Mitochondrial dynamics in myocardial ischemia/reperfusion injury: Effects of melatonin
Melatonin and mitochondrial quality control
Abstract
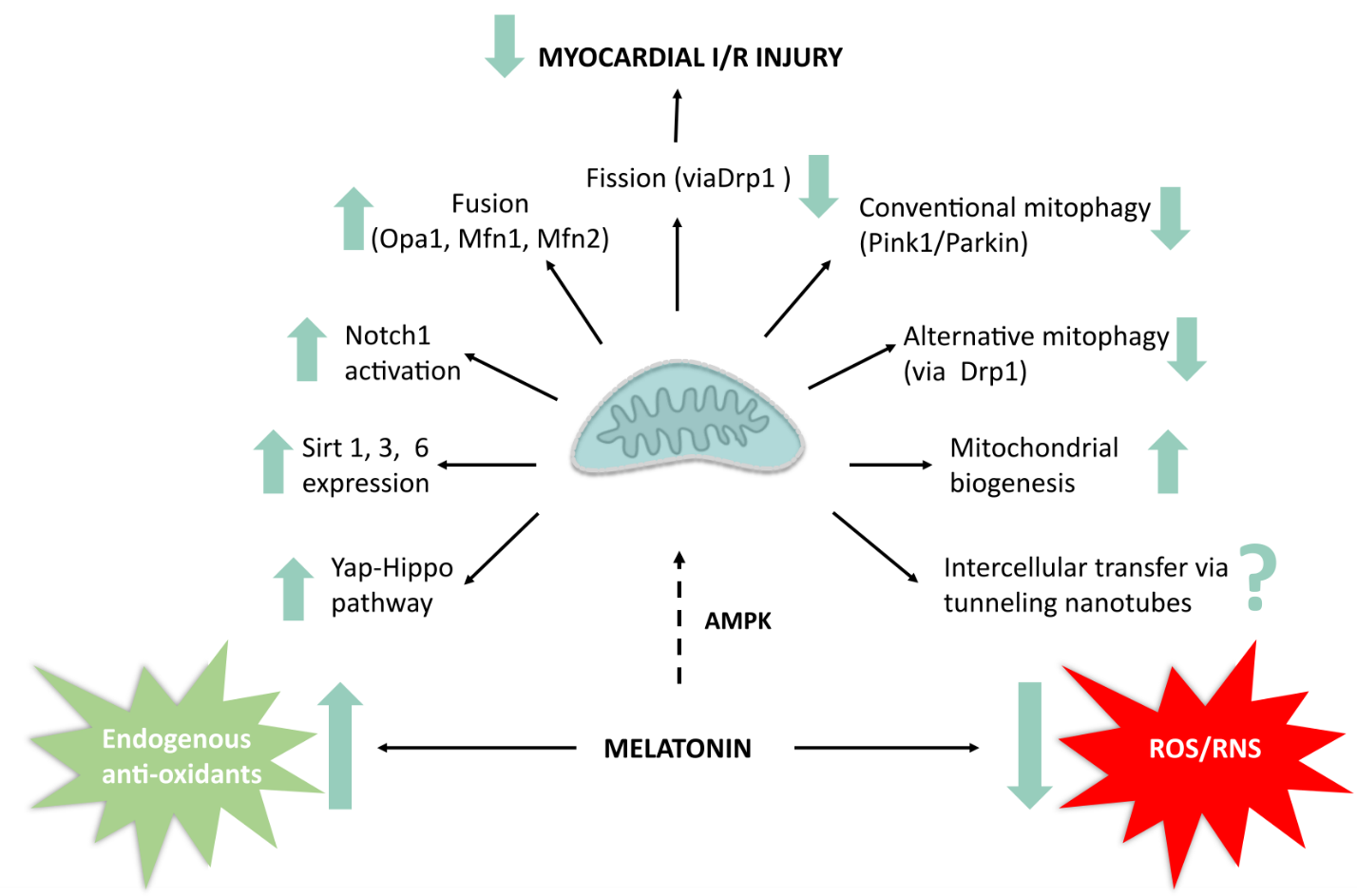
Timely reperfusion during myocardial infarction paradoxically leads to ischemia/reperfusion (I/R) injury. Mitochondrial quality control has emerged as a key participant in regulation of this process. The aims of this review are to briefly summarize current evidence for the role of mitochondrial quality control in I/R injury and to evaluate whether the cardioprotective actions of melatonin, a potent free radical scavenger and antioxidant, can be attributed to its effects on these processes. Using a variety of experimental models, in vivo and in vitro, melatonin-induced cardioprotection has been demonstrated to be associated with attenuation or reversal of the harmful effects of I/R on parameters of mitochondrial quality control as evidenced by (i) upregulation of mitochondrial fusion and inhibition of fission, particularly Drp1 expression and translocation from the cytosol to the mitochondria; (ii) changes in both the conventional and alternative mitophagy pathways. Melatonin significantly upregulates mitochondrial biogenesis and the expression of sirtuins 1, 3 and 6 and has a beneficial effect on mitochondrial-endoplasmic reticulum interaction in I/R. A novel observation is the ability of melatonin to stimulate intercellular transfer of mitochondria between damaged cells, although this has not yet been demonstrated in myocardial I/R. Melatonin treatment has profound effects on the diabetic heart: it reverses the significant reduction in function and inhibits the progression of diabetic cardiomyopathy which was associated with a significant effect on mitochondrial quality control, as evidenced by a reduction in fission. In summary, available evidence supports a major role for mitochondrial quality control in the beneficial actions of melatonin in the I/R heart.
References
2. Yellon DM, Hausenloy DJ (2007) Myocardial reperfusion injury. N. Engl. J. Med. 357 (11): 1121-1135. doi:10.1056/NEJMra071667.
3. Kurian GA, Rajagopal R, Vedantham S, Rajesh M (2016) The role of oxidative stress in myocardial ischemia and reperfusion injury and remodeling: Revisited. Oxid. Med. Cell Longev. 2016: 1656450. doi:10.1155/2016/1656450.
4. Kulek AR, Anzell A, Wider JM, Sanderson TH, Przyklenk K (2020) Mitochondrial quality control: role in cardiac models of lethal ischemia-reperfusion injury. Cells 9 (1): 214. doi:10.3390/cells9010214.
5. Murphy E, Steenbergen C (2021) Regulation of mitochondrial Ca(2+) uptake. Annu. Rev. Physiol. 83: 107-126. doi:10.1146/annurev-physiol-031920-092419.
6. Lesnefsky EJ, Chen Q, Tandler B, Hoppel CL (2017) Mitochondrial dysfunction and myocardial ischemia-reperfusion: Implications for novel therapies. Annu. Rev. Pharmacol. Toxicol. 57: 535-565. doi:10.1146/annurev-pharmtox-010715-103335.
7. Marin W, Marin D, Ao X, Liu Y (2021) Mitochondria as a therapeutic target for cardiac ischemia reperfusion injury (Review). Int. J. Mol. Med. 47 (2): 485-499. doi:10.3892/ijmm.2020.4823.
8. Kalogeris T, Baines CP, Krenz M, Korthuis RJ (2012) Cell biology of ischemia/reperfusion injury. Int. Rev. Cell Mol. Biol. 298 : 229-317. doi:10.1016/B978-0-12-394309-5.00006-7.
9. Gottlieb RA. (2011) Cell death pathways in acute ischemia/reperfusion injury. J. Cardiovasc. Pharmacol. Ther. 16 (3-4): 233-238. doi:10.1177/1074248411409581.
10. Hausenloy DJ, Yellon DM (2013) Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J. Clin. Invest. 123 (1): 92-100. doi:10.1172/JCI62874.
11. Ong SB, Hall AR, Hausenloy DJ (2013) Mitochondrial dynamics in cardiovascular health and disease. Antioxid. Redox. Signal. 19 (4): 400-414. doi:10.1089/ars.2012.4777.
12. Le Page S, Niro M, Fauconnier J, et al. (2016) Increase in cardiac ischemia-reperfusion injuries in Opa1+/- mouse model. PLoS One 11 (10): e0164066. doi:10.1371/journal.pone.0164066.
13. Ong SB, Kalkhoran SB, Hernández-Reséndiz S, et al.(2017) Mitochondrial-Shaping proteins in cardiac health and disease - the long and the short of it! Cardiovasc. Drugs Ther. 31 (1) : 87-107. doi:10.1007/s10557-016-6710-1.
14. Ong SB, Subrayan S, Lim SY, Yellon DM, et al. (2010) Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation 121 (18): 2012-2022. doi:10.1161/CIRCULATIONAHA.109.906610.
15. Wai T, García-Prieto J, Baker MJ, et al. (2015) Imbalanced OPA1 processing and mitochondrial fragmentation cause heart failure in mice. Science 350 (6265): aad0116. doi:10.1126/science.aad0116.
16. Vásquez-Trincado C, García-Carvajal I, Pennanen C, et al. (2016) Mitochondrial dynamics, mitophagy and cardiovascular disease. J. Physiol. 594 (3): 509-525. doi:10.1113/JP271301.
17. Wang W, Fernandez-Sanz C, Sheu SS (2018) Regulation of mitochondrial bioenergetics by the non-canonical roles of mitochondrial dynamics proteins in the heart. Biochim. Biophys. Acta Mol. Basis Dis. 1864 (5 Pt B): 1991-2001. doi:10.1016/j.bbadis.2017.09.004.
18. Chen Y, Liu Y, Dorn GW 2nd (2011) Mitochondrial fusion is essential for organelle function and cardiac homeostasis. Circ. Res. 109 (12):1327-1331. doi:10.1161/CIRCRESAHA.111.258723.
19. Song M, Gong G, Burelle Y, et al. (2015) Interdependence of parkin-mediated mitophagy and mitochondrial fission in adult mouse hearts. Circ. Res. 117 (4): 346-351. doi:10.1161/CIRCRESAHA.117.306859.
20. Mishra P, Carelli V, Manfredi G, Chan DC (2014) Proteolytic cleavage of Opa1 stimulates mitochondrial inner membrane fusion and couples fusion to oxidative phosphorylation. Cell Metab. 19 (4): 630-641. doi:10.1016/j.cmet.2014.03.011.
21. Mishra P, Chan DC (2016) Metabolic regulation of mitochondrial dynamics. J. Cell Biol. 212 (4): 379-387. doi:10.1083/jcb.201511036.
22. Cipolat S, Martins de Brito O, Dal Zilio B, Scorrano L (2004) OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc. Natl. Acad. Sci. U. S. A. 101 (45): 15927-15932. doi:10.1073/pnas.0407043101.
23. Varanita T, Soriano ME, Romanello V, et al. (2015) The OPA1-dependent mitochondrial cristae remodeling pathway controls atrophic, apoptotic, and ischemic tissue damage. Cell Metab. 21 (6): 834-844. doi:10.1016/j.cmet.2015.05.007.
24. Frezza C, Cipolat S, Martins de Brito O, et al. (2006) OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell 126 (1): 177-189. doi:10.1016/j.cell.2006.06.025.
25. Ishihara N, Fujita Y, Oka T, Mihara K (2006) Regulation of mitochondrial morphology through proteolytic cleavage of OPA1. EMBO J. 25 (13): 2966-2977. doi:10.1038/sj.emboj.7601184.
26. Yamaguchi R, Lartigue L, Perkins G, et al. (2008) Opa1-mediated cristae opening is Bax/Bak and BH3 dependent, required for apoptosis, and independent of Bak oligomerization. Mol. Cell 31 (4): 557-569. doi:10.1016/j.molcel.2008.07.010.
27. Arnoult D, Grodet A, Lee YJ, Estaquier J, Blackstone C (2005) Release of OPA1 during apoptosis participates in the rapid and complete release of cytochrome c and subsequent mitochondrial fragmentation. J. Biol. Chem. 280 (42): 35742-35750. doi:10.1074/jbc.M505970200.
28. Dorn GW 2nd (2015) Mitochondrial dynamism and heart disease: changing shape and shaping change. EMBO Mol. Med. 7 (7): 865-877. doi:10.15252/emmm.201404575.
29. Landes T, Martinou JC (2011) Mitochondrial outer membrane permeabilization during apoptosis: the role of mitochondrial fission. Biochim Biophys. Acta. 1813 (4): 540-545. doi:10.1016/j.bbamcr.2011.01.021.
30. Cassidy-Stone A, Chipuk JE, Ingerman E, et al. (2008) Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev. Cell. 14 (2): 193-204. doi:10.1016/j.devcel.2007.11.019.
31. Losón OC, Song Z, Chen H, Chan DC. (2013) Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Mol. Biol. Cell. 24 (5): 659-667. doi:10.1091/mbc.E12-10-0721.
32. Palmer CS, Elgass KD, Parton RG, Osellame LD, Stojanovski D, Ryan MT (2013) Adaptor proteins MiD49 and MiD51 can act independently of Mff and Fis1 in Drp1 recruitment and are specific for mitochondrial fission. J. Biol. Chem. 288 (38): 27584-27593. doi:10.1074/jbc.M113.479873.
33. Yu R, Jin SB, Lendahl U, Nistér M, Zhao J (2019) Human Fis1 regulates mitochondrial dynamics through inhibition of the fusion machinery. EMBO J. 38 (8): e99748. doi:10.15252/embj.201899748.
34. Osellame LD, Singh AP, Stroud DA, et al. (2016) Cooperative and independent roles of the Drp1 adaptors Mff, MiD49 and MiD51 in mitochondrial fission. J. Cell Sci. 129 (11): 2170-2181. doi:10.1242/jcs.185165.
35. Zhou H, Hu S, Jin Q, et al. (2017) Mff-dependent mitochondrial fission contributes to the pathogenesis of cardiac microvasculature ischemia/reperfusion injury via induction of mROS-Mediated Cardiolipin Oxidation and HK2/VDAC1 disassociation-involved mPTP opening. J. Am. Heart Assoc. 6 (3): e005328. doi:10.1161/JAHA.116.005328.
36. Jin Q, Li R, Hu N, et al. (2018) DUSP1 alleviates cardiac ischemia/reperfusion injury by suppressing the Mff-required mitochondrial fission and Bnip3-related mitophagy via the JNK pathways. Redox. Biol. 14: 576-587. doi:10.1016/j.redox.2017.11.004.
37. Hernandez-Resendiz S, Prunier F, Girao H, et al. (2020) Targeting mitochondrial fusion and fission proteins for cardioprotection. J. Cell Mol. Med. 24 (12): 6571-6585. doi:10.1111/jcmm.15384.
38. Hall AR, Burke N, Dongworth RK, et al. (2016) Hearts deficient in both Mfn1 and Mfn2 are protected against acute myocardial infarction. Cell Death Dis. 7 (5): e2238. doi:10.1038/cddis.2016.139.
39. de Brito OM, Scorrano L (2008) Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456 (7222): 605-610. doi:10.1038/nature07534.
40. Chen Y, Csordás G, Jowdy C, et al. (2012) Mitofusin 2-containing mitochondrial-reticular microdomains direct rapid cardiomyocyte bioenergetic responses via interorganelle Ca(2+) crosstalk. Circ. Res. 111 (7): 863-875. doi:10.1161/CIRCRESAHA.112.266585.
41. Nan J, Nan C, Ye J, et al. (2019) EGCG protects cardiomyocytes against hypoxia-reperfusion injury through inhibition of OMA1 activation. J. Cell Sci. 132 (2): jcs220871. doi:10.1242/jcs.220871.
42. Disatnik MH, Ferreira JCB, Campos JC, et al. (2013) Acute inhibition of excessive mitochondrial fission after myocardial infarction prevents long-term cardiac dysfunction. J. Am. Heart Assoc. 2 (5): e000461. doi:10.1161/JAHA.113.000461.
43. Tong M, Zablocki D, Sadoshima J (2020) The role of Drp1 in mitophagy and cell death in the heart. J. Mol. Cell. Cardiol. 142: 138-145. doi:10.1016/j.yjmcc.2020.04.015.
44. Zhang H, Wang P, Bisetto S, et al. (2017) A novel fission-independent role of dynamin-related protein 1 in cardiac mitochondrial respiration. Cardiovasc. Res. 113 (2): 160-170. doi:10.1093/cvr/cvw212.
45. Wu D, Dasgupta A, Chen KH, et al. (2020) Identification of novel dynamin-related protein 1 (Drp1) GTPase inhibitors: Therapeutic potential of Drpitor1 and Drpitor1a in cancer and cardiac ischemia-reperfusion injury. FASEB J. 34 (1): 1447-1464. doi:10.1096/fj.201901467R.
46. Coronado M, Fajardo G, Nguyen K, et al. (2018) Physiological Mitochondrial Fragmentation Is a Normal Cardiac Adaptation to Increased Energy Demand. Circ. Res. 122 (2): 282-295. doi:10.1161/CIRCRESAHA.117.310725.
47. Ikeda Y, Shirakabe A, Maejima Y, et al. (2015) Endogenous Drp1 mediates mitochondrial autophagy and protects the heart against energy stress. Circ. Res. 116 (2): 264-278. doi:10.1161/CIRCRESAHA.116.303356.
48. Bordt EA, Clerc P, Roelofs BA, et al. (2017) The putative Drp1 inhibitor mdivi-1 is a reversible mitochondrial Complex I inhibitor that modulates reactive oxygen species. Dev. Cell. 40 (6): 583-594.e6. doi:10.1016/j.devcel.2017.02.020.
49. Kageyama Y, Hoshijima M, Seo K, et al. (2014) Parkin-independent mitophagy requires Drp1 and maintains the integrity of mammalian heart and brain. EMBO J. 33 (23): 2798-2813. doi:10.15252/embj.201488658.
50. Lee Y, Lee HY, Hanna RA, Gustafsson ÅB (2011) Mitochondrial autophagy by Bnip3 involves Drp1-mediated mitochondrial fission and recruitment of Parkin in cardiac myocytes. Am. J. Physiol. Heart Circ. Physiol. 301 (5): H1924-31. doi:10.1152/ajpheart.00368.2011.
51. Twig G, Elorza A, Molina AJA, et al. (2008) Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 27 (2): 433-446. doi:10.1038/sj.emboj.7601963.
52. Gomes LC, Di Benedetto G, Scorrano L (2011) During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat. Cell Biol. 13 (5): 589-598. doi:10.1038/ncb2220.
53. Rambold AS, Kostelecky B, Elia N, Lippincott-Schwartz J (2011) Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. Proc. Natl. Acad. Sci. U. S. A. 108 (25):10190-10195. doi:10.1073/pnas.1107402108.
54. Saito T, Hamano K, Sadoshima J (2021) Molecular mechanisms and clinical implications of multiple forms of mitophagy in the heart. Cardiovasc. Res. 117 (14): 2730-2741. doi:10.1093/cvr/cvaa340.
55. Anzell AR, Maizy R, Przyklenk K, Sanderson TH (2018) Mitochondrial quality control and disease: insights into ischemia-reperfusion injury. Mol. Neurobiol. 55 (3): 2547-2564. doi:10.1007/s12035-017-0503-9.
56. Bingol B, Sheng M (2016) Mechanisms of mitophagy: PINK1, Parkin, USP30 and beyond. Free Radic. Biol. Med. 100: 210-222. doi:10.1016/j.freeradbiomed.2016.04.015.
57. Yoo SM, Jung YK (2018) A molecularapproach to mitophagy and mitochondrial dynamics. Mol. Cells 41 (1):18-26. doi:10.14348/molcells.2018.2277.
58. Kane LA, Lazarou M, Fogel AI, et al. (2014) PINK1 phosphorylates ubiquitin to activate Parkin E3 ubiquitin ligase activity. J. Cell Biol. 205 (2): 143-153. doi:10.1083/jcb.201402104.
59. Koyano F, Okatsu K, Kosako H, et al. (2014) Ubiquitin is phosphorylated by PINK1 to activate parkin. Nature 510 (7503): 162-166. doi:10.1038/nature13392.
60. Chen Y, Dorn GW 2nd (2013) PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science 340 (6131): 471-475. doi:10.1126/science.1231031.
61. Lazarou M, Sliter DA, Kane LA, et al. (2015) The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature 524 (7565): 309-314. doi:10.1038/nature14893.
62. Klionsky DJ, Schulman BA (2014) Dynamic regulation of macroautophagy by distinctive ubiquitin-like proteins. Nat. Struct. Mol. Biol. 21 (4): 336-345. doi:10.1038/nsmb.2787.
63. Feng Y, He D, Yao Z, Klionsky DJ (2014) The machinery of macroautophagy. Cell Res. 24 (1): 24-41. doi:10.1038/cr.2013.168.
64. Kubli DA, Quinsay MN, Huang C, Lee Y, Gustafsson AB (2008) Bnip3 functions as a mitochondrial sensor of oxidative stress during myocardial ischemia and reperfusion. Am. J. Physiol. Heart Circ. Physiol. 295 (5): H2025-31. doi:10.1152/ajpheart.00552.2008.
65. Hamacher-Brady A, Brady NR, Logue SE, et al. (2007) Response to myocardial ischemia/reperfusion injury involves Bnip3 and autophagy. Cell Death Differ. 14 (1): 146-157. doi:10.1038/sj.cdd.4401936
66. Azad MB, Chen Y, Henson ES, et al. (2008) Hypoxia induces autophagic cell death in apoptosis-competent cells through a mechanism involving BNIP3. Autophagy 4 (2): 195-204. doi:10.4161/auto.5278.
67. Houtkooper RH, Vaz FM (2008) Cardiolipin, the heart of mitochondrial metabolism. Cell. Mol. Life Sci. 65 (16): 2493-2506. doi:10.1007/s00018-008-8030-5.
68. Chu CT, Ji J, Dagda RK, et al. (2013) Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells. Nat. Cell. Biol. 15 (10): 1197-1205. doi:10.1038/ncb2837.
69. Kagan VE, Jiang J, Huang Z, et al. (2016) NDPK-D (NM23-H4)-mediated externalization of cardiolipin enables elimination of depolarized mitochondria by mitophagy. Cell Death Differ. 23 (7): 1140-1151. doi:10.1038/cdd.2015.160.
70. Kubli DA, Ycaza JE, Gustafsson AB (2007) Bnip3 mediates mitochondrial dysfunction and cell death through Bax and Bak. Biochem. J. 405 (3): 407-415. doi:10.1042/BJ20070319.
71. Chen M, Chen Z, Wang Y, et al. (2016) Mitophagy receptor FUNDC1 regulates mitochondrial dynamics and mitophagy. Autophagy 12 (4): 689-702. doi:10.1080/15548627.2016.1151580.
72. Sciarretta S, Maejima Y, Zablocki D, Sadoshima J (2018) The role of autophagy in the heart. Annu. Rev. Physiol. 80: 1-26. doi:10.1146/annurev-physiol-021317-121427.
73. Zaha VG, Young LH (2012) AMP-activated protein kinase regulation and biological actions in the heart. Circ. Res. 111 (6): 800-814. doi:10.1161/CIRCRESAHA.111.255505.
74. Sciarretta S, Hariharan N, Monden Y, Zablocki D, Sadoshima J (2011) Is autophagy in response to ischemia and reperfusion protective or detrimental for the heart? Pediatr. Cardiol. 32 (3): 275-281. doi:10.1007/s00246-010-9855-x.
75. Hirota Y, Yamashita S, Kurihara Y, et al. (2015) Mitophagy is primarily due to alternative autophagy and requires the MAPK1 and MAPK14 signaling pathways. Autophagy 11 (2): 332-343. doi:10.1080/15548627.2015.1023047.
76. Honda S, Arakawa S, Nishida Y, Yamaguchi H, et al. (2014) Ulk1-mediated Atg5-independent macroautophagy mediates elimination of mitochondria from embryonic reticulocytes. Nat. Commun. 5: 4004. doi:10.1038/ncomms5004.
77. Saito T, Nah J, Oka SI, et al. (2019) An alternative mitophagy pathway mediated by Rab9 protects the heart against ischemia. J. Clin Invest. 129 (2): 802-819. doi:10.1172/JCI122035.
78. Shirakabe A, Zhai P, Ikeda Y, et al. 2016) Drp1-dependent mitochondrial autophagy plays a protective role against pressure overload-induced mitochondrial dysfunction and heart failure. Circulation 133 (13): 1249-1263. doi:10.1161/CIRCULATIONAHA.115.020502.
79. Egan DF, Shackelford DB, Mihaylova MM, et al. (2011) Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 331 (6016): 456-461. doi:10.1126/science.1196371.
80. Lochner A, Marais E, Huisamen B (2018) Melatonin and cardioprotection against ischaemia/reperfusion injury: What’s new? A review. J. Pineal Res. 65 (1): e12490. doi:10.1111/jpi.12490.
81. Jockers R, Delagrange P, Dubocovich MM, Markus RP, et al. (2016) Update on melatonin receptors. Br. J. Pharmacol. 173 (18): 2702-2725. doi:10.1111/bph13536.
82. Yang Y, Duan W, Jin Z, et al. (2013) JAK2/STAT3 activation by melatonin attenuates mitochondrial oxidative damage induced by ischemia/reperfusion. J. Pineal Res. 55 (3): 275-286. doi: 10.1111/jpi12070.
83. Petrosillo G, Moro N, Ruggiero FM, Paradies G (2009) Melatonin inhibits cardiolipin peroxidation in mitochondria and prevents the mitochondrial permeability transition and cytochrome C release. Free Rad. Biol. Med. 47 (7): 969-974. doi: 10.1016/j.freeradbiomed.2009.06.032.
84. Reiter RJ, Tan DX, Rosales-Corral S, et al. (2018) Mitochondria: Central Organelles for Melatonin’s Antioxidant and Anti-Aging Actions. Molecules 23 (2): 509. doi:10.3390/molecules23020509.
85. Reiter RJ, Sharma R, Pires de Campos Zuccari DA, de Almeida Chuffa LG, et al. (2021) Melatonin synthesis in and uptake by mitochondria: implications for diseased cells with dysfunctional mitochondria. Future Med. Chem. 13 (4): 335-339. doi:10.4155/fmc-2020-0326.
86. Huo X, Wang C, Yu Z, Peng Y, et al. (2017) Human transporters, PEPT1/2 facilitate melatonin transportation into mitochondria of cancer cells: an implication of the therapeutic potential. J. Pineal Res. 62 (4): e12390.
87. Hevia D, González-Menéndez P, Quiros-González I, et al. (2015) Melatonin uptake through glucose transporters: a new target for melatonin inhibition of cancer. J. Pineal Res. 58 (2): 234-250. doi:10.1111/jpi.12210.
88. Suofu Y, Li W, Jean-Alphonse FG, et al. (2017) Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc. Natl. Acad. Sci. U S A. 114 (38): E7997-E8006. doi:10.1073/pnas.1705768114.
89. Reiter RJ, Mayo JC, Tan DX, et al. (2016) Melatonin as anti-oxidant: under promises but over-delivers. J Pineal Res 61 (3): 259-278. doi:10.1111/jpi.12360.
90. Tan DX, Manchester LC, Qin L, et al. (2016) Melatonin: a mitochondrial targeting molecule involving mitochondrial protection and dynamics. Int J Mol Sci 17 (12): 2124. doi:10.3390?ijms17122124.
91. Mayo JC, Sainz RM, Gonzales-Mendez P, et al. (2017) Melatonin and sirtuins: a not-so unexpected relationship. J. Pineal Res. 62 (2):e 12391.doi:10.1111/jpi.12391.
92. Venegas C, Garcia JA, Escames G, et al. (2012) Extrapineal melatonin: analysis of its subcellular distribution and daily fluctuations. J. Pineal Res. 52 (2):217-227. doi: 10.1111/j.1600-079x.2011.00931.x.
93. Van der Bliek AM, Sedensky MM, Morgan PG (2017) Cell biology of the mitochondrion. Genetics 207 (3): 843-871. doi:10.1534/genetics.117.300262.
94. Reiter RJ, Tan DX, Burkhardt S (2002) Reactive oxygen and nitrogen species and cellular and organismal decline: amelioration with melatonin. Mech. Ageing Dev. 123 (8): 1007-1019. doi:10.1016/s0047-6374(01)00384-0.
95. Tan D Xian, Reiter RJ, Manchester LC, et al. (2002) Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr. Top Med. Chem. 2 (2): 181-197. doi:10.2174/1568026023394443.
96. Hardeland R (2005) Antioxidative protection by melatonin: multiplicity of mechanisms from radical detoxification to radical avoidance. Endocrine 27 (2): 119-130. doi:10.1385/endo:27:2:119.
97. Galano A, Tan DX, Reiter RJ (2011) Melatonin as a natural ally against oxidative stress: a physicochemical examination. J. Pineal Res. 51 (1): 1-16. doi:10.1111/j.1600-079X.2011.00916.x.
98. Reiter RJ, Tan DX (2003) Melatonin: a novel protective agent against oxidative injury of the ischemic/reperfused heart. Cardiovasc. Res. 58 (1): 10-19. doi:10.1016/s0008-6363(02)00827-1.
99. Antolín I, Rodríguez C, Saínz RM, et al. (1996) Neurohormone melatonin prevents cell damage: effect on gene expression for antioxidant enzymes. FASEB. J. 10 (8): 882-890. doi:10.1096/fasebj.10.8.8666165.
100. Okatani Y, Wakatsuki A, Shinohara K, et al. (2001) Melatonin stimulates glutathione peroxidase activity in human chorion. J. Pineal Res. 30 (4): 199-205. doi:10.1034/j.1600-079x.2001.300402.x.
101. Urata Y, Honma S, Goto S, et al. (1999) Melatonin induces gamma-glutamylcysteine synthetase mediated by activator protein-1 in human vascular endothelial cells. Free Radic. Biol. Med. 27 (7-8): 838-847. doi:10.1016/s0891-5849(99)00131-8.
102. Galano A, Reiter RJ (2018) Melatonin and its metabolites vs oxidative stress: From individual actions to collective protection. J. Pineal Res. 65 (1): e12514. doi:10.1111/jpi.12514.
103. Reiter RJ, Rosales-Corral S, Tan DX, et al. (2017) Melatonin as a mitochondria-targeted antioxidant: one of evolution’s best ideas. Cell. Mol. Life Sci. 74 (21): 3863-3881. doi:10.1007/s00018-017-2609-7.
104. Reiter RJ, Sharma R, Rosales-Corral S, et al. (2022) Melatonin: a mitochondrial resident with a diverse skill set. Life Sci 301: 120612. doi: 10.1016/j.lfs.2022.120612.
105. Reiter RJ, Ma Q, Sharma R (2020) Melatonin in mitochondria: mitigating clear and present dangers. Physiology (Bethesda) 35 (2): 86-95. doi:10.1152/physiol.00034.2019.
106. Paradies G, Paradies V, Ruggiero FM, Petrosillo G (2017) Mitochondrial bioenergetics during aging: beneficial effects of melatonin. Cell Mol. Life Sci. 74 (21): 3897-3911. doi: 10.1007/s00018-017-2619-5.
107. Acuna-Castroviejo D, Lopez LC, Escames G, et al. (2011) Curr. Top. Med. Chem. 11: 221-240. doi:10.2174/156802611794863517.
108. Hardeland R (2017) Melatonin and the electron transport chain. Cell. Mol. Life Sci. 74: 3883-3896. doi: 10.1007/s000018-017-2615-9.
109. Reiter RJ, Sharma R, Ma Q (2021) Switching diseased cells from cytosolic aerobic glycolysis to mitochondrial oxidative phosphorylation: a metabolic rhythm regulated by melatonin? J. Pineal Res. 70: e12677. doi:10.1111/jpi12677.
110. Tengattini S, Reiter RJ, Tan DX, Terron MP, et al. (2008) Cardiovascular diseases: protective effects of melatonin. J. Pineal Res. (1): 16-25. doi:10.1111/j.1600-079X.2007.00518.x.
111. Randhawa PK, Gupta MK (2020) Melatonin as a protective agent in cardiac ischemia-reperfusion injury: Vision/Illusion? Eur. J. Pharmacol. 885: 173506. doi:10.1016/j.ejphar.2020.173506.
112. Zhou H, Ma Q, Zhu P, Ren J, et al. (2018) Protective role of melatonin in cardiac ischemia-reperfusion injury: From pathogenesis to targeted therapy. J. Pineal Res. 64 (3): e12471. doi:10.1111/jpi.12471.
113. Pourhanifeh MH, Dehdashtian E, Hosseinzadeh A, Sezavar SH, Mehrzadi S (2022) Clinical application of melatonin in thetreatment of cardiovascular diseases: current evidence and new insights into the cardioprotective and cardiotherapeutic properties. Cardiovasc. Drugs Ther. 36 (1): 131-155. doi:10.1007/s10557-020-07052-3.
114. Tobeiha M, Jafari A, Fadaei S, Mirazimi SMA, et al. (2022) Evidence for the benefits of melatonin in cardiovascular disease. Front. Cardiovasc. Med. 9: 888319.doi:10.3389/fcvm.2022.888319.
115. Veiga EC de A, Simões RDS, Caviola LL, et al. (2021) Melatonin and the cardiovascular system in animals: systematic review and meta-analysis. Clinics (Sao Paulo). 76: e2863. doi:10.6061/clinics/2021/e2863.
116. Sewerynek E, Reiter RJ, Melchiorri D, et al. (1996) Oxidative damage in the liver induced by ischemia-reperfusion: protection by melatonin. Hepatogastroenterology 43 (10): 898-905.
117. Zaoualí MA, Reiter RJ, Padrissa-Altés S, et al. (2011) Melatonin protects steatotic and nonsteatotic liver grafts against cold ischemia and reperfusion injury. J. Pineal Res. 50 (2): 213-221. doi:10.1111/j.1600-079X.2010.00831.x.
118. Tai SH, Chen HY, Lee EJ, et al. (2010) Melatonin inhibits postischemic matrix metalloproteinase-9 (MMP-9) activation via dual modulation of plasminogen/plasmin system and endogenous MMP inhibitor in mice subjected to transient focal cerebral ischemia. J. Pineal Res. 49 (4): 332-341. doi:10.1111/j.1600-079X.2010.00797.x.
119. Li Z, Nickkholgh A, Yi X, et al. (2009) Melatonin protects kidney grafts from ischemia/reperfusion injury through inhibition of NF-kB and apoptosis after experimental kidney transplantation. J. Pineal Res. 46 (4): 365-372. doi:10.1111/j.1600-079X.2009.00672.x.
120. Ates B, Yilmaz I, Geckil H, Iraz M, Birincioglu M, Fiskin K (2004) Protective role of melatonin given either before ischemia or prior to reperfusion on intestinal ischemia-reperfusion damage. J. Pineal Res. 37 (3): 149-152. doi:10.1111/j.1600-079X.2004.00148.x.
121. Yip HK, Chang YC, Wallace CG, et al. (2013) Melatonin treatment improves adipose-derived mesenchymal stem cell therapy for acute lung ischemia-reperfusion injury. J. Pineal Res. 54 (2): 207-221. doi:10.1111/jpi.12020.
122. Koksal M, Oğuz E, Baba F, et al. (2012) Effects of melatonin on testis histology, oxidative stress and spermatogenesis after experimental testis ischemia-reperfusion in rats. Eur. Rev. Med. Pharmacol. Sci. 16 (5): 582-588.
123. Nduhirabandi F, Du Toit EF, Blackhurst D, Marais D, Lochner A (2011) Chronic melatonin consumption prevents obesity-related metabolic abnormalities and protects the heart against myocardial ischemia and reperfusion injury in a prediabetic model of diet-induced obesity. J. Pineal Res. 50 (2): 171-182. doi:10.1111/j.1600-079X.2010.00826.x.
124. Dube K, Dhanabalan K, Salie R, Blignaut M, et al. (2019) Melatonin has profound effects on mitochondrial dynamics in myocardial ischaemia/reperfusion. Heliyon 5 (10): e02659. doi:10.1016/j.heliyon.2019.e02659.
125. Lamont KT, Somers S, Lacerda L, Opie LH, Lecour S (2011) Is red wine a SAFE sip away from cardioprotection? Mechanisms involved in resveratrol- and melatonin-induced cardioprotection. J. Pineal Res. 50 (4): 374-380. doi:10.1111/j.1600-079X.2010.00853.x.
126. Yang JB, Kang YM, Zhang C, Yu XJ, Chen WS (2019) Infusion of melatonin into the paraventricular nucleus ameliorates myocardial ischemia-reperfusion injury by regulating oxidative stress and inflammatory cytokines. J. Cardiovasc. Pharmacol. 74 (4): 336-347. doi:10.1097/FJC.0000000000000711.
127. Diez ER, Prados LV, Carrión A, et al. (2009) A novel electrophysiologic effect of melatonin on ischemia/reperfusion-induced arrhythmias in isolated rat hearts. J. Pineal Res. 46 (2): 155-160. doi:10.1111/j.1600-079X.2008.00643.x.
128. Chen Z, Chua CC, Gao J, et al. (2009) Prevention of ischemia/reperfusion-induced cardiac apoptosis and injury melatonin is independent of glutathione peroxdiase 1. J. Pineal Res. 46 (2): 235-241. doi:10.1111/j.1600-079X.2008.00654.x.
129. Petrosillo G, Colantuono G, Moro N, et al. (2009) Melatonin protects against heart ischemia-reperfusion injury by inhibiting mitochondrial permeability transition pore opening. Am. J. Physiol. Heart Circ. Physiol. 297 (4): H1487-93. doi:10.1152/ajpheart.00163.2009.
130. Feng J, Chen X, Liu R, et al. (2018) Melatonin protects against myocardial ischemia-reperfusion injury by elevating Sirtuin3 expression and manganese superoxide dismutase activity. Free Radic. Res. 52 (8): 840-849. doi:10.1080/10715762.2018.1461215.
131. Yu L, Liang H, Dong X, et al. (2015) Reduced silent information regulator 1 signaling exacerbates myocardial ischemia-reperfusion injury in type 2 diabetic rats and the protective effect of melatonin. J. Pineal Res. 59 (3): 376-390. doi:10.1111/jpi.12269.
132. Chen WR, Liu H Bin, Chen YD, et al. (2018) Melatonin attenuates myocardial ischemia/reperfusion injury by inhibiting autophagy via an AMPK/mTOR signaling pathway. Cell. Physiol. Biochem.. 47 (5): 2067-2076. doi:10.1159/000491474.
133. He B, Zhao Y, Xu L, et al. (2016) The nuclear melatonin receptor RORα is a novel endogenous defender against myocardial ischemia/reperfusion injury. J. Pineal Res. 60 (3): 313-326. doi:10.1111/jpi.12312.
134. Liu C, Jia Z, Zhang X, et al. (2012) Involvement of melatonin in autophagy-mediated mouse hepatoma H22 cell survival. Int. Immunopharmacol. 12 (2): 394-401. doi:10.1016/j.intimp.2011.12.012.
135. Niki E (2009) Lipid peroxidation: physiological levels and dual biological effects. Free Radic. Biol. Med. 47 (5): 469-484. doi:10.1016/j.freeradbiomed.2009.05.032.
136. Paradies G, Paradies V, Ruggiero FM, Petrosillo G (2018) Mitochondrial bioenergetics and cardiolipin alterations in myocardial ischemia-reperfusion injury: implications for pharmacological cardioprotection. Am. J. Physiol. Heart Circ. Physiol. 315 (5): H1341-H1352. doi:10.1152/ajpheart.00028.2018.
137. Dudek J (2017) Role of Cardiolipin in Mitochondrial Signaling Pathways. Front. cell Dev. Biol. 5: 90. doi:10.3389/fcell.2017.00090.
138. Kameoka S, Adachi Y, Okamoto K, Iijima M, Sesaki H (2018) Phosphatidic Acid and Cardiolipin Coordinate Mitochondrial Dynamics. Trends Cell. Biol. 28 (1): 67-76. doi:10.1016/j.tcb.2017.08.011.
139. Stepanyants N, Macdonald PJ, Francy CA, Mears JA, et al. (2015) Cardiolipin’s propensity for phase transition and its reorganization by dynamin-related protein 1 form a basis for mitochondrial membrane fission. Mol. Biol. Cell 26 (17): 3104-3116. doi:10.1091/mbc.E15-06-0330.
140. Ding M, Feng N, Tang D, et al. (2018) Melatonin prevents Drp1-mediated mitochondrial fission in diabetic hearts through SIRT1-PGC1α pathway. J. Pineal Res. 65 (2): e12491. doi:10.1111/jpi.12491.
141. Zhou H, Zhang Y, Hu S, et al. (2017) Melatonin protects cardiac microvasculature against ischemia/reperfusion injury via suppression of mitochondrial fission-VDAC1-HK2-mPTP-mitophagy axis. J. Pineal Res. 63 (1): e12413. doi:10.1111/jpi.12413.
142. Zhang Y, Wang Y, Xu J, et al. (2019) Melatonin attenuates myocardial ischemia-reperfusion injury via improving mitochondrial fusion/mitophagy and activating the AMPK-OPA1 signaling pathways. J. Pineal Res. 66 (2): e12542. doi:10.1111/jpi.12542.
143. Qi X, Wang J (2020) Melatonin improves mitochondrial biogenesis through the AMPK/PGC1α pathway to attenuate ischemia/reperfusion-induced myocardial damage. Aging (Albany NY). 12 (8): 7299-7312. doi:10.18632/aging.103078.
144. Chen WR, Zhou YJ, Sha Y, et al. (2020) Melatonin attenuates vascular calcification by inhibiting mitochondria fission via an AMPK/Drp1 signalling pathway. J. Cell. Mol. Med. 24 (11): 6043-6054. doi:10.1111/jcmm.15157.
145. Ma X, Wang S, Cheng H, et al. (2022) Melatonin attenuates ischemia/reperfusion-induced oxidative stress by activating mitochondrialfusion in cardiomyocytes. Oxid. Med. Cell. Longev. 2022: 7105181. doi:10.1155/2022/7105181.
146. Wu J, Yang Y, Gao Y, et al. (2020) Melatonin attenuates anoxia/reoxygenation injury by inhibiting excessive mitophagy through the MT2/SIRT3/FoxO3a signaling pathway in H9c2 Cells. Drug Des. Devel. Ther. 14: 2047-2060. doi:10.2147/DDDT.S248628.
147. Ma S, Dong Z (2019) Melatonin attenuates cardiac reperfusion stress by improving OPA1-related mitochondrial fusion in a Yap-Hippo pathway-dependent manner. J. Cardiovasc. Pharmacol. 73 (1): 27-39. doi:10.1097/FJC.0000000000000626.
148. Dai SH, Wu QC, Zhu RR, et al. (2020) Notch1 protects against myocardial ischaemia-reperfusion injury via regulating mitochondrial fusion and function. J. Cell. Mol. Med. 24 (5): 3183-3191. doi:10.1111/jcmm.14992.
149. Liu D, Ma Z, Di S, et al. (2018) AMPK/PGC1α activation by melatonin attenuates acute doxorubicin cardiotoxicity via alleviating mitochondrial oxidative damage and apoptosis. Free Radic. Biol. Med. 129: 59-72. doi:10.1016/j.freeradbiomed.2018.08.032.
150. Pei H, Du J, Song X, et al. (2016) Melatonin prevents adverse myocardial infarction remodeling via Notch1/Mfn2 pathway. Free Radic. Biol. Med. 97: 408-417. doi:10.1016/j.freeradbiomed.2016.06.015.
151. Yu LM, Dong X, Xue XD, et al. (2021) Melatonin attenuates diabetic cardiomyopathy and reduces myocardial vulnerability to ischemia-reperfusion injury by improving mitochondrial quality control: Role of SIRT6. J. Pineal Res. 70 (1): e12698. doi:10.1111/jpi.12698.
152. Singhanat K, Apaijai N, Jaiwongkam T, Kerdphoo S, et al. (2021) Melatonin as a therapy in cardiac ischemia-reperfusion injury: Potential mechanisms by which MT2 activation mediates cardioprotection. J. Adv. Res. 29: 33-44. doi:10.1016/j.jare.2020.09.007.
153. Yu L, Gong B, Duan W, et al. (2017) Melatonin ameliorates myocardial ischemia/reperfusion injury in type 1 diabetic rats by preserving mitochondrial function: role of AMPK-PGC-1α-SIRT3 signaling. Sci. Rep. 7: 41337. doi:10.1038/srep41337.
154. Guan L, Che Z, Meng X, et al. (2019) MCU up-regulation contributes to myocardial ischemia-reperfusion injury through calpain/OPA-1-mediated mitochondrial fusion/mitophagy Inhibition. J. Cell. Mol. Med. 23 (11): 7830-7843. doi:10.1111/jcmm.14662.
155. Naaz S, Mishra S, Pal PK, Chattopadhyay A, et al. (2020) Activation of SIRT1/PGC 1α/SIRT3 pathway by melatonin provides protection against mitochondrial dysfunction in isoproterenol induced myocardial injury. Heliyon. 6 (10): e05159. doi:10.1016/j.heliyon.2020.e05159.
156. Camara AKS, Bienengraeber M, Stowe DF (2011) Mitochondrial approaches to protect against cardiac ischemia and reperfusion injury. Front. Physiol. 2: 13. doi:10.3389/fphys.2011.00013.
157. Camara AKS, Lesnefsky EJ, Stowe DF (2010) Potential therapeutic benefits of strategies directed to mitochondria. Antioxid. Redox. Signal. 13 (3): 279-347. doi:10.1089/ars.2009.2788.
158. Petrosillo G, Di Venosa N, Pistolese M, et al. (2006) Protective effect of melatonin against mitochondrial dysfunction associated with cardiac ischemia- reperfusion: role of cardiolipin. FASEB. J. 20 (2): 269-276. doi:10.1096/fj.05-4692com.
159. Lesnefsky EJ, Moghaddas S, Tandler B, et al. (2001) Mitochondrial dysfunction in cardiac disease: ischemia--reperfusion, aging, and heart failure. J. Mol. Cell. Cardiol. 33 (6): 1065-1089. doi:10.1006/jmcc.2001.1378.
160. Paradies G, Petrosillo G, Pistolese M, et al. (2004) Decrease in mitochondrial complex I activity in ischemic/reperfused rat heart: involvement of reactive oxygen species and cardiolipin. Circ. Res. 94 (1): 53-59. doi:10.1161/01.RES.0000109416.56608.64.
161. López A, García JA, Escames G, et al. (2009) Melatonin protects the mitochondria from oxidative damage reducing oxygen consumption, membrane potential, and superoxide anion production. J. Pineal Res. 46 (2): 188-198. doi:10.1111/j.1600-079X.2008.00647.x.
162. Acuna-Castroviejo D, Escames G, Rodriguez MI, Lopez LC (2007) Melatonin role in the mitochondrial function. Front. Biosci. 12: 947-963. doi:10.2741/2116.
163. Dong Y, Undyala VVR, Przyklenk K (2016) Inhibition of mitochondrial fission as a molecular target for cardioprotection: critical importance of the timing of treatment. Basic Res. Cardiol. 111 (5): 59. doi:10.1007/s00395-016-0578-x.
164. Zaja I, Bai X, Liu Y, et al. (2014) Cdk1, PKCδ and calcineurin-mediated Drp1 pathway contributes to mitochondrial fission-induced cardiomyocyte death. Biochem. Biophys. Res. Commun. 453 (4): 710-721. doi:10.1016/j.bbrc.2014.09.144.
165. Bai Y, Yang Y, Gao Y, et al. (2021) Melatonin postconditioning ameliorates anoxia/reoxygenation injury by regulating mitophagy and mitochondrial dynamics in a SIRT3-dependent manner. Eur. J. Pharmacol. 904: 174157. doi:10.1016/j.ejphar.2021.174157
166. Wang JX, Jiao JQ, Li Q, Long B, et al. (2011) miR-499 regulates mitochondrial dynamics by targeting calcineurin and dynamin-related protein 1. Nat. Med. 17: 71-78. doi:10.1038/nm.2282.
167. Maneechote C, Palee S, Chattipakorn SC, Chattipakorn N (2017) Roles of mitochondrial dynamics modulators in cardiac ischaemia/reperfusion injury. J. Cell. Mol. Med. 21 (11) : 2643-2653. doi:10.1111/jcmm.13330.
168. Arinno A, Maneechote C, Khuanjing T, et al. (2021) Cardioprotective effects of melatonin and metformin against doxorubicin-induced cardiotoxicity in rats are through preserving mitochondrial function and dynamics. Biochem. Pharmacol. 192: 114743. doi:10.1016/j.bcp.2021.114743.
169. Pei H-F, Hou J-N, Wei F-P, et al. (2017) Melatonin attenuates postmyocardial infarction injury via increasing TOM70 expression. J. Pineal Res. 62 (1): e12371.
170. Kato H, Lu Q, Rapaport D, Kozjak-Pavlovic V (2013) Tom70 is essential for PINK1 import into mitochondria. PLoS One. 8 (3): e58435. doi:10.1371/journal.pone.0058435.
171. Zhou H, Li D, Zhu P, et al. (2017) Melatonin suppresses platelet activation and function against cardiac ischemia/reperfusion injury via PPARgamma/FUNDC1/mitiphagy pathways. J. Pineal Res. 63: e12438. doi:10.1111/jpi12438.
172. Yang J, He J, Ismail M, et al. (2019) HDAC inhibition induces autophagy and mitochondrial biogenesis to maintain mitochondrial homeostasis during cardiac ischemia/reperfusion injury. J. Mol. Cell. Cardiol. 130: 36-48. doi:10.1016/j.yjmcc.2019.03.008.
173. Sanz MN, Farine E, Niederberger P, et al. (2019) Cardioprotective reperfusion strategies differentially affect mitochondria: Studies in an isolated rat heart model of donation after circulatory death (DCD). Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 19 (2): 331-344. doi:10.1111/ajt.15024.
174. Niu YJ, Zhou W, Nie ZW, Shin KT, et al. (2020) Melatonin enhances mitochondrial biogenesis and protects against rotenone-induced mitochondrial deficiency in early porcine embryos. J. Pineal Res. 68 (2): e12627. doi:10.1111/jpi.12627.
175. Jiang LL, Zhang FP, He YF, et al. (2019) Melatonin regulates mitochondrial function and biogenesis during rat dental papilla cell differentiation. Eur. Rev. Med. Pharmacol. Sci. 23 (13):5967-5979. doi:10.26355/eurrev_201907_18343.
176. Wang CF, Song CY, Wang X, et al. (2019) Protective effects of melatonin on mitochondrial biogenesis and mitochondrial structure and function in the HEK293-APPswe cell model of Alzheimer’s disease. Eur. Rev. Med. Pharmacol. Sci. 23 (8): 3542-3550. doi:10.26355/eurrev_201904_17723.
177. Wang Z V, Hill JA (2015) Diabetic cardiomyopathy: catabolism driving metabolism. Circulation 131 (9): 771-773. doi:10.1161/CIRCULATIONAHA.115.015357.
178. Boudina S, Abel ED (2007) Diabetic cardiomyopathy revisited. Circulation 115 (25): 3213-3223. doi:10.1161/CIRCULATIONAHA.106.679597.
179. Wang S, Zhao Z, Feng X, et al. (2018) Melatonin activates Parkin translocation and rescues the impaired mitophagy activity of diabetic cardiomyopathy through Mst1 inhibition. J. Cell. Mol. Med. 22 (10): 5132-5144. doi:10.1111/jcmm.13802.
180. Kahn BB, Alquier T, Carling D, Hardie DG (2005) AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell. Metab. 1 (1): 15-25. doi:10.1016/j.cmet.2004.12.003.
181. Zhai M, Li B, Duan W, et al. (2017) Melatonin ameliorates myocardial ischemia reperfusion injury through SIRT3-dependent regulation of oxidative stress and apoptosis. J. Pineal Res. 63 (2): e12419. doi:10.1111/jpi.12419.
182. Hsu CP, Zhai P, Yamamoto T, et al. (2010) Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation 122 (21): 2170-2182. doi:10.1161/CIRCULATIONAHA.110.958033.
183. Tong C, Morrison A, Mattison S, et al. (2013) Impaired SIRT1 nucleocytoplasmic shuttling in the senescent heart during ischemic stress. FASEB J. 27 (11): 4332-4342. doi:10.1096/fj.12-216473.
184. Brunet A, Sweeney LB, Sturgill JF, et al. (2004) Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 303 (5666): 2011-2015. doi:10.1126/science.1094637.
185. Daitoku H, Hatta M, Matsuzaki H, et al. (2004) Silent information regulator 2 potentiates Foxo1-mediated transcription through its deacetylase activity. Proc. Natl. Acad. Sci. U. S. A. 101 (27): 10042-10047. doi:10.1073/pnas.0400593101.
186. Yu L, Sun Y, Cheng L, et al. (2014) Melatonin receptor-mediated protection against myocardial ischemia/reperfusion injury: role of SIRT1. J. Pineal Res. 57 (2): 228-238. doi:10.1111/jpi.12161.
187. Kanwal A, Pillai VB, Samant S, et al. (2019) The nuclear and mitochondrial sirtuins, Sirt6 and Sirt3, regulate each other’s activity and protect the heart from developing obesity-mediated diabetic cardiomyopathy. FASEB. J. 33 (10): 10872-10888. doi:10.1096/fj.201900767R.
188. Meng F, Qian M, Peng B, et al. (2020) Synergy between SIRT1 and SIRT6 helps recognize DNA breaks and potentiates the DNA damage response and repair in humans and mice. Elife 9: e55828. doi:10.7554/eLife.55828.
189. Pi H, Xu S, Reiter RJ, et al. (2015) SIRT3-SOD2-mROS-dependent autophagy in cadmium-induced hepatotoxicity and salvage by melatonin. Autophagy 11 (7): 1037-1051. doi:10.1080/15548627.2015.1052208.
190. Reiter RJ, Tan DX, Rosales-Corral S, Galano A, et al. (2018) Melatonin mitigates mitochondrial meltdown: interactions with SIRT3. Int. J. Mol. Sci. 19 (8): 2439. doi:10.3390/ijms19082439.
191. Zheng Y, Shi B, Ma M, et al. (2019) The novel relationship between Sirt3 and autophagy in myocardial ischemia-reperfusion. J. Cell. Physiol. 234 (5): 5488-5495. doi:10.1002/jcp.27329.
192. Zhang M, Lin J, Wang S, et al. (2017) Melatonin protects against diabetic cardiomyopathy through Mst1/Sirt3 signaling. J. Pineal Res. 63(2): e12418. doi:10.1111/jpi.12418.
193. Yu L, Liang H, Lu Z, et al. (2015) Membrane receptor-dependent Notch1/Hes1 activation by melatonin protects against myocardial ischemia-reperfusion injury: in vivo and in vitro studies. J. Pineal Res. 59 (4): 420-433. doi:10.1111/jpi.12272.
194. Zhou XL, Wu X, Xu QR, et al. (2019) Notch1 provides myocardial protection by improving mitochondrial quality control. J. Cell. Physiol. 234 (7): 11835-11841. doi:10.1002/jcp.27892.
195. Chen J, Zhong J, Wang LL, Chen YY (2021) Mitochondrial transfer in cardiovascular disease: from mechanisms to therapeutic implications. Front. Cardiovasc. Med. 8: 771298. doi:10.3389/fcvm.2021.771298.
196. Reiter RJ, Rosales-Corral S (2021) Melatonin, tunneling nanotubes and anastasis: Cheating cell death. Melatonin Res. 4 (4): 566-580. doi:10.32794/mr112500112.
197. Luchetti F, Carloni S, Nasoni MG, Reiter RJ, et al. (2022) Tunnelling nanotubes and mesenchymal stem cells: new insights into the role of melatonin in neuronal recovery. J. Pineal Research. 73 (1): e12800. doi: 10.111/jpi12800.
198. Martins-Marques T, Hausenloy DJ, Sluijter JPG, et al. (2021) Intercellular communication in the heart: therapeutic opportunities for cardiac ischemia. Trends Mol. Med. 27 (3): 248-262. doi:10.1016/j.molmed.2020.10.002.
199. Batista-Almeida D (2020) Ischaemia impacts TNT-mediated communications between cardiac cells. Curr. Res. Cell. Biol. 1: 100001.
200. Han H, Hu J, Yan Q, et al. (2016) Bone marrow -derived mesenchymal stenm cells rescue injured H9c2 cells via transferring intact mitochondria through tunneling nanotubes in an in vitro simulated ischemia/reperfusion model. Mol. Med. Rep. 13: 1517-1524. doi: 10.3892/mmr.2015.4726.
201. Zhang Y, Yu Z, Jiang D, et al. (2016) iPSC-MSCs with high intrinsic MIRO1 and sensitivity to TNF-α yield efficacious mitochondrial transfer to rescue Anthracycline-induced cardiomyopathy. Stem Cell Reports 7 (4): 749-763. doi:10.1016/j.stemcr.2016.08.009.
202. Figeac F, Lesault P-F, LeCoz O, Damy T, et al. (2014) Nanotubular crosstalk with distressed cardiomyocytes stimulates the paracrine repair function of mesenchymal stem cells. Stem Cells 32: 216. doi: 10.1002/stem.1560.
203. Nasoni MG, Carloni S, Canonico B, et al. (2021) Melatonin reshapes the mitochondrial network and promotes intercellular mitochondrial transfer via tunneling nanotubes after ischemic-like injury in hippocampal HT22 cells. J. Pineal Res. 71 (1): e12747. doi:10.1111/jpi.12747.
204. Yip HK, Dubey NK, Lin KC, et al. (2021) Melatonin rescues cerebral ischemic events through upregulated tunneling nanotube-mediated mitochondrial transfer and downregulated mitochondrial oxidative stress in rat brain. Biomed. Pharmacother. 139: 111593. doi:10.1016/j.biopha.2021.111593.
205. Wang J, Toan S, Li R, Zhou H (2020) Melatonin fine-tunes intracellular calcium signals and eliminates myocardial damage through the IP3R/MCU pathways in cardiorenal syndrome type 3. Biochem. Pharmacol. 174: 113832. doi:10.1016/j.bcp.2020.113832.
206. Viola HM, Livia CH (2014) How does calcium regulate mitochondrial energetics in the heart? New insights. Heart Lung Circ. 23: 602. doi:10.1016/j.hlc 2014.02.009
207. Kwong JQ (2017) The mitochondrial calcium uniporter in the heart. J. Physiol. 595: 3743. doi:10.1113/JP273059192.
208. Hughes WE, Beyer AM, Gutterman DD (2020) Vascular autophagy in health and disease. Basic Res. Cardiol. 115 (4): 41. doi:10.1007/s00395-020-0802-6.
209. Yang M, Li C, Yang S, et al. (2020) Mitochondria-associated ER membranes - the origin site of autophagy. Front. cell. Dev. Biol. 8: 595. doi:10.3389/fcell.2020.00595.
210. Wilson EL, Metzakopian E (2021) ER-mitochondria contact sites in neurodegeneration: genetic screening approaches to investigate novel disease mechanisms. Cell Death Differ. 28 (6): 1804-1821. doi:10.1038/s41418-020-00705-8.
211. Eisner V, Csordas G, Hajnoczky G (2013) Interactions between sarcoplasmic reticulum and mitochondria in cardiac and skeletal muscle-pivotal roles in Ca2+ and reactive oxygen species signaling. J. Cell Science 126: 2965-2978. doi:10.1242/jcs 093609.
212. Li W, Liu B, Wang L, Liu J, et al. (2021) Melatonin attenuates cardiac ischemia-reperfusion injury through modulation of IP3R-mediated mitochondria-ER contact. Oxid. Med. Cell. Longev. 2021: 1370862. doi:10.1155/2021/1370862.
213. Yeung HM, Hung MW, Fung ML (2008) Melatonin ameliorates calcium homeostasis in myocardial and ischemia-reperfusion injury in chronically hypoxic rats. J. Pineal Res. 45 (4): 373-382. doi:10.1111/j.1600-079X.2008.00601.x.
214. Hu S, Zhu P, Zhou H, et al. (2018) melatonin-induced protective effects on cardiomyocytes against reperfusion injury partly through modulation of IP3R and SERCA2a via activation of ERK1. Arq. Bras. Cardiol. 110 (1): 44-51. doi:10.5935/abc.20180008.
215. Kho C, Lee A, Jeong D, et al. (2015) Small-molecule activation of SERCA2a SUMOylation for the treatment of heart failure. Nat. Commun. 6:7229. doi:10.1038/ncomms8229.
216. Hayashi T, Su TP (2007) Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell 131 (3): 596-610. doi:10.1016/j.cell.2007.08.036.
217. Zhou H, Wang J, Zhu P,Hu S, et al. (2018) Ripk3 regulates cardiac microvascular reperfusion injury: the role of IP3R-dependent calcium overload, XO-mediated oxidative stress and F-action/filopodia based cellular migration. Cell Signal. 45: 12-22. doi:10.1016/j.cellsig.2018.01.020.
218. Zhang Y, Zhou H, Wu W, et al. (2016) Liraglutide protects cardiac microvascular endothelial cells against hypoxia/reoxygenation injury through the suppression of the SR-[Ca2+]-XO-ROS axis via activation of the GLP-IR/PI3K/Akt/survivin pathways. Free Radic. Biol. Med. 95: 278. doi:10.1016/j.freeradbiomed.2016.03.035.
219. Zhu H, Jin Q, Li Y, et al. (2018) Melatonin protected cardiac microvascular endothelial cells against oxidative stress via suppression of IP3R-[Ca2+]c/VDAC-[Ca2+]m by activation of MAPK/ERK signaling pathway. Cell Stress Chaperones 23: 101. doi:10.1007/512192-017-0827-4.
220. Ding M, Ning J, Feng N, Li Z, et al. (2018). Dynamin-related protein 1 mediated mitochondrial fission contributes to post-traumatic cardiac dysfunction in rats and the protective effect of melatonin. J. Pineal Res. 64: 12447. doi:10.1111/jpi 12447.
221. Bindoli A, Rigobello MP, Deeble DJ (1992) Biochemical and toxicological properties of the oxidation products of catecholamines. Free Radic. Biol. Med. 13 (4): 391-405. doi:10.1016/0891-5849(92)90182-g.
222. Chattopadhyay A, Biswas S, Bandyopadhyay D, et al. (2003) Effect of isoproterenol on lipid peroxidation and antioxidant enzymes of myocardial tissue of mice and protection by quinidine. Mol. Cell. Biochem. 245 (1-2): 43-49. doi:10.1023/a:1022808224917.
223. Mukherjee D, Roy SG, Bandyopadhyay A, et al. (2010) Melatonin protects against isoproterenol-induced myocardial injury in the rat: antioxidative mechanisms. J. Pineal Res. 48 (3): 251-262. doi:10.1111/j.1600-079X.2010.00749.x.
224. Khalil MI, Ahmmed I, Ahmed R, et al. (2015) Amelioration of isoproterenol-induced oxidative damage in rat myocardium by Withania somnifera leaf extract. Biomed. Res. Int. 2015: 624159. doi:10.1155/2015/624159.
225. Mukherjee D, Ghosh AK, Dutta M, et al. (2015) Mechanisms of isoproterenol-induced cardiac mitochondrial damage: protective actions of melatonin. J. Pineal Res. 58 (3): 275-290. doi:10.1111/jpi.12213.
226. Chen Y, McMillan-Ward E, Kong J, Israels SJ, Gibson SB (2007) Mitochondrial electron-transport-chain inhibitors of complexes I and II induce autophagic cell death mediated by reactive oxygen species. J. Cell. Sci. 120 (Pt 23): 4155-4166. doi:10.1242/jcs.011163.
227. Li B, Chauvin C, De Paulis D, et al. (2012) Inhibition of complex I regulates the mitochondrial permeability transition through a phosphate-sensitive inhibitory site masked by cyclophilin D. Biochim. Biophys. Acta 1817 (9): 1628-1634. doi:10.1016/j.bbabio.2012.05.011.
228. Pei W, Liou AKF, Chen J (2003) Two caspase-mediated apoptotic pathways induced by rotenone toxicity in cortical neuronal cells. FASEB. J. 17 (3): 520-522. doi:10.1096/fj.02-0653fje.
229. Joselin AP, Hewitt SJ, Callaghan SM, et al. (2012) ROS-dependent regulation of Parkin and DJ-1 localization during oxidative stress in neurons. Hum. Mol. Genet. 21 (22): 4888-4903. doi:10.1093/hmg/dds325.
230. Niu Y, Zhou W, Nie Z-W, et al. (2020) Melatonin enhances mitochondrial biogenesis and protects against rotenone-induced mitochondrial deficiency in early porcine embryos. J. Pineal Res. 68: e12627. doi:10.1111/jpi12627.
231. Chen L, Tian Q, Shi Z, et al. (2021) Melatonin alleviates cardiac function in sepsis-caused myocarditis via maintenance of mitochondrial function. Front. Nutr. 8: 754235. doi:10.3389/fnut.2021.754235.
232. Zhang J, Wang L, Xie W, Hu S, et al. (2019). Melatonin attenuates ER stress and mitochondrial damage in septic cardiomyopathy: a new mechanism involving BAP31 upregulation and MAPK-ERK pathway. J. Cell. Physiol. 235: 2847-2856. doi: 10.1002/jcp.29190.
233. Namba T (2019) BAP31 regulates mitochondrial function via interaction with TOM40 within ER-mitochondrial contact sites. Sci. Adv. 5: eaaw1386-1-eaaw1386-12. doi: 10.1126/sciadv.1386.
234. Zhang WX, He BM, Wu Y, Qiao JF, et al. (2019) Melatonin protects against sepsis-induced cardiac dysfunction by regulating apoptosis and autophagyvia activation of SIRT1 in mice. Life Sci. 217: 8-15. doi: 10.1016/j.lfs.2018.11.055.
235. Cuny GD, Degterev A (2021) RIPK protein kinase family: atypical lives of a typical kinase. Semin. Cell. Dev. Biol. 109: 96-105. doi 1o.1016/j.semcdb.2020.06.0114.
236. Zhong J, Tan Y, Lu J, Liu J, et al. (2019) Therapeutic contribution of melatonin ton treatment of septic cardiomyopathy: a novel mechnanism linking Ripk3-modified mitochondrial performance and endoplasmic reticulum function. Redox. Biol. 26: 101287.doi: 10.1016/j.redox.2019.101287.
237. Cucielo MS, Cesário RC, Silveira HS, Gaiotte LB, et al. (2022). Melatonin reverses the Warburg-type metabolism and reduces mitochondrial membrane potential of ovarian cancer Cells independent of MT1 Receptor Activation. Molecules 27 (14): 4350. doi: 10.3390/molecules27144350.
238. Anderson G, Rodriguez M, Reiter RJ (2019) Multiple Sclerosis: Melatonin, orexin, and ceramide interact with platelet activation coagulation factors and gut-microbiome-derived butyrate in the circadian dysregulation of mitochondria in glia and Immune cells. Int. J. Mol. Sci. 20 (21): 5500. doi: 10.3390/ijms20215500.
239. Anderson G, Maes M (2020) Gut dysbiosis dysregulates central and systemic homeostasis via suboptimal mitochondrial function: assessment, treatment and classification Implications. Curr. Top. Med. Chem. 20 (7): 524-539. doi: 10.2174/1568026620666200131094445.
240. Yu Z, Han J, Chen H, Wang Y, et al. (2021) Oral supplementation with butyrate improves myocardial ischemia/reperfusion injuryviaa gut-brain neural circuit. Front Cardiovasc. Med. 8: 718674. doi: 10.3389/fcvm.2021.718674.
241. Baltatu OC, Senar S, Campos LA, Cipolla-Neto J (2019) Cardioprotective Melatonin: Translating from Proof-of-Concept Studies to Therapeutic Use. Int. J. Mol. Sci. 20 (18). doi:10.3390/ijms20184342.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


