Autoxidation of melatonin at excited state: mechanism proposal for formation of N1-acetyl-N2-formyl-5-methoxykynuramine
Autoxidation of melatonin
Abstract
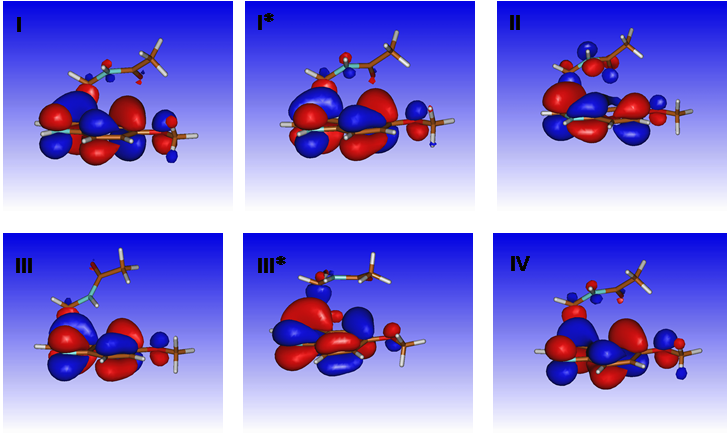
N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) is one of the primary oxidation products of melatonin. There is growing evidence of its beneficial biological properties, including antioxidant features and modulators of cytokines and enzymes involved in the inflammatory process. Here, the autoxidation of melatonin mediated by UVC was studied regarding the formation of AFMK and the reaction mechanism. The parameters evaluated were irradiation, pH, dissolved oxygen, superoxide radical anion, and hydroxyl radical. We found that the AFMK yield is directly correlated with UVC irradiation. The AFMK concentration decreased 95% when a 280 nm cutoff filter blocked the irradiation. By removing the dissolved oxygen from the medium, the decrease was 90%. Superoxide dismutase, acting as a scavenger of superoxide radical anion, caused a 64% reduction. At pH 7.0, the AFMK yield was just 14% of those obtained at pH 10. These findings are consistent with a typical autoxidation reaction. In addition, the low yield of AFMK in the absence of UVC irradiation suggested that electronically excited melatonin is the species involved in the initial electron transfer. Density Functional Theory (DFT) calculations were performed to strengthen the proposal. Corroborant with the experimental results, the theoretical analyses revealed that electron transfer from melatonin to molecular oxygen is only energetically feasible in the excited state. In conclusion, the direct autoxidation of melatonin at excited state in alkaline pH is a straightforward approach to producing AFMK.
References
2. Do Amaral FG, Cipolla-Neto J (2018) A brief review about melatonin, a pineal hormone. Arch. Endocrinol. Metab. 62: 472–479.
3. Arnao MB, Hernández-Ruiz J (2015) Functions of melatonin in plants: a review. J. Pineal Res. 59: 133–150.
4. Murch SJ, Erland LAE (2021) A Systematic review of melatonin in plants: an example of evolution of literature. Front. Plant Sci. 12: 1-24.
5. Yang S, Zhao Y, Qin X, Ding C, Chen Y, Tang Z, Huang Y, Reiter RJ, Yuan S, Yuan M (2022) New insights into the role of melatonin in photosynthesis. J. Exp. Bot. 1-10.
6. Tan DX, Hardeland R, Manchester LC, Paredes SD, Korkmaz A, Sainz TM, Mayo JC, Fuentes-Broto L, Reiter RJ (2010) The changing biological roles of melatonin during evolution: from an antioxidant to signals of darkness, sexual selection and fitness. Biol. Rev. Camb. Philos. Soc. 85: 607–623.
7. Kanwar MK, Yu J, Zhou J (2018) Phytomelatonin: Recent advances and future prospects. J. Pineal Res. 65: e12526.
8. Nabavi SM, Nabavi SF, Sureda A, Xiao J, Dehpour AR, Shirooie S, Silva AS, Baldi A, Khan H, Daglia M (2019) Anti-inflammatory effects of melatonin: A mechanistic review. Crit. Rev. Food Sci. Nutr. 59: 4–16.
9. Pan S, Guo Y, Hong F, Xu P, Zhai Y (2022) Therapeutic potential of melatonin in colorectal cancer: Focus on lipid metabolism and gut microbiota. Biochim. Biophys. Acta. Mol. Basis Dis. 1868: 166281.
10. Tang Y, Groom K, Chamley L, Chen Q (2021) Melatonin, a potential therapeutic agent for preeclampsia, reduces the extrusion of toxic extracellular vesicles from preeclamptic placentae. Cells 10: 1904.
11. Acuña-Castroviejo D, Escames G, Venegas C, Díaz-Casado ME, Lima-Cabello E, López LC, Rosales-Corral S, Tan DX, Reiter RJ (2014) Extrapineal melatonin: Sources, regulation, and potential functions. Cell. Mol. Life Sci. 71: 2997–3025.
12. Reiter RJ, Paredes SD, Korkmaz A, Manchester LC, Tan DX (2008), Melatonin in relation to the “strong” and “weak” versions of the free radical theory of aging. Adv. Med. Sci. 53: 119–129.
13. Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre-Jimenez M, Qin L (2016) Melatonin as an antioxidant: under promises but over delivers. J. Pineal Res. 61: 253–278.
14. Chrustek A, Olszewska-Słonina D (2021) Melatonin as a powerful antioxidant. Acta Pharm. 71: 335–354.
15. Hardeland R, Tan DX, Reiter RJ (2009) Kynuramines, metabolites of melatonin and other indoles: the resurrection of an almost forgotten class of biogenic amines. J. Pineal Res. 47: 109–126.
16. Bonnefont-Rousselot D, Collin F, Jore D, Gardès-Albert M (2011) Reaction mechanism of melatonin oxidation by reactive oxygen species in vitro. J. Pineal Res. 50: 328–335.
17. Back (2021) Melatonin metabolism, signaling and possible roles in plants. Plant J. 105: 376–391.
18. Tan DX, Manchester LC, Terron MP, Flores LJ, Reiter (2007) One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 42: 28–42.
19. Ximenes VF, Catalani LH, Campa A (2001) Oxidation of melatonin and tryptophan by an HRP cycle involving compound III. Biochem. Biophys. Res. Commun. 287: 130-134.
20. Ximenes, VF, Pessoa AS, Padovan CZ, Abrantes DC, Gomes FHF, Maticoli MA, de Menezes ML (2009) Oxidation of melatonin by AAPH-derived peroxyl radicals: Evidence of a pro-oxidant effect of melatonin. Biochim. Biophys. Acta - Gen. Subj. 1790: 787-792.
21. Ximenes VF, Rodrigues AP, Cabello C, Menezes MLD, Fernandes JR (2008) The co-catalytic effect of chlorpromazine on peroxidase-mediated oxidation of melatonin: Enhanced production of N1-acetyl-N2-formyl- 5-methoxykynuramine. J. Pineal Res. 44: 115-120.
22. Galano A, Tan DX, Reiter RJ (2013) On the free radical scavenging activities of melatonin’s metabolites, AFMK and AMK. J. Pineal Res. 54: 245–257.
23. Mayo JC, Sainz RM, Tan DX, Hardeland R, Leon J, Rodriguez C, Reiter RJ (2005) Anti-inflammatory actions of melatonin and its metabolites, N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) and N1-acetyl-5-methoxykynuramine (AMK), in macrophages. J. Neuroimmunol. 165: 139–149.
24. Fernández AS, Gago AG, Naveda FA, Calleja JG, Zawadzka A, Czarnocki Z, Barrallo JCM, Menéndez RMS, Rodríguez-González P, Alonso JIG (2022) Evaluation of different internal standardization approaches for the quantification of melatonin in cell culture samples by multiple heart-cutting two dimensional liquid chromatography tandem mass spectrometry. J. Chromatogr. A 1663: 462752.
25. Iwashita H, Matsumoto Y, Maruyama Y, Watanabe K, Chiba A, Hattori A (2021) The melatonin metabolite N1-acetyl-5-methoxykynuramine facilitates long-term object memory in young and aging mice. J. Pineal Res. 70: e12703.
26. de Castro TB, Bordin-Junior NA, de Almeida, de Campos Zuccari DAP (2018) Evaluation of melatonin and AFMK levels in women with breast cancer. Endocrine 62: 242–249.
27. Kim TK, Lin Z, Tidwell WJ, Li W, Slominski AT (2015) Melatonin and its metabolites accumulate in the human epidermis in vivo and inhibit proliferation and tyrosinase activity in epidermal melanocytes in vitro. Mol. Cell. Endocrinol. 404: 1–8.
28. Ximenes VF, Padovan CZ, Carvalho DA, Fernandes JR (2010) Oxidation of melatonin by taurine chloramine. J. Pineal Res. 49: 115-122.
29. Ximenes VF, Silva SO, Rodrigues MR, Catalani LH, Maghzal GJ, Kettle AJ, Campa A (2005) Superoxide-dependent oxidation of melatonin by myeloperoxidase. J. Biol. Chem. 280: 38160-38169.
30. Ferry G, Ubeaud C, Lambert PH, Bertin S, Cogé F, Chomarat P, Delagrange P, Serkiz B, Bouchet JP, Truscott RJW, Boutin JA (2005) Molecular evidence that melatonin is enzymatically oxidized in a different manner than tryptophan: investigations with both indoleamine 2,3-dioxygenase and myeloperoxidase. Biochem. J. 388: 205–215.
31. De Almeida EA, Martinez GR, Klitzke CF, De Medeiros MHG, Di Mascio P (2003) Oxidation of melatonin by singlet molecular oxygen (O2(1deltag)) produces N1-acetyl-N2-formyl-5-methoxykynurenine. J. Pineal Res. 35: 131–137.
32. Dinç E, Ragno G, Baleanu D, De Luca M, Ioele G (2012) Fractional wavelet transform–continous wavelet transform for the quantification of melatonin and its photodegradation product. 45: 337–343.
33. Andrisano V, Bertucci C, Battaglia A, Cavrini V (2000) Photostability of drugs: Photodegradation of melatonin and its determination in commercial formulations. J. Pharm. Biomed. Anal. 23: 15–23.
34. Maharaj DS, Anoopkumar-Dukie S, Glass BD, Antunes EM, Lack B, Walker RB, Daya S (2002) The identification of the UV degradants of melatonin and their ability to scavenge free radicals. J. Pineal Res. 32: 257–261.
35. Ditchfield R, Hehre WJ, Pople JÁ (2003) Self‐consistent molecular‐orbital methods. IX. An extended gaussian‐type basis for molecular‐orbital studies of organic molecules. J. Chem. Phys. 54, 724.
36. Perdew JP, Ernzerhof M, Burke K (1998) Rationale for mixing exact exchange with density functional approximations. J. Chem. Phys. 105: 9982.
37. Grimme S, Ehrlich S, Goerigk L (2011) Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 32: 1456–1465.
38. Tomasi L, Mennucci B, Cammi R (2005) Quantum mechanical continuum solvation models. Chem. Rev. 105: 2999–3093.
39. Frisch MARMJ, Trucks GW, Schlegel HB, Scuseria GE (1998) Gaussian 98.
40. Ramasarma T, Rao AVS, Maya Devi M, Omkumar RV, Bhagyashree KS, Bhat SV (2015) New insights of superoxide dismutase inhibition of pyrogallol autoxidation. Mol. Cell. Biochem. 400: 277–285.
41. Welch KD, Davis TZ, Aust SD (2002) Iron autoxidation and free radical generation: effects of buffers, ligands, and chelators. Arch. Biochem. Biophys. 397: 360–369.
42. Mochizuki M, Yamazaki SI, Kano K, Ikeda T (2002) Kinetic analysis and mechanistic aspects of autoxidation of catechins. Biochim. Biophys. Acta - Gen. Subj. 1569: 35–44.
43. Daescu M, Toulbe N, Baibarac M, Mogos A, Lőrinczi A, Logofatu C (2020) Photoluminescence as a complementary tool for UV-VIS spectroscopy to highlight the photodegradation of drugs: A case study on melatonin. Molecules 25: 3820.
44. Miura YH, Tomita I, Watanabe T, Hirayama T, Fukui S (1998) Active oxygens generation by flavonoids. Biol. Pharm. Bull. 21: 93–96.
45. Bruck R, Aeed H, Shirin H, Matas Z, Zaidel L, Avni Y, Halpern Z (1999) The hydroxyl radical scavengers dimethylsulfoxide and dimethylthiourea protect rats against thioacetamide-induced fulminant hepatic failure. J. Hepatol. 31: 27–38.
46. Campos-Shimada LB, Gilglioni EH, Garcia RF, Martins-Maciel ER, Ishii-Iwamoto EL, Salgueiro-Pagadigorria CL (2020) Superoxide dismutase: a review and a modified protocol for activities measurements in rat livers. Physiol. Biochem. 126: 292–299.
47. Seever K, Hardeland R (2008) Novel pathway for N1-acetyl-5-methoxykynuramine: UVB-induced liberation of carbon monoxide from precursor N 1-acetyl-N 2-formyl-5-methoxykynuramine. J. Pineal Res. 44: 450–455.
48. He H, Lin M, Han Z, Muroya Y, Kudo H, Katsumura Y (2005) The formation and properties of the melatonin radical: a photolysis study of melatonin with 248 nm laser light. Org. Biomol. Chem. 3: 1568–1574.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


