Pleiotropic role of melatonin in brain mitochondria of obese mice
Melatonin on obese brain mitochondria
Abstract
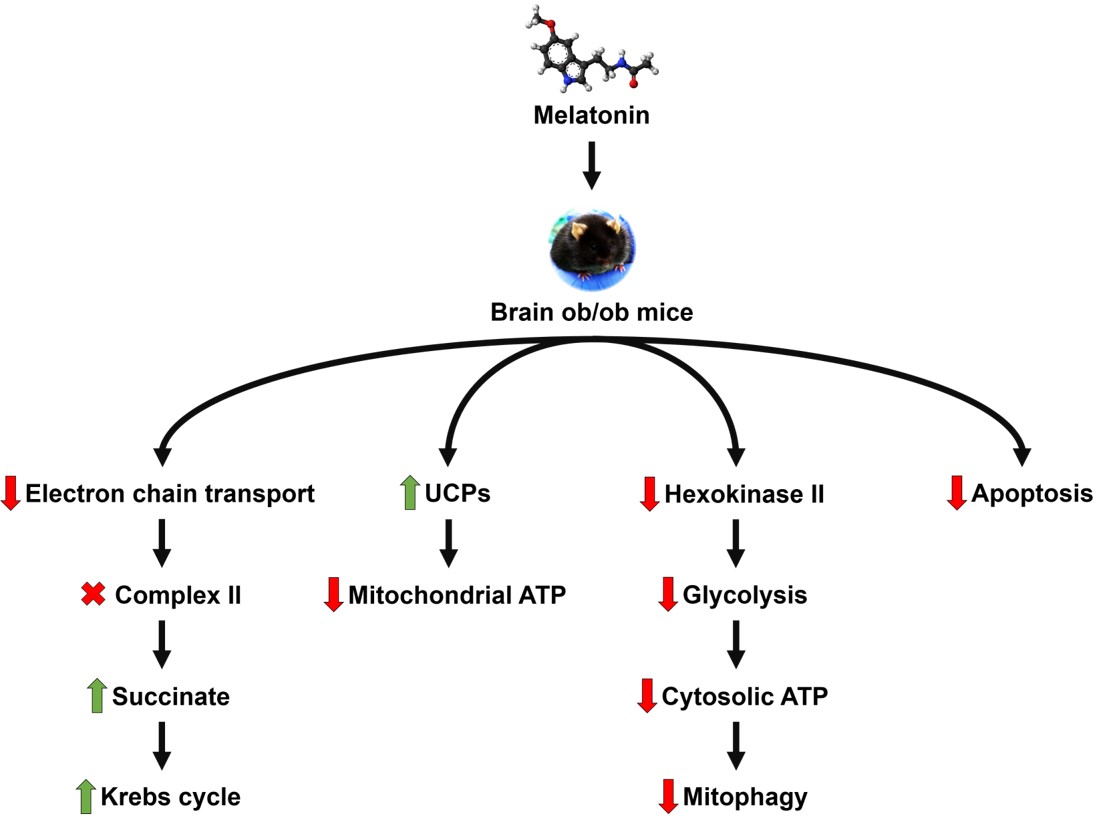
Obesity induced by a leptin deficiency causes extensive damage in brain due to a large increase in oxidative stress. Mitochondria have a central role in this neural damage. Leptin receptors have a wide expression in the brain and its absence is associated with reduced mitochondrial respiration by a decoupled electron transport chain and a significantly increased complex II activity which is major player in mitochondrial free radical generation. The consequences are an abrupt reduction in ATP production and a reduced activity of the tricarboxylic acid (TCA) cycle, evidence of dysfunction of processes related to energy production, the reduction of which contributes to multiple brain pathologies. Melatonin, a major protector of mitochondria against free radicals with a significant influence on glucose metabolism, has been shown to counteract these conditions. In the present study the main respirasome expression was recovered by melatonin, with a reduction in complex II activity and the complex II dependent free radical generation. Additionally, melatonin normalized the TCA cycle. Reduction in ATP synthesis was caused by UCP2 activation. The uninterrupted sensation of starvation due to leptin deficiency involves impairments in glucose metabolism, which was reversed by melatonin via negatively acting on hexokinase II, a key regulator of glycolysis and major contributor to the Warburg effect. Hexokinase II reduction was accompanied by a significant Bcl-2 reduction, inducing a delicate readjustment of pro and anti-apoptotic proteins in the mitochondria to preserve cell survival, which was associated with a marked reduction of the Bax activator, Puma, observed in obese animals treated by melatonin.
References
2. Tan X, et al. (2012) Leptin deficiency contributes to the pathogenesis of alcoholic fatty liver disease in mice. Am. J. Pathol. 181 (4): 1279-1286.
3. Jo YH, Buettner C (2014) Why leptin keeps you warm. Mol. Metab. 3 (8): 779-780.
4. Holmstrom MH, Tom RZ, Bjornholm M, Garcia-Roves PM, Zierath JR (2013) Effect of leptin treatment on mitochondrial function in obese leptin-deficient ob/ob mice. Metabolism 62 (9): 1258-1267.
5. Lee TH, Cheng KK, Hoo RL, Siu PM, Yau SY (2019) The novel perspectives of adipokines on brain health. Int. J. Mol. Sci. 20 (22): 5638.
6. Kershaw EE, Flier JS (2004) Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 89 (6): 2548-2556.
7. Harvey J, Solovyova N, Irving A (2006) Leptin and its role in hippocampal synaptic plasticity. Prog. Lipid Res. 45 (5): 369-378.
8. Leuner B, Gould E (2010) Structural plasticity and hippocampal function. Annu. Rev. Psychol. 61: 111-140,C111-113.
9. Tan DX, Xu B, Zhou X, Reiter RJ (2018) Pineal calcification, melatonin production, aging, associated health consequences and rejuvenation of the pineal gland. Molecules 23 (2): 301.
10. Spinedi E, Cardinali DP (2019) Neuroendocrine-metabolic dysfunction and sleep disturbances in neurodegenerative disorders: focus on Alzheimer's disease and melatonin. Neuroendocrinology 108 (4): 354-364.
11. Wolden-Hanson T, et al. (2000) Daily melatonin administration to middle-aged male rats suppresses body weight, intraabdominal adiposity, and plasma leptin and insulin independent of food intake and total body fat. Endocrinology 141 (2): 487-497.
12. Prunet-Marcassus B, et al. (2003) Melatonin reduces body weight gain in Sprague Dawley rats with diet-induced obesity. Endocrinology 144 (12): 5347-5352.
13. Alonso-Vale MI, et al. (2005) Melatonin enhances leptin expression by rat adipocytes in the presence of insulin. Am. J. Physiol. Endocrinol. Metab. 288 (4): E805-812.
14. Yang W, Tang K, Wang Y, Zhang Y, Zan L (2017) Melatonin promotes triacylglycerol accumulation via MT2 receptor during differentiation in bovine intramuscular preadipocytes. Sci. Rep. 7 (1): 15080.
15. Buonfiglio D, et al. (2018) Melatonin absence leads to long-term leptin resistance and overweight in rats. Front. Endocrinol. (Lausanne). 9: 122.
16. Alghamdi BS (2018) The neuroprotective role of melatonin in neurological disorders. J. Neurosci. Res. 96 (7): 1136-1149.
17. Shukla M, Chinchalongporn V, Govitrapong P, Reiter RJ (2019) The role of melatonin in targeting cell signaling pathways in neurodegeneration. Ann. N. Y. Acad. Sci. 1443 (1): 75-96.
18. Nelson RJ, Chbeir S (2018) Dark matters: effects of light at night on metabolism. Proc. Nutr. Soc. 77 (3): 223-229.
19. Rubio-Gonzalez A, et al. (2018) Melatonin prevents the harmful effects of obesity on the brain, including at the behavioral level. Mol. Neurobiol. 55 (7): 5830-5846.
20. de Luxan-Delgado B, et al. (2014) Melatonin administration decreases adipogenesis in the liver of ob/ob mice through autophagy modulation. J. Pineal Res. 56 (2): 126-133.
21. de Luxan-Delgado B, et al. (2016) Melatonin reduces endoplasmic reticulum stress and autophagy in liver of leptin-deficient mice. J. Pineal Res. 61 (1): 108-123.
22. Anjum I, Fayyaz M, Wajid A, Sohail W, Ali A (2018) Does obesity increase the risk of dementia: A literature review. Cureus 10 (5): e2660.
23. McLean FH, et al. (2019) A high-fat diet induces rapid changes in the mouse hypothalamic proteome. Nutr. Metab. (Lond) 16: 26.
24. Roberts DJ, Tan-Sah VP, Ding EY, Smith JM, Miyamoto S (2014) Hexokinase-II positively regulates glucose starvation-induced autophagy through TORC1 inhibition. Mol. Cell 53 (4): 521-533.
25. Roberts DJ, Miyamoto S (2015) Hexokinase II integrates energy metabolism and cellular protection: Akting on mitochondria and TORCing to autophagy. Cell Death Differ. 22 (2): 248-257.
26. Tan VP, et al. (2019) Dissociation of mitochondrial HK-II elicits mitophagy and confers cardioprotection against ischemia. Cell Death Dis. 10 (10): 730.
27. de Mello AH, Costa AB, Engel JDG, Rezin GT (2018) Mitochondrial dysfunction in obesity. Life Sci. 192: 26-32.
28. Nedergaard J, Ricquier D, Kozak LP (2005) Uncoupling proteins: current status and therapeutic prospects. EMBO Rep. 6 (10): 917-921.
29. Garrett RH, Grisham CM (2013) Biochemistry Fifth Edition, International ed. p668.
30. Giralt M, Villarroya F (2017) Mitochondrial uncoupling and the regulation of glucose homeostasis. Curr. Diabetes Rev. 13 (4): 386-394.
31. da-Silva WS, et al. (2004) Mitochondrial bound hexokinase activity as a preventive antioxidant defense: steady-state ADP formation as a regulatory mechanism of membrane potential and reactive oxygen species generation in mitochondria. J. Biol. Chem. 279 (38): 39846-39855.
32. Jong CJ, Yeung J, Tseung E, Karmazyn M (2019) Leptin-induced cardiomyocyte hypertrophy is associated with enhanced mitochondrial fission. Mol. Cell Biochem. 454 (1-2): 33-44.
33. Reiter RJ, et al. (2017) Melatonin as a mitochondria-targeted antioxidant: one of evolution's best ideas. Cell. Mol. Life Sci. 74 (21): 3863-3881.
34. Reiter RJ, et al. (2018) Mitochondria: Central organelles for melatonin's antioxidant and anti-aging actions. Molecules 23 (2): 509.
35. Mortezaee K, et al. (2019) Modulation of apoptosis by melatonin for improving cancer treatment efficiency: An updated review. Life Sci. 228: 228-241.
36. Pastorino JG, Shulga N, Hoek JB (2002) Mitochondrial binding of hexokinase II inhibits Bax-induced cytochrome c release and apoptosis. J. Biol. Chem. 277 (9): 7610-7618.
37. Robey RB, Hay N (2006) Mitochondrial hexokinases, novel mediators of the antiapoptotic effects of growth factors and Akt. Oncogene 25 (34): 4683-4696.
38. Kaeidi A, Hajializadeh Z, Jahandari F, Fatemi I (2019) Leptin attenuates oxidative stress and neuronal apoptosis in hyperglycemic condition. Fundam. Clin. Pharmacol. 33 (1): 75-83.
39. Goldstein N, et al. (2019) Leptin stimulates autophagy/lysosome-related degradation of long-lived proteins in adipocytes. Adipocyte 8 (1): 51-60.
40. Dickson BM, Roelofs AJ, Rochford JJ, Wilson HM, De Bari C (2019) The burden of metabolic syndrome on osteoarthritic joints. Arthritis Res. Ther. 21 (1): 289.
41. Modzelewska P, Chludzinska S, Lewko J, Reszec J (2019) The influence of leptin on the process of carcinogenesis. Contemp. Oncol. (Pozn) 23 (2): 63-68.
42. Coto-Montes A, et al. (2012) Role of melatonin in the regulation of autophagy and mitophagy: a review. Mol. Cell Endocrinol. 361 (1-2): 12-23.
43. Boga JA, et al. (2019) Therapeutic potential of melatonin related to its role as an autophagy regulator: A review. J. Pineal Res. 66 (1): e12534.
44. Reiter RJ, Sharma R, Ma Q, Rorsales-Corral S, de Almeida Chuffa LG (2020) Melatonin inhibits Warburg-dependent cancer by redirecting glucose oxidation to the mitochondria: a mechanistic hypothesis. Cell. Mol. Life Sci. 77 (13): 2527-2542.
45. Tobore TO (2020) Towards a comprehensive theory of obesity and a healthy diet: The causal role of oxidative stress in food addiction and obesity. Behav. Brain Res. 384: 112560.
46. Formolo DA, et al. (2019) Deep Brain Stimulation for Obesity: A review and future directions. Front. Neurosci. 13: 323.
47. Silva AM, Oliveira PJ (2012) Evaluation of respiration with clark type electrode in isolated mitochondria and permeabilized animal cells. Meth. Mol. Biol. 810: 7-24.
48. Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analyt. Biochem. 72: 248-254.
49. Oliván M, et al. (2016) Identification of biomarkers of stress in meat of pigs managed under different mixing treatments. Brit. Biotechnol. J. 11: 1-13.
50. Vincent HK & Taylor AG (2006) Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. Int J Obes (Lond) 30 (3): 400-418.
51. Gu J, et al. (2016) The architecture of the mammalian respirasome. Nature 537 (7622): 639-643.
52. Bezawork-Geleta A, Rohlena J, Dong L, Pacak K, Neuzil J (2017) Mitochondrial complex II: at the crossroads. Trends Biochem. Sci. 42 (4): 312-325.
53. Lussey-Lepoutre C, et al. (2015) Loss of succinate dehydrogenase activity results in dependency on pyruvate carboxylation for cellular anabolism. Nat. Commun. 6: 8784.
54. Benaroya H (2020) Brain energetics, mitochondria, and traumatic brain injury. Rev. Neurosci. 31 (4): 363-390.
55. Giachin G, Bouverot R, Acajjaoui S, Pantalone S, Soler-Lopez M (2016) Dynamics of human mitochondrial complex I assembly: Implications for neurodegenerative diseases. Front. Mol. Biosci. 3: 43.
56. Holper L, Ben-Shachar D, Mann JJ (2019) Multivariate meta-analyses of mitochondrial complex I and IV in major depressive disorder, bipolar disorder, schizophrenia, Alzheimer disease, and Parkinson disease. Neuropsychopharmacology 44 (5): 837-849.
57. Fisar Z, et al. (2019) Activities of mitochondrial respiratory chain complexes in platelets of patients with Alzheimer's disease and depressive disorder. Mitochondrion 48: 67-77.
58. Pho H, et al. (2016) The effect of leptin replacement on sleep-disordered breathing in the leptin-deficient ob/ob mouse. J. Appl. Physiol. 120 (1): 78-86.
59. Yao Q, et al. (2016) Localizing effects of leptin on upper airway and respiratory control during sleep. Sleep 39 (5): 1097-1106.
60. Berger S, Polotsky VY (2018) Leptin and leptin resistance in the pathogenesis of obstructive sleep apnea: A possible link to oxidative stress and cardiovascular complications. Oxid. Med. Cell Longev. 2018: 5137947.
61. Hardeland R (2017) Melatonin and the electron transport chain. Cell. Mol. Life Sci. 74 (21): 3883-3896.
62. Reiter RJ, Ma Q, Sharma R (2020) Melatonin in mitochondria: Mitigating clear and present dangers. Physiology (Bethesda) 35 (2): 86-95.
63. Acuna-Castroviejo D, Escames G, Leon J, Carazo A, Khaldy H (2003) Mitochondrial regulation by melatonin and its metabolites. Adv. Exp. Med. Biol. 527: 549-557.
64. Hardeland R (2018) Recent findings in melatonin research and their relevance to the CNS. Cent. Nerv. Syst. Agents Med. Chem. 18 (2): 102-114.
65. Cipolla-Neto J, Amaral FG, Afeche SC, Tan DX, Reiter RJ (2014) Melatonin, energy metabolism, and obesity: a review. J. Pineal Res. 56 (4): 371-381.
66. Qian J, Scheer F (2016) Circadian system and glucose metabolism: implications for physiology and disease. Trends Endocrinol. Metab. 27 (5): 282-293.
67. Garaulet M, et al. (2020) Melatonin effects on glucose metabolism: Time to unlock the controversy. Trends Endocrinol. Metab. 31 (3): 192-204.
68. Liu K, et al. (2020) Scd1 controls de novo beige fat biogenesis through succinate-dependent regulation of mitochondrial complex II. Proc. Natl. Acad. Sci. USA 117 (5): 2462-2472.
69. Kluckova K, et al. (2015) Ubiquinone-binding site mutagenesis reveals the role of mitochondrial complex II in cell death initiation. Cell Death Dis. 6: e1749.
70. Navarro-Alarcon M, et al. (2014) Melatonin and metabolic regulation: A review. Food Funct. 5 (11): 2806-2832.
71. Bonomini F, Borsani E, Favero G, Rodella LF, Rezzani R (2018) Dietary melatonin supplementation could be a promising preventing/therapeutic approach for a variety of liver diseases. Nutrients 10 (9) 1135.
72. Fernandez Vazquez G, Reiter RJ, Agil A (2018) Melatonin increases brown adipose tissue mass and function in Zucker diabetic fatty rats: implications for obesity control. J. Pineal Res. 64 (4): e12472.
73. Warburg O, Dickens F, Kaiser B (1931) The Metabolism of Tumours: Investigations from the Kaiser Wilhelm Institute for Biology, Berlin-Dahlem. JAMA 96 (23):1982.
74. Stacpoole PW (2017) Therapeutic targeting of the pyruvate dehydrogenase complex/pyruvate dehydrogenase kinase (PDC/PDK) axis in cancer. J. Natl. Cancer Inst. 109 (11): djx071.
75. Sun L, Shukair S, Naik TJ, Moazed F, Ardehali H (2008) Glucose phosphorylation and mitochondrial binding are required for the protective effects of hexokinases I and II. Mol. Cell Biol. 28 (3): 1007-1017.
76. Murakawa T, et al. (2015) Bcl-2-like protein 13 is a mammalian Atg32 homologue that mediates mitophagy and mitochondrial fragmentation. Nat. Commun. 6: 7527.
77. Zhang Y, et al. (2019) Melatonin attenuates myocardial ischemia-reperfusion injury via improving mitochondrial fusion/mitophagy and activating the AMPK-OPA1 signaling pathways. J. Pineal Res. 66 (2): e12542.
78. Tait SW & Green DR (2013) Mitochondrial regulation of cell death. Cold Spring Harb Perspect Biol. 5 (9): a008706.
79. Reed JC (2008) Bcl-2-family proteins and hematologic malignancies: history and future prospects. Blood 111 (7): 3322-3330.
80. Tiong YL, Ng KY, Koh RY, Ponnudurai G, Chye SM (2019) Melatonin prevents oxidative stress-induced mitochondrial dysfunction and apoptosis in high glucose-treated schwann cells via upregulation of Bcl2, NF-kappaB, mTOR, wnt signalling pathways. Antioxidants (Basel) 8 (7): 198.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


