, Xie W, Hu S, Zhou H, Zhu P, Zhu H (2020) Melatonin attenuates ER stress and mitochondrial damage in septic cardiomyopathy: A new mechanism involving BAP31 upregulation and MAPK-ERK pathway. J. Cell Physiol. 235 (3): 2847‐2856. DOI:10.1002/jcp.29190.
63. Shi C, Zeng J, Li Z, Chen Q, Hang W, Xia L, Wu Y, Chen J, Shi A (2018) Melatonin mitigates kainic acid-induced neuronal tau hyperphosphorylation and memory deficits through alleviating ER stress. Front. Mol. Neurosci. 11: 5. DOI:10.3389/fnmol.2018. 00005.
64. Bermejo-Millo JC, Guimarães MRM, de Luxán-Delgado B, Potes Y, Perez-Martinez Z, Diaz-Luis A, Caballero B, Solano JJ, Vega-Naredo I, Coto-Montes A (2018) High-Fructose Consumption Impairs the Redox System and Protein Quality Control in the Brain of Syrian Hamsters: Therapeutic Effects of Melatonin. Mol. Neurobiol. 55 (10): 7973‐7986. DOI:10.1007/s12035-018-0967-2.
65. Rubio-González A, Bermejo-Millo JC, de Luxán-Delgado B, Potes Y, Perez-Martinez Z, Boga JA, Vega-Naredo I, Caballero B, Solano JJ, Coto-Montes A (2018) Melatonin prevents the harmful effects of obesity on the brain, including at the behavioral level. Mol. Neurobiol. 55 (7): 5830‐5846. DOI:10.1007/s12035-017-0796-8.
66. Kleber A, Kubulus D, Rössler D, Wolf B, Volk T, Speer T, Fink T (2014) Melatonin modifies cellular stress in the liver of septic mice by reducing reactive oxygen species and increasing the unfolded protein response. Exp. Mol. Pathol. 97 (3): 565‐571. DOI:10.1016/j.yexmp.2014.10.009.
67. Ouyang H, Zhong J, Lu J, Zhong Y, Hu Y, Tan Y (2019) Inhibitory effect of melatonin on Mst1 ameliorates myocarditis through attenuating ER stress and mitochondrial dysfunction. J. Mol. Histol. 50 (5): 405–415. DOI:10.1007/s10735-019-09836-w.
68. Tuñón MJ, San-Miguel B, Crespo I, Laliena A, Vallejo D, Álvarez M, Prieto J, González-Gallego J (2013) Melatonin treatment reduces endoplasmic reticulum stress and modulates the unfolded protein response in rabbits with lethal fulminant hepatitis of viral origin. J. Pineal Res. 55 (3): 221–222DOI:10.1111/jpi.12063.
69. Estornes, Y, Aguileta, MA, Dubuisson, C, De Keyser, J, Goossens, V, Kersse, K, Samali, A, Vandenabeele, P, Bertrand, MJM (2014) RIPK1 promotes death receptor-independent caspase-8-mediated apoptosis under unresolved ER stress conditions. Cell Death Dis. 6 (6): e1798. DOI: 10.1038/cddis.2015.175.
70. Fiege JK, Stone IA, Dumm RE, Waring BM, Fife BT, Agudo j, Brown BD, Heaton NS, Langlois RA (2019) Long-term surviving influenza infected cells evade CD8+ T cell mediated clearance. PLoS Pathog. 15 (9): e1008077.DOI:10.1371/journal.ppat.1008077.
71. Takezaki A, Tsukumo SI, Setoguchi Y, Ledford JG, Goto H, Hosomichi K, Uehara H, Nishioka Y, Yasutomo K (2019) A homozygous SFTPA1 mutation drives necroptosis of type II alveolar epithelial cells in patients with idiopathic pulmonary fibrosis. J. Exp. Med. 216 (12): 2724‐2735. DOI:10.1084/jem.20182351.
72. Hsu YH, Cho LC, Wang LS, Chen LK, Lee JJ, Yang HH. (2006) Acute respiratory distress syndrome associated with rabies: a case report. Kaohsiung J. Med. Sci. 22 (2): 94‐98. DOI:10.1016/S1607-551X(09)70227-X.
73. Casola A, Burger N, Liu T, Jamaluddin M, Brasier AR, Garofalo RP. (2001) Oxidant tone regulates RANTES gene expression in airway epithelial cells infected with respiratory syncytial virus: Role in viral-induced interferon regulatory factor activation. J. Biol. Chem. 276 (23): 19715‐19722. DOI:10.1074/jbc.M101526200.
74. Richter K and Kietzmann T (2016) Reactive oxygen species and fibrosis: further evidence of a significant liaison. Cell Tissue Res. 365: 591–605.
75. Khomich OA, Kochetkov SN, Bartosch B, and Ivanov AV (2018) Redox biology of respiratory viral infections. Viruses 10 (8): 392.
76. Kropski JA, Blackwell TS (2018) Endoplasmic reticulum stress in the pathogenesis of fibrotic disease. J. Clin. Invest. 128 (1): 64‐73. DOI:10.1172/JCI93560.
77. Abouhashem AS, Singh K, Azzazy HME, Sen CK (2020) Is low alveolar type II cell SOD3 in the lungs of elderly linked to the observed severity of COVID-19? Antioxid. Redox Signal. 10.1089/ars.2020.8111. DOI:10.1089/ars.2020.8111
78. Borok Z, Horie M, Flodby P, Wang H, Liu Y, Ganesh S, Firth AL, Minoo P, Li C, Beers MF, Lee AS, Zhou B (2020) Grp78 loss in epithelial progenitors reveals an age-linked role for endoplasmic reticulum stress in pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 201 (2): 198‐211. DOI:10.1164/rccm.201902-0451OC.
79. Tanaka K, Ishihara T, Azuma A, Kudoh S, Ebina M, Nukiwa T, Sugiyama Y, Tasaka Y, Namba T, Ishihara T, Sato K, Mizushima Y, and Mizushima T (2010) Therapeutic effect of lecithinized superoxide dismutase on bleomycin-induced pulmonary fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 298: L348–L360.
80. Mileva M, Dimitrova A, Krastev D, Alexandrova A, Tsvetanova E, Georgieva A, Galabov A (2020) Oseltamivir and S-adenosyl-l-methionine combination as effective therapeutic strategy for suppression of oxidative damage in lung caused by influenza virus infection in mice. Drug Res. (Stuttg). 10.1055/a-1147-8824. DOI:10.1055/a-1147-8824.
81. He B, Zhang W, Qiao J, Peng Z, Chai X (2019) Melatonin protects against COPD by attenuating apoptosis and endoplasmic reticulum stress via upregulating SIRT1 expression in rats. Can. J. Physiol. Pharmacol. 97 (5): 386‐391. DOI:10.1139/cjpp-2018-0529.
82. He B, Chen Q, Zhou D, Wang L, Liu Z (2019) Role of reciprocal interaction between autophagy and endoplasmic reticulum stress in apoptosis of human bronchial epithelial cells induced by cigarette smoke extract. IUBMB Life 71 (1): 66‐80. DOI:10.1002/iub.1937.
83. Magrone T, Magrone M, Jirillo E (2020) Focus on receptors for coronaviruses with special reference to angiotensin-converting enzyme 2 as a potential drug target - a perspective. Endocr. Metab. Immune Disord. Drug Targets. 10.2174/1871530320666200427112902. DOI:10.2174/1871530320666200427112902.
84. Zou X, Chen K, Zou J, Han P, Hao J, Han Z (2020) Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 14 (2): 185‐192. DOI:10.1007/s11684-020-0754-0.
85. Farnoosh G, Ghanei M, Khorramdelazad H, Alishiri G, Farahani AJ, Shahriary A, Zijoud SRH (2020) Are Iranian Sulfur mustard-exposed survivors more vulnerable to SARS-CoV-2: some similarity in their pathogenesis. Disaster Med. Public Health Prep. 1‐12. DOI:10.1017/dmp.2020.156.
86. Dalamaga M, Karampela I, Mantzoros CS (2020) Commentary: Could iron chelators prove to be useful as an adjunct to COVID-19 treatment regimens? Metabolism. 108: 154260. DOI:10.1016/j.metabol.2020.154260.
87. Messina G, Polito R, Monda V, Cipolloni L, Nunno ND, Mizio GD, Murabito P, Carotenuto M, Messina A, Pisanelli D, Valenzano A, Cibelli G, Scarinci A, Monda M, Sessa F (2020) Functional role of dietary intervention to improve the outcome of COVID-19: A hypothesis of work. Int. J. Mol. Sci. 21 (9): E3104. DOI:10.3390/ijms21093104.
88. Shneider A, Kudriavtsev A, Vakhrusheva A (2020) Can melatonin reduce the severity of COVID-19 pandemic? Int. Rev. Immunol. 1‐10. DOI:10.1080/08830185.2020.1756284.
89. Scriven P, Brown NJ, Pockley AG, Wyld L (2007) The unfolded protein response and cancer: a brighter future unfolding? J. Mol. Med. 85: 331-341. DOI: 10.1007/s00109-006-0150-5.
90. Lin JH, Walter P, Yen TS (2008) Endoplasmic reticulum stress in disease pathogenesis. Annu. Rev. Pathol. 3: 399- 425.
91. Yorimitsu T, Nair U, Yang Z, Klionsky DJ (2006) Endoplasmic reticulum stress triggers autophagy. J. Biol. Chem. 281 (40): 30299‐30304. DOI:10.1074/jbc.M607007200.
92. Allen EA, Baehrecke EH (2020) Autophagy in animal development. Cell Death Differ. 27 (3): 903‐918. DOI:10.1038/s41418-020-0497-0.
93. Horton R, Wileman T, Rushworth SA (2020) Autophagy driven extracellular vesicles in the leukaemic microenvironment Curr. Cancer Drug Targets 10.2174/1568009620666200428111051. DOI:10.2174/1568009620666200428111051.
94. He C, Klionsky DJ (2009) Regulation mechanisms and signaling pathways of autophagy. Annu. Rev. Genet. 43: 67‐93. DOI:10.1146/annurev-genet-102808-114910.
95. Kulkarni AS, Gubbi S, Barzilai N (2020) Benefits of metformin in attenuating the hallmarks of aging. Cell Metab. S1550-4131(20)30183-2. DOI:10.1016/j.cmet.2020.04.001.
96. Yorimitsu T, Klionsky DJ (2007) Endoplasmic reticulum stress: a new pathway to induce autophagy. Autophagy 3 (2): 160‐162. DOI:10.4161/auto.3653.
97. Wang D, Zhang P, Xu X, Wang J, Wang D, Peng P, Zheng C, Meng Q-J, Yang L, Luo Z (2019) Knockdown of cytokeratin 8 overcomes chemoresistance of chordoma cells by aggravating endoplasmic reticulum stress through PERK/eIF2α arm of unfolded protein response and blocking autophagy. Cell Death Dis. 10 (12): 887. DOI: 10.1038/s41419-019-2125-9.
98. Maeyashiki C, Melhem H, Hering L, Baebler K, Cosin-Roger J, Schefer F, Weder B, Hausmann M, Scharl M, Rogler G, de Vallière C, Ruiz PA (2020) Activation of pH-sensing receptor OGR1 (GPR68) induces ER stress via the IRE1α/JNK pathway in an intestinal epithelial cell model. Sci. Rep. 10 (1): 1438. DOI:10.1038/s41598-020-57657-9.
99. Qin L, Wang Z, Tao L, Wang Y (2010) ER stress negatively regulates AKT/TSC/mTOR pathway to enhance autophagy. Autophagy 6 (2): 239‐247. DOI:10.4161/auto.6.2.11062.
100. Dong X and Levine B (2013) Autophagy and viruses: adversaries or allies? J. Innate Immun. 5: 480–493. DOI: 10.1159/000346388.
101. Paulus GL, Xavier RJ (2015) Autophagy and checkpoints for intracellular pathogen defense. Curr. Opin. Gastroenterol. 31: 14‐23. DOI: 10.1097/MOG.0000000000000134.
102. Orvedahl A, MacPherson S, Sumpter R Jr, Talloczy Z, Zou Z, Levine B (2010) Autophagy protects against Sindbis virus infection of the central nervous system. Cell Host. Microbe 7: 115‐127. DOI: 10.1016/j.chom.2010.01.007.
103. Huang SC, Chang CL, Wang PS, Tsai Y, Liu HS (2009) Enterovirus 71‐induced autophagy detected in vitro and in vivo promotes viral replication. J. Med. Virol. 81: 1241‐1252. DOI: 10.1016/j.chom.2010.01.007.
104. O'Donnell V, Pacheco JM, LaRocco M, Burrage T, Jackson W, Rodriguez LL, Borca MV, Baxt B (2011) Foot‐and‐mouth disease virus utilizes an autophagic pathway during viral replication. Virology 410: 142‐150. DOI: 10.1016/j.virol.2010.10.042.
105. Granato M, Santarelli R, Farina A, Gonnella R, Lotti LV, Faggioni A, Cirone M (2014) Epstein‐barr virus blocks the autophagic flux and appropriates the autophagic machinery to enhance viral replication. J. Virol. 88: 12715‐12726. DOI: 10.1128/JVI.02199-14.
106. Casais R, Molleda LG, Machín A, del Barrio G, García Manso A, Dalton KP, Coto A, Martin Alonso JM, Prieto M, Parra F (2008) Structural and functional analysis of virus factories purified from rabbit vesivirus-infected Vero cells. Virus Res. 137 (1): 112‐121. DOI:10.1016/j.virusres.2008.06.009.
107. McLean JE, Wudzinska A, Datan E, Quaglino D, Zakeri Z (2011) Flavivirus NS4A‐induced autophagy protects cells against death and enhances virus replication. J. Biol. Chem. 286: 22147‐22159. DOI: 10.1074/jbc.M110.192500.
108. Jackson WT, Giddings TH Jr, Taylor MP, Mulinyawe S, Rabinovitch M, Kopito RR, Kirkegaard K (2005) Subversion of cellular autophagosomal machinery by RNA viruses. PLoS Biol. 3: e156. DOI: 10.1371/journal.pbio.0030156.
109. Chen X, Wang K, Xing Y, Tu J, Yang X, Zhao Q, Li K, Chen Z (2014) Coronavirus membrane-associated papain-like proteases induce autophagy through interacting with Beclin1 to negatively regulate antiviral innate immunity. Protein Cell. 5: 912-27. DOI: 10.1007/s13238-014-0104-6.
110. Gassen NC, Niemeyer D, Muth D, Corman VM, Martinelli S, Gassen A, Hafner K, Papies J, Mösbauer K, Zellner A, Zannas AS, Herrmann A, Holsboer F, Brack-Werner R, Boshart M, Müller-Myhsok B, Drosten C, Müller MA, Rein T (2019) SKP2 attenuates autophagy through Beclin1-ubiquitination and its inhibition reduces MERS-Coronavirus infection. Nat. Commun. 10 (1): 5770. DOI:10.1038/s41467-019-13659-4.
111. Prentice E, McAuliffe J, Lu X, Subbarao K, Denison MR (2004a) Identification and characterization of severe acute respiratory syndrome coronavirus replicase proteins. J. Virol. 78: 9977-86.
112. Prentice E, Jerome WG, Yoshimori T, Mizushima N, Denison MR (2004b) Coronavirus replication complex formation utilizes components of cellular autophagy. J. Biol. Chem. 279: 10136-10141. DOI: 10.1074/jbc.M306124200.
113. Yang N, Shen HM (2020) Targeting the endocytic pathway and autophagy process as a novel therapeutic strategy in COVID-19. Int. J. Biol. Sci. 16 (10): 1724‐1731. DOI:10.7150/ijbs.45498.
114. Shibutani ST, Saitoh T, Nowag H, Munz C, Yoshimori T (2015) Autophagy and autophagy-related proteins in the immune system. Nat. Immunol. 16: 1014–1024. DOI: 10.1038/ni.3273.
115. Rathinam VA, Vanaja SK, Fitzgerald KA (2012) Regulation of inflammasome signaling. Nat. Immunol. 13: 333–342. DOI: 10.1038/ni.2237.
116. Jang YJ, Kim JH, Byun S (2019) Modulation of autophagy for controlling immunity. Cells 8 (2): 138. DOI:10.3390/cells8020138.
117. San-Miguel B, Crespo I, Vallejo D, Álvarez M, Prieto J, Gonzalez-Gallego J, Tuñón MJ (2014) Melatonin modulates the autophagic response in acute liver failure induced by the rabbit hemorrhagic disease virus. J. Pineal Res. 56 (3): 313–321. DOI:10.1111/jpi.12124.
118. Vega-Naredo I, Caballero B, Sierra V, García-Macia M, de Gonzalo- Calvo D, Oliveira PJ, Rodríguez-Colunga MJ, Coto-Montes A (2012) Melatonin modulates autophagy through a redox-mediated action in female Syrian hamster Harderian gland controlling cell types and gland activity. J. Pineal Res. 52 (1): 80‐92. DOI:10.1111/j.1600-079X.2011.00922.x.
119. Maiese K (2020) The mechanistic target of rapamycin (mTOR): Novel considerations as an antiviral treatment and possibilities for COVID-19 Curr. Neurovasc. Res. 10.2174/1567202617666200425205122. DOI:10.2174/1567202617666200425205122.
120. Tan, DX, Hardeland R (2020) Potential utility of melatonin in deadly infectious diseases related to the overreaction of innate immune response and destructive inflammation: focus on COVID-19. Melatonin Res. 3 (1): 120-143. DOI:10.32794/mr11250052.
121. Zhang R, Wang X, Ni L, Di X, Ma B, Niu S, Liu C, Reiter RJ (2020) COVID-19: Melatonin as a potential adjuvant treatment. Life Sci. 250: 117583. DOI:10.1016/j.lfs.2020.117583.
122. Shneider A, Kudriavtsev A, Vakhrusheva A (2020) Can melatonin reduce the severity of COVID-19 pandemic? Int. Rev. Immunol. 1‐10. DOI:10.1080/08830185.2020.1756284
123. Martín Giménez VM, Inserra F, Tajer CD, Mariani J, Ferder L, Reiter RJ, Manucha W (2020) Lungs as target of COVID-19 infection: Protective common molecular mechanisms of vitamin D and melatonin as a new potential synergistic treatment. Life Sci. 254: 117808. DOI:10.1016/j.lfs.2020.117808.
124. Zhou Y, Hou Y, Shen J, Huang Y, Martin W, Cheng F (2020) Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov. 6: 14. DOI:10.1038/s41421-020-0153-3.
125. Anderson G, Reiter RJ (2020) Melatonin: Roles in influenza, Covid-19, and other viral infections. Rev. Med. Virol. 30 (3): e2109. DOI:10.1002/rmv.2109.
126. Reiter RJ, Sharma R, Ma Q, Dominquez-Rodriguez A, Marik PE, Abreu-Gonzalez P (2020) Melatonin inhibits COVID-19-induced cytokine storm by reversing aerobic glycolysis in immune cells: a mechanistic analysis. Med. Drug Discov. 6: 100044. DOI:10.1016/j.medidd.2020.100044.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


