Melatonin receptor-mediated attenuation of excitotoxic cell death in cultured spinal cord slices
Melatonin receptor-mediated neuroprotection
Abstract
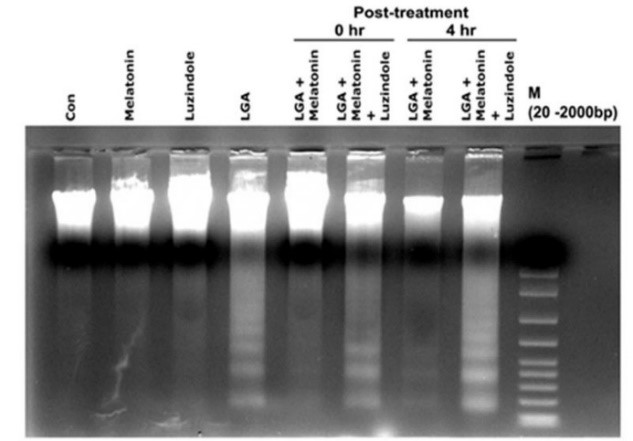
Recent studies suggest ex vivo modeling of neuronal injury is a robust approach for the mechanistic study of neurodegeneration. Melatonin, an indolamine, is a versatile molecule with antioxidative, antiapoptotic, neuroprotective, and anti-inflammatory properties. While melatonin has been studied as a therapeutic agent for spinal cord injury (SCI) related neuronal cell loss, its actions in organotypic slice cultures approximating SCI effects are less well understood. The actions of melatonin were therefore examined following exposure of cultured rat spinal cord slices to glutamate excitotoxicity. Exposure to glutamate (500 μM) for 4 hours induced neuronal degeneration that was prevented by 0.5 μM melatonin (applied immediately or 4 hours following glutamate exposure). Decreased internucleosomal DNA fragmentation, Bax:Bcl-2 and calpain:calpastatin ratios, caspase 8, 9 and 3 activities in slice cultures were measured following melatonin treatment. Melatonin receptor (MTR1, MTR2) mRNA levels were increased in the melatonin treated spinal cord slices. To confirm melatonin receptor-mediated protection, slice cultures were treated with 10 or 25 μM luzindole (melatonin receptor antagonist) at 0 and 4 hours, respectively, after glutamate exposure. Luzindole significantly decreased the ability of melatonin to prevent cell death in the sliced culture model. These results suggest melatonin receptors may provide a pathway for therapeutic applications to prevent penumbral neuron loss following SCI.
References
2. Fitch MT, Doller C, Combs CK, Landreth GE, Silver J (1999) Cellular and molecular mechanisms of glial scarring and progressive cavitation: in vivo and in vitro analysis of inflammation-induced secondary injury after CNS trauma. J. Neurosci. 19: 8182-8198.
3. Dumont RJ, Okonkwo DO, Verma S, Hurlbert RJ, Boulos PT, Ellegala DB, Dumont AS (2001) Acute spinal cord injury, part I: pathophysiologic mechanisms. Clin. Neuropharmacol. 24: 254-264.
4. Xu GY, Hughes MG, Zhang L, Cain L, McAdoo DJ (2005) Administration of glutamate into the spinal cord at extracellular concentrations reached post-injury causes functional impairments. Neurosci. lett. 384: 271-276.
5. Ray SK, Hogan EL, Banik NL (2003) Calpain in the pathophysiology of spinal cord injury: neuroprotection with calpain inhibitors. Brain Res. Rev. 42: 169-185.
6. Wu HY, Tomizawa K, Oda Y, Wei FY, Lu YF, Matsushita M, Li ST, Moriwaki A, Matsui H (2004) Critical role of calpain-mediated cleavage of calcineurin in excitotoxic neurodegeneration. J. Biol. Chem. 279: 4929-4940.
7. Blomgren K, Zhu C, Wang X, Karlsson JO, Leverin AL, Bahr BA, Mallard C, Hagberg H (2001) Synergistic activation of caspase-3 by m-calpain after neonatal hypoxia-ischemia: a mechanism of "pathological apoptosis"? J. Biol. Chem. 276: 10191-10198.
8. Wood DE, Thomas A, Devi LA, Berman Y, Beavis RC, Reed JC, Newcomb EW (1998) Bax cleavage is mediated by calpain during drug-induced apoptosis. Oncogene 17: 1069-1078.
9. Ray SK, Neuberger TJ, Deadwyler G, Wilford G, DeVries GH, Banik NL (2002) Calpain and calpastatin expression in primary oligodendrocyte culture: preferential localization of membrane calpain in cell processes. J. Neurosci. Res. 70: 561-569.
10. Nath R, Raser KJ, Stafford D, Hajimohammadreza I, Posner A, Allen H, Talanian RV, Yuen P, Gilbertsen RB, Wang KK (1996) Non-erythroid alpha-spectrin breakdown by calpain and interleukin 1 beta-converting-enzyme-like protease(s) in apoptotic cells: contributory roles of both protease families in neuronal apoptosis. Biochem. J. 319 ( Pt 3): 683-690.
11. Goll DE, Thompson VF, Li H, Wei W, Cong J (2003) The calpain system. Physiol. Rev. 83: 731-801.
12. Bevers MB, Neumar RW (2008) Neumar, Mechanistic role of calpains in postischemic neurodegeneration. J. Cereb. Blood Flow Metab. 28:655-673.
13. Vosler PS, Brennan CS, Chen J (2008) Calpain-mediated signaling mechanisms in neuronal injury and neurodegeneration. Mol. Neurobiol. 38: 78-100.
14. Sribnick EA, Ray SK, Banik NL (2006) Banik, Estrogen prevents glutamate-induced apoptosis in C6 glioma cells by a receptor-mediated mechanism. Neuroscience 137: 197-209.
15. Das A, Sribnick EA, Wingrave JM, Del Re AM, Woodward JJ, Appel SH, Banik NL, Ray SK (2005) Calpain activation in apoptosis of ventral spinal cord 4.1 (VSC4.1) motoneurons exposed to glutamate: calpain inhibition provides functional neuroprotection. J. Neurosci. Res. 81: 551-562.
16. McCracken E, Hunter AJ, Patel S, Graham DI, Dewar D (1999) Calpain activation and cytoskeletal protein breakdown in the corpus callosum of head-injured patients. J. Neurotrauma 16: 749-761.
17. Ray SK, Wilford GG, Crosby CV, Hogan EL, Banik NL (1999) Banik, Diverse stimuli induce calpain overexpression and apoptosis in C6 glioma cells. Brain Res. 829: 18-27.
18. Ray SK, Matzelle DD, Wilford GG, Hogan EL, Banik NL (2001) Cell death in spinal cord injury (SCI) requires de novo protein synthesis. Calpain inhibitor E-64-d provides neuroprotection in SCI lesion and penumbra. Ann. NY Acad. Sci. 939: 436-449.
19. Allegra M, Reiter RJ, Tan DX, Gentile C, Tesoriere L, Livrea MA (2003) The chemistry of melatonin's interaction with reactive species. J. Pineal Res. 34: 1-10.
20. Cuzzocrea S, Reiter RJ (2002) Pharmacological actions of melatonin in acute and chronic inflammation. Curr. Top. Med. Chem. 2: 153-165.
21. Guerrero JM, Reiter RJ (2002) Melatonin-immune system relationships. Curr. Top. Med. Chem. 2: 167-179.
22. Reiter RJ, Tan DX, Manchester LC, Qi W (2001) Biochemical reactivity of melatonin with reactive oxygen and nitrogen species: a review of the evidence. Cell. Biochem. Biophys. 34: 237-256.
23. Reiter RJ, Tan DX, Leon J, Kilic U, Kilic E (2005) When melatonin gets on your nerves: its beneficial actions in experimental models of stroke. Exp. Biol. Med. (Maywood) 230: 104-117.
24. Jou MJ, Peng TI, Reiter RJ, Jou SB, Wu HY, Wen ST (2004) Visualization of the antioxidative effects of melatonin at the mitochondrial level during oxidative stress-induced apoptosis of rat brain astrocytes. J. Pineal Res. 37: 55-70.
25. Sugai F, Yamamoto Y, Sakoda S (2004) [Organotypic spinal cord culture using mice]. Nihon Yakurigaku Zasshi 124: 19-23.
26. Das A, Belagodu A, Reiter RJ, Ray SK, Banik NL (2008) Cytoprotective effects of melatonin on C6 astroglial cells exposed to glutamate excitotoxicity and oxidative stress. J. Pineal Res. 45: 117-124.
27. Das A, McDowell M, Pava MJ, Smith JA, Reiter RJ, Woodward JJ, Varma AK, Ray SK, Banik NL (2010) The inhibition of apoptosis by melatonin in VSC4.1 motoneurons exposed to oxidative stress, glutamate excitotoxicity, or TNF-alpha toxicity involves membrane melatonin receptors. J. Pineal Res. 48: 157-169.
28. Tamtaji OR, Mirhosseini N, Reiter RJ, Azami A, Asemi Z (2019) Melatonin, a calpain inhibitor in the central nervous system: Current status and future perspectives. J. Cell Physiol. 234: 1001-1007.
29. Drexler B, Hentschke H, Antkowiak B, Grasshoff C (2010) Organotypic cultures as tools for testing neuroactive drugs - link between in-vitro and in-vivo experiments. Curr. Med. Chem. 17: 4538-4550.
30. Sundstrom L, Morrison B, 3rd, Bradley M, Pringle A (2005) Organotypic cultures as tools for functional screening in the CNS. Drug Discov. Today 10: 993-1000.
31. Samantaray S, Sribnick EA, Das A, Knaryan VH, Matzelle DD, Yallapragada AV, Reiter RJ, Ray SK, Banik NL (2008) Melatonin attenuates calpain upregulation, axonal damage and neuronal death in spinal cord injury in rats. J. Pineal Res. 44: 348-357.
32. Manchester LC, Coto-Montes A, Boga JA, Andersen LP, Zhou Z, Galano A, Vriend J, Tan DX, Reiter RJ (2015) Melatonin: an ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 59: 403-419.
33. Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre-Jimenez M, Qin L (2016) Melatonin as an antioxidant: under promises but over delivers. J. Pineal Res. 61: 253-278.
34. Benton RL, Ross CD, Miller KE (2000) Glutamine synthetase activities in spinal white and gray matter 7 days following spinal cord injury in rats. Neurosci. Lett. 291: 1-4.
35. Sribnick EA, Del Re AM, Ray SK, Woodward JJ, Banik NL (2009) Estrogen attenuates glutamate-induced cell death by inhibiting Ca2+ influx through L-type voltage-gated Ca2+ channels. Brain Res. 1276: 159-170.
36. Gumuslu E, Mutlu O, Sunnetci D, Ulak G, Celikyurt IK, Cine N, Akar F, Savli H, Erden F (2014) The Antidepressant agomelatine improves memory deterioration and upregulates CREB and BDNF gene expression levels in unpredictable chronic mild stress (UCMS)-exposed mice. Drug Target Insights 8: 11-21.
37. Tardito D, Perez J, Tiraboschi E, Musazzi L, Racagni G, Popoli M (2006) Signaling pathways regulating gene expression, neuroplasticity, and neurotrophic mechanisms in the action of antidepressants: a critical overview. Pharmacol. Rev. 58: 115-134.
38. Benitez-King G, Anton-Tay F (1993) Anton-Tay, Calmodulin mediates melatonin cytoskeletal effects. Experientia 49: 635-641.
39. Luchetti F, Canonico B, Betti M, Arcangeletti M, Pilolli F, Piroddi M, Canesi L, Papa S, Galli F (2010) Melatonin signaling and cell protection function. FASEB 24: 3603-3624.
40. Hardeland R (2017) Melatonin and the electron transport chain. Cell. Mol. Life Sci. 74: 3883-3896.
41. Mayo JC, Sainz RM, Gonzalez-Menendez P, Hevia D, Cernuda-Cernuda R (2017) Cernuda-Cernuda, Melatonin transport into mitochondria. Cell. Mol. Life Sci. 74: 3927-3940.
42. Suofu Y, Li W, Jean-Alphonse FG, Jia J, Khattar NK, Li J, Baranov SV, Leronni D, et al. (2017) Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc. Natl. Acad. Sci. USA 114: E7997-E8006.
43. Reiter RJ, Ma Q, Sharma R (2020) Melatonin in mitochondria: mitigating clear and present dangers. Physiol. 35: 86-95.
44. Chen M, Cecon E, Karamitri A, Gao W, Gerbier R, Ahmad R, Jockers R (2020) Melatonin MT1 and MT2 receptor ERK signaling is differentially dependent on Gi/o and Gq/11 proteins. J. Pineal Res. 68: e12641.
45. Zhang J, Wang L, Xie W, Hu S, Zhou H, Zhu P, Zhu H (2020) Melatonin attenuates ER stress and mitochondrial damage in septic cardiomyopathy: A new mechanism involving BAP31 upregulation and MAPK-ERK pathway. J. Cell. Physiol. 235: 2847-2856.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


