An insight into the importance of B vitamins and melatonin in the prevention of diabetes through modulation of the brain energy metabolism- a comprehensive review
Melatonin and B vitamins in brain metabolism and diabetes
Abstract
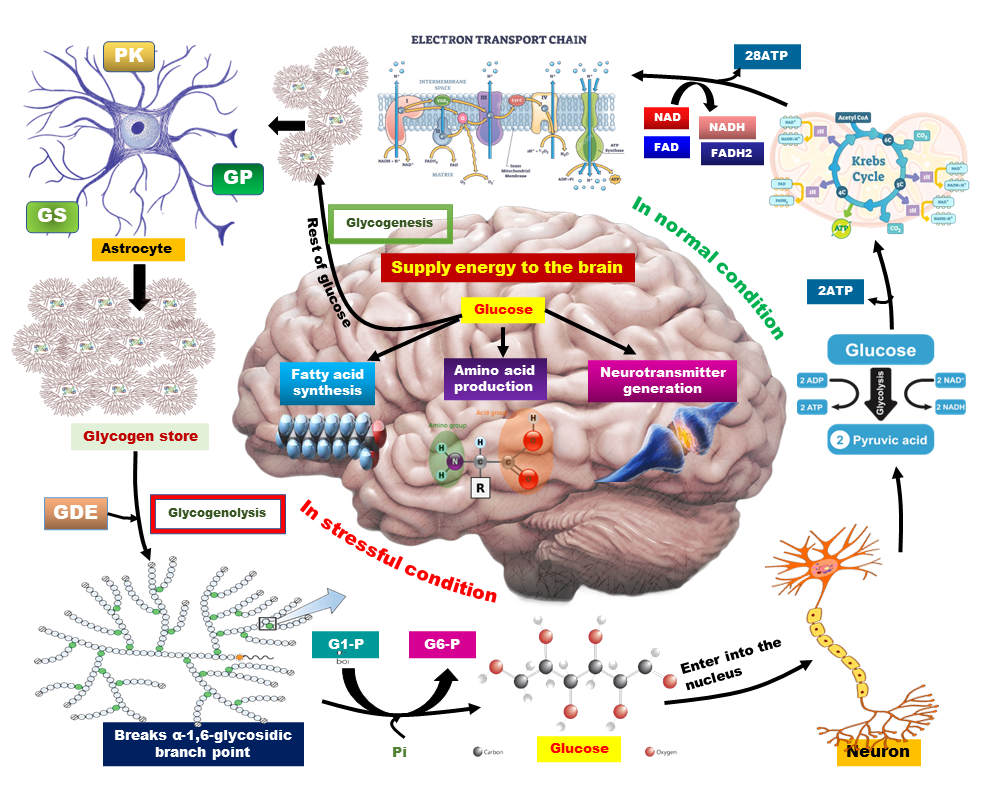
Energy metabolism is the biochemical pathway of converting macronutrients (carbohydrates, protein, and fat) to cellular energy for the maintenance of cell homeostasis. The brain is an organ that consumes unproportional energy compared to its size. Glucose (glycogen, in storage form of glucose) is the principal source of brain energy. Impairment in brain energy metabolism results in neuronal loss and subsequent neurodegenerative diseases including AD, PD, amyotrophic lateral sclerosis, Huntington’s disease, etc. However, metabolic disorders such as chronic hyperglycemia, and insulin resistance are also linked with neuronal activity. Dysregulation in neuronal transmission is associated with oxidative stress and brain insulin resistance. Diabetes mellitus jeopardizes brain function through various mechanisms including glucose toxicity, BBB damage, neuroinflammation, and gliosis. B vitamins as antioxidants and neuroprotective agents, can improve brain glucose metabolism. Melatonin is a potent free radical scavenger and it can also modulate cellular cytokine levels and prevent insulin resistance. The neuroprotective and antihyperglycemic effects of melatonin improve the brain's antioxidant defense system, decrease brain NOS activity, and prevent glucose toxicity. Hence this review suggests a therapeutic use of a combination of melatonin and B vitamins to improve brain functioning disrupted by diabetes.
References
2. Attwell D, Laughlin SB (2001) An energy budget for signaling in the grey matter of the brain. J. Cereb. Blood Flow Metab. 21 (10): 1133–1145. DOI: 10.1097/00004647-200110000-00001.
3. Howarth C, Gleeson P, Attwell D (2012) Updated energy budgets for neural computation in the neocortex and cerebellum. J. Cereb. Blood Flow Metab. 32 (7): 1222–1232. DOI: 10.1038/jcbfm.2012.35.
4. Mergenthaler P, Lindauer U, Dienel GA, Meisel A (2013) Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci. 36 (10): 587–597. DOI: 10.1016/j.tins.2013.07.001.
5. Brown AM, Ransom BR (2007) Astrocyte glycogen and brain energy metabolism. Glia 55 (12): 1263–1271. DOI: 10.1002/glia.20557.
6. Zoccoli G, Silvani A, Franzini C 2017. (2017) Sleep and the peripheral vascular system. In: Reference Module in Neuroscience and Biobehavioral Psychology. Elsevier; 2017. p. 563–567. DOI: 10.1016/B978-0-12-809324-5.00964-0.
7. FA. Beltrán, AI. Acuña MM and MC 2012. (2012) Brain energy metabolism in health and disease. In: Neuroscience - Dealing With Frontiers. In Tech; 2012. DOI: 10.5772/36092.
8. Mazziotta JC, Phelps ME, Pahl JJ, Huang S-C, Baxter LR, Riege WH, Hoffman JM, Kuhl DE, Lanto AB, Wapenski JA, Markham CH (1987) Reduced cerebral glucose metabolism in asymptomatic subjects at risk for huntington’s disease. N. Engl. J. Med. 316 (7): 357–362. DOI: 10.1056/NEJM198702123160701.
9. Mosconi L, Tsui WH, De Santi S, Li J, Rusinek H, Convit A, Li Y, Boppana M, de Leon MJ (2005) Reduced hippocampal metabolism in MCI and AD: Automated FDG-PET image analysis. Neurology 64 (11): 1860–1867. DOI: 10.1212/01.WNL.0000163856.13524.08.
10. Leenders KL, Frackowiak RSJ, Quinn N, Marsden CD (1986) Brain energy metabolism and dopaminergic function in Huntington’s disease measured in vivo using positron emission tomography. Mov. Disord. 1 (1): 69–77. DOI: 10.1002/mds.870010110.
11. Hattingen E, Magerkurth J, Pilatus U, Mozer A, Seifried C, Steinmetz H, Zanella F, Hilker R (2009) Phosphorus and proton magnetic resonance spectroscopy demonstrates mitochondrial dysfunction in early and advanced Parkinson’s disease. Brain 132 (12): 3285–3297. DOI: 10.1093/brain/awp293.
12. Duarte J (2014) Metabolic alterations associated to brain dysfunction in diabetes. Aging Dis. 6 (5): 304–321. DOI: 10.14336/ad.2014.1104.
13. Frisardi V, Solfrizzi V, Seripa D, Capurso C, Santamato A, Sancarlo D, Vendemiale G, Pilotto A, Panza F (2010) Metabolic-cognitive syndrome: A cross-talk between metabolic syndrome and Alzheimer’s disease. Ageing Res. Rev. 9 (4): 399–417. DOI: 10.1016/j.arr.2010.04.007.
14. Spauwen PJJ, Köhler S, Verhey FRJ, Stehouwer CDA, van Boxtel MPJ (2013) Effects of type 2 diabetes on 12-year cognitive change. Diabetes Care. 36 (6): 1554–1561. DOI: 10.2337/dc12-0746.
15. Craft S, Peskind E, Schwartz MW, Schellenberg GD, Raskind M, Porte D (1998) Cerebrospinal fluid and plasma insulin levels in Alzheimer’s disease. Neurology 50 (1): 164–168. DOI: 10.1212/WNL.50.1.164.
16. Hurrle S, Hsu WH (2017) The etiology of oxidative stress in insulin resistance. Biomed. J. 40 (5): 257–262. DOI: 10.1016/j.bj.2017.06.007.
17. Dugan L, Sensi S, Canzoniero L, Handran S, Rothman S, Lin T, Goldberg M, Choi D (1995) Mitochondrial production of reactive oxygen species in cortical neurons following exposure to N-methyl-D-aspartate. J. Neurosci. 15 (10): 6377–6388. DOI: 10.1523/JNEUROSCI.15-10-06377.1995.
18. Jackson GR, Werrbach-Perez K, Pan Z, Sampath D, Perez-Polo JR (1994) Neurotrophin regulation of energy homeostasis in the central nervous system. Dev. Neurosci. 16 (5–6): 285–290. DOI: 10.1159/000112121.
19. Garcia-Serrano AM, Duarte JMN (2020) Brain metabolism alterations in type 2 diabetes: what did we learn from diet-induced diabetes models? Front. Neurosci. 14 (229): 1–11. DOI: 10.3389/fnins.2020.00229.
20. Muriach M, Flores-Bellver M, Romero FJ, Barcia JM (2014) Diabetes and the brain: oxidative stress, inflammation, and autophagy. Oxid. Med. Cell. Longev. 2014 (102158): 1–9. DOI: 10.1155/2014/102158.
21. Mukhopadhyay M, Majumder R, Banerjee A, Bandyopadhyay D (2022) A comparative overview on the role of melatonin and vitamins as potential antioxidants against oxidative stress induced degenerative infirmities: An emerging concept. Melatonin Res. 5 (3): 254–277. DOI: 10.32794/mr112500131.
22. Tan D-X, Manchester LC, Hardeland R, Lopez-Burillo S, Mayo JC, Sainz RM, Reiter RJ (2003) Melatonin: a hormone, a tissue factor, an autocoid, a paracoid, and an antioxidant vitamin. J. Pineal Res. 34 (1): 75–78. DOI: 10.1034/j.1600-079X.2003.02111.x.
23. Darnton-Hill I (2019) Public health aspects in the prevention and control of vitamin deficiencies. Curr. Dev. Nutr. 3 (9): 1–14. DOI: 10.1093/cdn/nzz075.
24. Kennedy D (2016) B vitamins and the Brain: mechanisms, dose and efficacy—A review. Nutrients 8 (2): 68. DOI: 10.3390/nu8020068.
25. Smith AG, Croft MT, Moulin M, Webb ME (2007) Plants need their vitamins too. Curr. Opin. Plant Biol. 10 (3): 266–275. DOI: 10.1016/j.pbi.2007.04.009.
26. Homocysteine Studies Collaboration (2002) Homocysteine and risk of ischemic heart disease and stroke. JAMA 288 (16): 2015. DOI: 10.1001/jama.288.16.2015.
27. Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D’Agostino RB, Wilson PWF, Wolf PA (2002) Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N. Engl. J. Med. 346 (7): 476–483. DOI: 10.1056/NEJMoa011613.
28. Smulders YM, Blom HJ (2011) The homocysteine controversy. J. Inherit. Metab. Dis. 34 (1): 93–99. DOI: 10.1007/s10545-010-9151-1.
29. Reppart SM, Weaver DR, Godson C (1996) Melatonin receptors step into the light: cloning and classification of subtypes. Trends Pharmacol. Sci. 17 (3): 100–102. DOI: 10.1016/0165-6147(96)10005-5.
30. Costa EJX, Lopes RH, Lamy-Freund MT (1995) Permeability of pure lipid bilayers to melatonin. J. Pineal Res. 19 (3): 123–126. DOI: 10.1111/j.1600-079X.1995.tb00180.x.
31. Tan DX, Reiter R, Manchester L, Yan M, El-Sawi M, Sainz R, Mayo J, Kohen R, Allegra M, Hardelan R (2002) Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr. Top. Med. Chem. 2 (2): 181–197. DOI: 10.2174/1568026023394443.
32. Reiter R, Tan DX, Manchester L, Terrón MP, Flores LJ, Koppisepi S (2007) Medical implications of melatonin: receptor-mediated and receptor-independent actions. Adv. Med. Sci. 52 : 11–28.
33. Leston J, Harthé C, Brun J, Mottolese C, Mertens P, Sindou M, Claustrat B (2010) Melatonin is released in the third ventricle in humans. A study in movement disorders. Neurosci. Lett. 469 (3): 294–297. DOI: 10.1016/j.neulet.2009.12.008.
34. Uz T, Ahmed R, Akhisaroglu M, Kurtuncu M, Imbesi M, Dirim Arslan A, Manev H (2005) Effect of fluoxetine and cocaine on the expression of clock genes in the mouse hippocampus and striatum. Neuroscience 134 (4): 1309–1316. DOI: 10.1016/j.neuroscience.2005.05.003.
35. Uz T, Qu T, Sugaya K, Manev H (2002) Neuronal expression of arylalkylamine N-acetyltransferase (AANAT) mRNA in the rat brain. Neurosci. Res. 42 (4): 309–316. DOI: 10.1016/S0168-0102(02)00011-1.
36. Tan D-X, Manchester L, Esteban-Zubero E, Zhou Z, Reiter R (2015) Melatonin as a potent and inducible endogenous antioxidant: synthesis and metabolism. Molecules 20 (10): 18886–18906. DOI: 10.3390/molecules201018886.
37. Acuña-Castroviejo D, Escames G, LeÓn J, Carazo A, Khaldy H 2003. (2003) Mitochondrial regulation by melatonin And its metabolites. In: Advances in Experimental Medicine and Biology. 2003. p. 549–557. DOI: 10.1007/978-1-4615-0135-0_63.
38. Rehman S, Ikram M, Ullah N, Alam S, Park H, Badshah H, Choe K, Ok Kim M (2019) Neurological enhancement effects of melatonin against brain injury-induced oxidative stress, neuroinflammation, and neurodegeneration via AMPK/CREB signaling. Cells 8 (7): 760. DOI: 10.3390/cells8070760.
39. Turgut M, Kaplan S (2011) Effects of melatonin on peripheral nerve regeneration. Recent Pat. Endocr. Metab. Immune Drug Discov. 5 (2): 100–108. DOI: 10.2174/187221411799015336.
40. Banerjee A, Chattopadhyay A, Bandyopadhyay D (2021) Potentially synergistic effects of melatonin and metformin in alleviating hyperglycaemia: a comprehensive review. Melatonin Res. 4 (4): 522–550. DOI: 10.32794/mr112500110.
41. Erbsluh F, Bernsmeier A, Hillesheim HR (1958) The glucose consumption of the brain & its dependence on the liver. Arch. fur Psychiatr. und Nervenkrankheiten Ver. mit Zeitschrift fur die Gesamte Neurol. und Psychiatr. 196 (6): 611–626. DOI: 10.1007/BF00344388.
42. Mathieu C, de la Sierra-Gallay IL, Duval R, Xu X, Cocaign A, Léger T, Woffendin G, Camadro J-M, Etchebest C, Haouz A, Dupret J-M, Rodrigues-Lima F (2016) Insights into brain glycogen metabolism. J. Biol. Chem. 291 (35): 18072–18083. DOI: 10.1074/jbc.M116.738898.
43. Newgard CB, Hwang PK, Fletterick RJ (1989) The family of glycogen phosphorylases: structure and function. Crit. Rev. Biochem. Mol. Biol. 24 (1): 69–99. DOI: 10.3109/10409238909082552.
44. Hossain MI, Roulston CL, Stapleton DI (2014) Molecular basis of impaired glycogen metabolism during ischemic stroke and hypoxia. Coles JA, editor. PLoS One 9 (5): e97570. DOI: 10.1371/journal.pone.0097570.
45. Choi I-Y, Seaquist ER, Gruetter R (2003) Effect of hypoglycemia on brain glycogen metabolism in vivo. J. Neurosci. Res. 72 (1): 25–32. DOI: 10.1002/jnr.10574.
46. Swanson RA, Sagar SM, Sharp FR (1989) Regional brain glycogen stores and metabolism during complete global ischaemia. Neurol. Res. 11 (1): 24–28. DOI: 10.1080/01616412.1989.11739856.
47. Pfeiffer-Guglielmi B, Fleckenstein B, Jung G, Hamprecht B (2003) Immunocytochemical localization of glycogen phosphorylase isozymes in rat nervous tissues by using isozyme-specific antibodies. J. Neurochem. 85 (1): 73–81. DOI: 10.1046/j.1471-4159.2003.01644.x.
48. Pellegri G, Rossier C, Magistretti PJ, Martin J-L (1996) Cloning, localization and induction of mouse brain glycogen synthase. Mol. Brain Res. 38 (2): 191–199. DOI: 10.1016/0169-328X(95)00305-C.
49. Magistretti PJ, Sorg O, Martin J-L 1993. (1993) Regulation of glycogen metabolism in astrocytes: physiological, pharmacological, and pathological aspects. In: Astrocytes. Elsevier; 1993. p. 243–265. DOI: 10.1016/B978-0-12-511370-0.50015-1.
50. Adeva-Andany MM, González-Lucán M, Donapetry-García C, Fernández-Fernández C, Ameneiros-Rodríguez E (2016) Glycogen metabolism in humans. BBA Clin. 5 : 85–100. DOI: 10.1016/j.bbacli.2016.02.001.
51. Nadeau OW, Fontes JD, Carlson GM (2018) The regulation of glycogenolysis in the brain. J. Biol. Chem. 293 (19): 7099–7107. DOI: 10.1074/jbc.R117.803023.
52. Camandola S, Mattson MP (2017) Brain metabolism in health, aging, and neurodegeneration. EMBO J. 36 (11): 1474–1492. DOI: 10.15252/embj.201695810.
53. Shah K, DeSilva S, Abbruscato T (2012) The role of glucose transporters in brain disease: diabetes and alzheimer’s disease. Int. J. Mol. Sci. 13 (12): 12629–12655. DOI: 10.3390/ijms131012629.
54. Dwyer DS, Vannucci SJ, Simpson IA (2002) Expression, regulation, and functional role of glucose transporters (GLUTs) in brain. Int. Rev. Neurobiol. 51 : 159–188. DOI: 10.1016/S0074-7742(02)51005-9.
55. Reagan LP, Rosell DR, Alves SE, Hoskin EK, McCall AL, Charron MJ, McEwen BS (2002) GLUT8 glucose transporter is localized to excitatory and inhibitory neurons in the rat hippocampus. Brain Res. 932 (1–2): 129–134. DOI: 10.1016/S0006-8993(02)02308-9.
56. Vannucci SJ, Koehler-Stec EM, Li K, Reynolds TH, Clark R, Simpson IA (1998) GLUT4 glucose transporter expression in rodent brain: effect of diabetes. Brain Res. 797 (1): 1–11. DOI: 10.1016/S0006-8993(98)00103-6.
57. Joost H-G, Thorens B (2001) The extended GLUT-family of sugar/polyol transport facilitators: nomenclature, sequence characteristics, and potential function of its novel members. Mol. Membr. Biol. 18 (4): 247–256. DOI: 10.1080/09687680110090456.
58. Reagan LP, Magariños AM, Yee DK, Swzeda LI, Van Bueren A, McCall AL, McEwen BS (2000) Oxidative stress and HNE conjugation of GLUT3 are increased in the hippocampus of diabetic rats subjected to stress. Brain Res. 862 (1–2): 292–300. DOI: 10.1016/S0006-8993(00)02212-5.
59. Wright EM, Turk E, Hager K, Lescale-Matys L, Hirayama B, Supplisson S, Loo DD (1992) The Na+/glucose cotransporter (SGLT1). Acta Physiol. Scand. Suppl. 607 : 201–207.
60. Koepsell H (2020) Glucose transporters in brain in health and disease. Pflügers Arch. - Eur. J. Physiol. 472 (9): 1299–1343. DOI: 10.1007/s00424-020-02441-x.
61. Pardridge WM, Triguero D, Farrell CR (1990) Downregulation of blood-brain barrier glucose transporter in experimental diabetes. Diabetes 39 (9): 1040–1044. DOI: 10.2337/diabetes.39.9.1040.
62. Cornford EM, Hyman S, Cornford ME, Clare-Salzler M (1995) Down-regulation of blood-brain glucose transport in the hyperglycemic nonobese diabetic mouse. Neurochem. Res. 20 (7): 869–873. DOI: 10.1007/BF00969700.
63. Brownlee M (2001) Biochemistry and molecular cell biology of diabetic complications. Nature 414 (6865): 813–820. DOI: 10.1038/414813a.
64. Choi I-Y, Lee S-P, Kim S-G, Gruetter R (2001) In vivo measurements of brain glucose transport using the reversible michaelis–menten model and simultaneous measurements of cerebral blood flow changes during hypoglycemia. J. Cereb. Blood Flow Metab. 21 (6): 653–663. DOI: 10.1097/00004647-200106000-00003.
65. Gruetter R, Ugurbil K, Seaquist ER (2002) Steady-state cerebral glucose concentrations and transport in the human brain. J. Neurochem. 70 (1): 397–408. DOI: 10.1046/j.1471-4159.1998.70010397.x.
66. Ueno M, Chiba Y, Matsumoto K, Murakami R, Fujihara R, Kawauchi M, Miyanaka H, Nakagawa T (2016) Blood-brain barrier damage in vascular dementia. Neuropathology 36 (2): 115–124. DOI: 10.1111/neup.12262.
67. Edison P, Archer HA, Hinz R, Hammers A, Pavese N, Tai YF, Hotton G, Cutler D, Fox N, Kennedy A, Rossor M, Brooks DJ (2007) Amyloid, hypometabolism, and cognition in Alzheimer disease: An [11C]PIB and [18F]FDG PET study. Neurology 68 (7): 501–508. DOI: 10.1212/01.wnl.0000244749.20056.d4.
68. Chen Z, Zhong C (2013) Decoding Alzheimer’s disease from perturbed cerebral glucose metabolism: Implications for diagnostic and therapeutic strategies. Prog. Neurobiol. 108 : 21–43. DOI: 10.1016/j.pneurobio.2013.06.004.
69. Fernandez AM, Hernandez-Garzón E, Perez-Domper P, Perez-Alvarez A, Mederos S, Matsui T et al. (2017) Insulin regulates astrocytic glucose handling through cooperation with IGF-I. Diabetes 66 (1): 64–74. DOI: 10.2337/db16-0861.
70. Gibbs ME, Lloyd HGE, Santa T, Hertz L (2007) Glycogen is a preferred glutamate precursor during learning in 1-day-old chick: Biochemical and behavioral evidence. J. Neurosci. Res. 85 (15): 3326–3333. DOI: 10.1002/jnr.21307.
71. Duarte JMN, Morgenthaler FD, Gruetter R (2017) Glycogen supercompensation in the rat brain after acute hypoglycemia is independent of glucose levels during recovery. Neurochem. Res. 42 (6): 1629–1635. DOI: 10.1007/s11064-017-2178-z.
72. Lee JH, Jahrling JB, Denner L, Dineley KT (2018) Targeting insulin for Alzheimer’s disease: mechanisms, status and potential directions. Perry G, Avila J, Moreira PI, Sorensen AA, Tabaton M, editors. J. Alzheimer’s Dis. 64 (s1): S427–S453. DOI: 10.3233/JAD-179923.
73. Willette AA, Modanlo N, Kapogiannis D (2015) Insulin resistance predicts medial temporal hypermetabolism in mild cognitive impairment conversion to Alzheimer disease. Diabetes 64 (6): 1933–1940. DOI: 10.2337/db14-1507.
74. Heni M, Kullmann S, Preissl H, Fritsche A, Häring H-U (2015) Impaired insulin action in the human brain: causes and metabolic consequences. Nat. Rev. Endocrinol. 11 (12): 701–711. DOI: 10.1038/nrendo.2015.173.
75. Belenguer P, Duarte JMN, Schuck PF, Ferreira GC (2019) Mitochondria and the brain: bioenergetics and beyond. Neurotox. Res. 36 (2): 219–238. DOI: 10.1007/s12640-019-00061-7.
76. Santos R, Correia S, Alves M, Oliveira PF, Cardoso S, Carvalho C, Duarte AI, Santos MS, Moreira PI (2014) Insulin therapy modulates mitochondrial dynamics and biogenesis, autophagy and tau protein phosphorylation in the brain of type 1 diabetic rats. Biochim. Biophys. Acta - Mol. Basis Dis. 1842 (7): 1154–1166. DOI: 10.1016/j.bbadis.2014.04.011.
77. Guo L, Tian J, Du H (2017) Mitochondrial dysfunction and synaptic transmission failure in Alzheimer’s disease. J. Alzheimer’s Dis. 57 (4): 1071–1086. DOI: 10.3233/JAD-160702.
78. Sonnay S, Gruetter R, Duarte JMN (2017) How energy metabolism supports cerebral function: insights from 13C magnetic resonance studies in vivo. Front. Neurosci. 11: 288. DOI: 10.3389/fnins.2017.00288.
79. Alberini CM, Cruz E, Descalzi G, Bessières B, Gao V (2018) Astrocyte glycogen and lactate: New insights into learning and memory mechanisms. Glia 66 (6): 1244–1262. DOI: 10.1002/glia.23250.
80. Duran J, Gruart A, Varea O, López-Soldado I, Delgado-García JM, Guinovart JJ (2019) Lack of neuronal glycogen impairs memory formation and learning-dependent synaptic plasticity in mice. Front. Cell. Neurosci. 13 . DOI: 10.3389/fncel.2019.00374.
81. Soares AF, Nissen JD, Garcia‐Serrano AM, Nussbaum SS, Waagepetersen HS, Duarte JMN (2019) Glycogen metabolism is impaired in the brain of male type 2 diabetic Goto‐Kakizaki rats. J. Neurosci. Res. 97 (8): 1004–1017. DOI: 10.1002/jnr.24437.
82. Kelly Á, Laroche S, Davis S (2003) Activation of mitogen-activated protein kinase/extracellular signal-regulated kinase in Hippocampal circuitry is required for consolidation and reconsolidation of recognition memory. J. Neurosci. 23 (12): 5354–5360. DOI: 10.1523/JNEUROSCI.23-12-05354.2003.
83. Marinangeli C, Didier S, Vingtdeux V (2016) AMPK in neurodegenerative diseases: implications and therapeutic perspectives. Curr. Drug Targets 17 (8): 890–907. DOI: 10.2174/1389450117666160201105645.
84. Guilarte TR (2009) Vitamin B6 and cognitive development: recent research findings from human and animal studies. Nutr. Rev. 51 (7): 193–198. DOI: 10.1111/j.1753-4887.1993.tb03102.x.
85. Hutto BR (1997) Folate and cobalamin in psychiatric illness. Compr. Psychiatry 38 (6): 305–314. DOI: 10.1016/S0010-440X(97)90925-1.
86. Dror DK, Allen LH (2008) Effect of vitamin B12 deficiency on neurodevelopment in infants: current knowledge and possible mechanisms. Nutr. Rev. 66 (5): 250–255. DOI: 10.1111/j.1753-4887.2008.00031.x.
87. Spector R (2014) Vitamin transport diseases of brain: focus on folates, thiamine and riboflavin. Brain Disord. Ther. 03 (02). DOI: 10.4172/2168-975X.1000120.
88. Spector R, Johanson CE (2007) Vitamin transport and homeostasis in mammalian brain: focus on vitamins B and E. J. Neurochem. 103 (2): 425–438. DOI: 10.1111/j.1471-4159.2007.04773.x.
89. Uchida Y, Ito K, Ohtsuki S, Kubo Y, Suzuki T, Terasaki T (2015) Major involvement of Na + -dependent multivitamin transporter (SLC5A6/SMVT) in uptake of biotin and pantothenic acid by human brain capillary endothelial cells. J. Neurochem. 134 (1): 97–112. DOI: 10.1111/jnc.13092.
90. Laforenza U, Patrini C, Rindi G (1988) Distribution of thiamine, thiamine phosphates, and thiamine metabolizing enzymes in neuronal and glial cell enriched fractions of rat brain. J. Neurochem. 51 (3): 730–735. DOI: 10.1111/j.1471-4159.1988.tb01805.x.
91. Rao VLR, Richardson JS, Butterworth RF (1993) Decreased activities of thiamine diphosphatase in frontal and temporal cortex in Alzheimer’s disease. Brain Res. 631 (2): 334–336. DOI: 10.1016/0006-8993(93)91554-6.
92. Héroux M, Raghavendra Rao VL, Lavoie J, Richardson JS, Butterworth RF (1996) Alterations of thiamine phosphorylation and of thiamine-dependent enzymes in Alzheimer’s disease. Metab. Brain Dis. 11 (1): 81–88. DOI: 10.1007/BF02080933.
93. Torvik A (1985) Two types of brain lesions in Wernicke’s encephalopathy. Neuropathol. Appl. Neurobiol. 11 (3): 179–190. DOI: 10.1111/j.1365-2990.1985.tb00016.x.
94. Butterworth RF (1989) Effects of thiamine deficiency on brain metabolism: implications for the pathogenesis of the wernicke-korsakoff syndrome. Alcohol Alcohol. 24 (4): 271–279. DOI: 10.1093/oxfordjournals.alcalc.a044913.
95. Vindedzis SA, Stanton KG, Sherriff JL, Dhaliwal SS (2008) Thiamine deficiency in diabetes — is diet relevant? Diabetes Vasc. Dis. Res. 5 (3): 215–215. DOI: 10.3132/dvdr.2008.035.
96. Jermendy G (2006) Evaluating thiamine deficiency in patients with diabetes. Diabetes Vasc. Dis. Res. 3 (2): 120–121. DOI: 10.3132/dvdr.2006.014.
97. Kohda Y, Shirakawa H, Yamane K, Otsuka K, Kono T, Terasaki F, Tanaka T (2008) Prevention of incipient diabetic cardiomyopathy by high-dose thiamine. J. Toxicol. Sci. 33 (4): 459–472. DOI: 10.2131/jts.33.459.
98. Mee L, Nabokina SM, Sekar VT, Subramanian VS, Maedler K, Said HM (2009) Pancreatic beta cells and islets take up thiamin by a regulated carrier-mediated process: studies using mice and human pancreatic preparations. Am. J. Physiol. Liver Physiol. 297 (1): G197–G206. DOI: 10.1152/ajpgi.00092.2009.
99. Page GLJ, Laight D, Cummings MH (2011) Thiamine deficiency in diabetes mellitus and the impact of thiamine replacement on glucose metabolism and vascular disease. Int. J. Clin. Pract. 65 (6): 684–690. DOI: 10.1111/j.1742-1241.2011.02680.x.
100. Watson JD, Dako DY (1975) Erythrocyte transketolase activity in adult Ghanaian subjects. Clin. Chim. Acta. 59 (1): 55–61. DOI: 10.1016/0009-8981(75)90218-1.
101. Thornalley PJ, Babaei-Jadidi R, Al Ali H, Rabbani N, Antonysunil A, Larkin J, Ahmed A, Rayman G, Bodmer CW (2007) High prevalence of low plasma thiamine concentration in diabetes linked to a marker of vascular disease. Diabetologia 50 (10): 2164–2170. DOI: 10.1007/s00125-007-0771-4.
102. Thakur K, Tomar SK, Singh AK, Mandal S, Arora S (2017) Riboflavin and health: A review of recent human research. Crit. Rev. Food Sci. Nutr. 57 (17): 3650–3660. DOI: 10.1080/10408398.2016.1145104.
103. Alam MM, Iqbal S, Naseem I (2015) Ameliorative effect of riboflavin on hyperglycemia, oxidative stress and DNA damage in type-2 diabetic mice: Mechanistic and therapeutic strategies. Arch. Biochem. Biophys. 584 : 10–19. DOI: 10.1016/j.abb.2015.08.013.
104. Udhayabanu T, Manole A, Rajeshwari M, Varalakshmi P, Houlden H, Ashokkumar B (2017) Riboflavin responsive mitochondrial dysfunction in neurodegenerative diseases. J. Clin. Med. 6 (5): 52. DOI: 10.3390/jcm6050052.
105. Zhao R, Wang H, Qiao C, Zhao K (2018) Vitamin B2 blocks development of Alzheimer’s disease in APP/PS1 transgenic mice via anti-oxidative mechanism. Trop. J. Pharm. Res. 17 (6): 1049. DOI: 10.4314/tjpr.v17i6.10.
106. King GL (2008) The role of inflammatory cytokines in diabetes and its complications. J. Periodontol. 79 (8s): 1527–1534. DOI: 10.1902/jop.2008.080246.
107. Sauve AA (2008) NAD + and vitamin B 3 : from metabolism to therapies. J. Pharmacol. Exp. Ther. 324 (3): 883–893. DOI: 10.1124/jpet.107.120758.
108. Gasperi V, Sibilano M, Savini I, Catani M (2019) Niacin in the central nervous system: An update of biological aspects and clinical applications. Int. J. Mol. Sci. 20 (4): 974. DOI: 10.3390/ijms20040974.
109. Li F, Chong Z, Maiese K (2006) Cell life versus cell longevity: the mysteries surrounding the NAD+ precursor nicotinamide. Curr. Med. Chem. 13 (8): 883–895. DOI: 10.2174/092986706776361058.
110. Trammell SAJ, Brenner C (2015) NNMT: A bad actor in fat makes good in liver. Cell Metab. 22 (2): 200–201. DOI: 10.1016/j.cmet.2015.07.017.
111. Hong S, Moreno-Navarrete JM, Wei X, Kikukawa Y, Tzameli I, Prasad D, Lee Y, Asara JM, Fernandez-Real JM, Maratos-Flier E, Pissios P (2015) Nicotinamide N-methyltransferase regulates hepatic nutrient metabolism through Sirt1 protein stabilization. Nat. Med. 21 (8): 887–894. DOI: 10.1038/nm.3882.
112. Chong ZZ, Lin S-H, Maiese K (2004) The NAD + precursor nicotinamide governs neuronal survival during oxidative stress through protein kinase B coupled to FOXO3a and mitochondrial membrane potential. J. Cereb. Blood Flow Metab. 24 (7): 728–743. DOI: 10.1097/01.WCB.0000122746.72175.0E.
113. Julius U, Fischer S (2013) Nicotinic acid as a lipid-modifying drug – A review. Atheroscler. Suppl 14 (1): 7–13. DOI: 10.1016/j.atherosclerosissup.2012.10.036.
114. Tavintharan S, Woon K, Pek LT, Jauhar N, Dong X, Lim SC, Sum CF (2011) Niacin results in reduced monocyte adhesion in patients with type 2 diabetes mellitus. Atherosclerosis 215 (1): 176–179. DOI: 10.1016/j.atherosclerosis.2010.12.020.
115. Picone S, Ariganello P, Mondì V, Di Palma F, Martini L, Marziali S, Fariello G, Paolillo P (2019) A solution based on melatonin, tryptophan, and vitamin B6 (Melamil Tripto©) for sedation in newborns during brain MRI. Ital. J. Pediatr. 45 (1): 122. DOI: 10.1186/s13052-019-0714-y.
116. Anitha M, Abraham PM, Paulose CS (2012) Striatal dopamine receptors modulate the expression of insulin receptor, IGF-1 and GLUT-3 in diabetic rats: Effect of pyridoxine treatment. Eur. J. Pharmacol. 696 (1–3): 54–61. DOI: 10.1016/j.ejphar.2012.09.006.
117. Leklem JE, Hollenbeck CB (1990) Acute ingestion of glucose decreases plasma pyridoxal 5’-phosphate and total vitamin B-6 concentration. Am. J. Clin. Nutr. 51 (5): 832–836. DOI: 10.1093/ajcn/51.5.832.
118. Satyanarayana A, Balakrishna N, Pitla S, Reddy PY, Mudili S, Lopamudra P, Suryanarayana P, Viswanath K, Ayyagari R, Reddy GB (2011) Status of B-vitamins and homocysteine in diabetic retinopathy: association with vitamin-B12 deficiency and hyperhomocysteinemia. PLoS One 6 (11): e26747. DOI: 10.1371/journal.pone.0026747.
119. Rogers KS, Higgins ES, Kline ES (1986) Experimental diabetes causes mitochondrial loss and cytoplasmic enrichment of pyridoxal phosphate and aspartate aminotransferase activity. Biochem. Med. Metab. Biol. 36 (1): 91–97. DOI: 10.1016/0885-4505(86)90111-8.
120. Spellacy WN, Buhi WC, Birk SA (1977) Vitamin B6 treatment of gestational diabetes mellitus. Am. J. Obstet. Gynecol. 127 (6): 599–602. DOI: 10.1016/0002-9378(77)90356-8.
121. Bennink HJTC, Schreurs WHP (1975) Improvement of oral glucose tolerance in gestational diabetes by pyridoxine. BMJ 3 (5974): 13–15. DOI: 10.1136/bmj.3.5974.13.
122. Connick JH, Stone TW (1985) The role of kynurenines in diabetes mellitus. Med. Hypotheses 18 (4): 371–376. DOI: 10.1016/0306-9877(85)90104-5.
123. Liu Z, Li P, Zhao Z-H, Zhang Y, Ma Z-M, Wang S-X (2016) Vitamin B6 prevents endothelial dysfunction, insulin resistance, and hepatic lipid accumulation in Apoe −/− mice fed with high-fat diet. J. Diabetes Res. 2016 : 1–8. DOI: 10.1155/2016/1748065.
124. Fernandez-Mejia C (2005) Pharmacological effects of biotin. J. Nutr. Biochem. 16 (7): 424–427. DOI: 10.1016/j.jnutbio.2005.03.018.
125. Tong L (2013) Structure and function of biotin-dependent carboxylases. Cell. Mol. Life Sci. 70 (5): 863–891. DOI: 10.1007/s00018-012-1096-0.
126. Via M (2012) The malnutrition of obesity: micronutrient deficiencies that promote diabetes. ISRN Endocrinol. 2012 (103472): 1–8. DOI: 10.5402/2012/103472.
127. Dakshinamurti K, Cheah-Tan C (1968) Biotin-mediated synthesis of hepatic glucokinase in the rat. Arch. Biochem. Biophys. 127 (1): 17–21. DOI: 10.1016/0003-9861(68)90195-1.
128. Romero-Navarro G (1999) Biotin regulation of pancreatic glucokinase and insulin in primary cultured rat Islets and in biotin- deficient rats. Endocrinology 140 (10): 4595–4600. DOI: 10.1210/en.140.10.4595.
129. Hemmati M, Babaei H, Abdolsalehei M (2013) Survey of the effect of biotin on glycemic control and plasma lipid concentrations in type 1 diabetic patients in kermanshah in Iran (2008-2009). Oman Med. J. 28 (3): 195–198. DOI: 10.5001/omj.2013.53.
130. Sone H, Ito M, Sugiyama K, Ohneda M, Maebashi M, Furukawa Y (1999) Biotin enhances glucose-stimulated insulin secretion in the isolated perfused pancreas of the rat. J. Nutr. Biochem. 10 (4): 237–243. DOI: 10.1016/S0955-2863(99)00003-0.
131. Sone H, Ito M, Shimizu M, Sasaki Y, Komai M FY (2000) Characteristics of the biotin enhancement of glucose-induced insulin release in pancreatic islets of the rat. Biosci. Biotechnol. Biochem. 64 (3): 550–554. DOI: 10.1271/bbb.64.550.
132. De la Vega LA, Stockert RJ (2000) Regulation of the insulin and asialoglycoprotein receptors via cGMP-dependent protein kinase. Am. J. Physiol. Physiol. 279 (6): C2037–C2042. DOI: 10.1152/ajpcell.2000.279.6.C2037.
133. Ferre T, Pujol A, Riu E, Bosch F, Valera A (1996) Correction of diabetic alterations by glucokinase. Proc. Natl. Acad. Sci. 93 (14): 7225–7230. DOI: 10.1073/pnas.93.14.7225.
134. Lazo de la Vega-Monroy ML, Larrieta E, German MS, Baez-Saldana A, Fernandez-Mejia C (2013) Effects of biotin supplementation in the diet on insulin secretion, islet gene expression, glucose homeostasis and beta-cell proportion. J. Nutr. Biochem. 24 (1): 169–177. DOI: 10.1016/j.jnutbio.2012.03.020.
135. Larrieta E, de la Vega-Monroy MLL, Vital P, Aguilera A, German MS, El Hafidi M, Fernandez-Mejia C (2012) Effects of biotin deficiency on pancreatic islet morphology, insulin sensitivity and glucose homeostasis. J. Nutr. Biochem. 23 (4): 392–399. DOI: 10.1016/j.jnutbio.2011.01.003.
136. Reddi A, DeAngelis B, Frank O, Lasker N, Baker H (1988) Biotin supplementation improves glucose and insulin tolerances in genetically diabetic KK mice. Life Sci. 42 (13): 1323–1330. DOI: 10.1016/0024-3205(88)90226-3.
137. Xiang X, Liu Y, Zhang X, Zhang W, Wang Z (2015) [Effects of biotin on blood glucose regulation in type 2 diabetes rat model]. Wei Sheng Yan Jiu. 44 (2): 185–189, 195.
138. Reynolds E (2006) Vitamin B12, folic acid, and the nervous system. Lancet Neurol. 5 (11): 949–960. DOI: 10.1016/S1474-4422(06)70598-1.
139. Rucker RB, Zempleni J, Suttie JW MD 2007. [Internet] (2007) Handbook of Vitamins. Rucker RB, Zempleni J, Suttie JW, McCormick DB, editors. CRC Press; 2007. DOI: 10.1201/9781420005806.
140. Molina M, Gonzalez R, Folgado J, Real JT, Martínez-Hervás S, Priego A, Lorente R, Chaves FJ, Ascaso JF (2013) Correlation between plasma concentrations of homocysteine and diabetic polyneuropathy evaluated with the Semmes-Weinstein monofilament test in patients with type 2 diabetes mellitus. Med. Clin. (Barc). 141 (9): 382–386. DOI: 10.1016/j.medcli.2012.09.042.
141. Ukinc K, Ersoz HO, Karahan C, Erem C, Eminagaoglu S, Hacihasanoglu AB, Yilmaz M, Kocak M (2009) Methyltetrahydrofolate reductase C677T gene mutation and hyperhomocysteinemia as a novel risk factor for diabetic nephropathy. Endocrine 36 (2): 255–261. DOI: 10.1007/s12020-009-9218-7.
142. Jianbo L, Yuche C, Ming S, Jingrong T, Qing D, Yu Z, Jiawei C, Hongxing W (2011) Association of homocysteine with peripheral neuropathy in Chinese patients with type 2 diabetes. Diabetes Res. Clin. Pract. 93 (1): 38–42. DOI: 10.1016/j.diabres.2011.03.020.
143. Smolek M, Notaroberto, Jaramillo, Pradillo (2013) Intervention with vitamins in patients with nonproliferative diabetic retinopathy: a pilot study. Clin. Ophthalmol. 7 : 1451. DOI: 10.2147/OPTH.S46718.
144. Polizzi F, Andican G, Çetin E, Civelek S, Yumuk V, Burçak G (2012) Increased DNA-glycation in type 2 diabetic patients: the effect of thiamine and pyridoxine therapy. Exp. Clin. Endocrinol. Diabetes 120 (06): 329–334. DOI: 10.1055/s-0031-1298016.
145. González-Ortiz M, Martínez-Abundis E, Robles-Cervantes JA, Ramírez-Ramírez V, Ramos-Zavala MG (2011) Effect of thiamine administration on metabolic profile, cytokines and inflammatory markers in drug-naïve patients with type 2 diabetes. Eur. J. Nutr. 50 (2): 145–149. DOI: 10.1007/s00394-010-0123-x.
146. Xiang D, Zhang Q, Wang Y-T (2020) Effectiveness of niacin supplementation for patients with type 2 diabetes. Medicine (Baltimore). 99 (29): e21235. DOI: 10.1097/MD.0000000000021235.
147. Unoki-Kubota H, Yamagishi S, Takeuchi M, Bujo H, Saito Y (2010) Pyridoxamine, an inhibitor of advanced glycation end product (AGE) formation ameliorates insulin resistance in obese, type 2 diabetic mice. Protein Pept. Lett. 17 (9): 1177–1181. DOI: 10.2174/092986610791760423.
148. Sahin K, Tuzcu M, Orhan C, Sahin N, Kucuk O, Ozercan IH, Juturu V, Komorowski JR (2013) Anti-diabetic activity of chromium picolinate and biotin in rats with type 2 diabetes induced by high-fat diet and streptozotocin. Br. J. Nutr. 110 (2): 197–205. DOI: 10.1017/S0007114512004850.
149. Cipolla-Neto J, Amaral FG, Afeche SC, Tan DX, Reiter RJ (2014) Melatonin, energy metabolism, and obesity: a review. J. Pineal Res. 56 (4): 371–381. DOI: 10.1111/jpi.12137.
150. Simonneaux V, Ribelayga C (2003) Generation of the melatonin endocrine message in mammals: A review of the complex regulation of melatonin synthesis by norepinephrine, peptides, and other pineal transmitters. Pharmacol. Rev. 55 (2): 325–395. DOI: 10.1124/pr.55.2.2.
151. Reiter RJ, Tan DX, Kim SJ, Cruz MHC (2014) Delivery of pineal melatonin to the brain and SCN: role of canaliculi, cerebrospinal fluid, tanycytes and Virchow–Robin perivascular spaces. Brain Struct. Funct. 219 (6): 1873–1887. DOI: 10.1007/s00429-014-0719-7.
152. Reiter RJ (1991) Melatonin: The chemical expression of darkness. Mol. Cell. Endocrinol. 79 (1–3): C153–C158. DOI: 10.1016/0303-7207(91)90087-9.
153. Scheer FAJL, Hilton MF, Mantzoros CS, Shea SA (2009) Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. 106 (11): 4453–4458. DOI: 10.1073/pnas.0808180106.
154. Esposito E, Cuzzocrea S (2010) Antiinflammatory Activity of Melatonin in Central Nervous System. Curr. Neuropharmacol. 8 (3): 228–242. DOI: 10.2174/157015910792246155.
155. Stefulj J, Hörtner M, Ghosh M, Schauenstein K, Rinner I, Wölfler A, Semmler J, Liebmann PM (2001) Gene expression of the key enzymes of melatonin synthesis in extrapineal tissues of the rat. J. Pineal Res. 30 (4): 243–247. DOI: 10.1034/j.1600-079X.2001.300408.x.
156. Arendt J, Aulinas A 2022. (2022) Physiology of the pineal gland and melatonin. In: Endotext. MDText.com, Inc.; 2022.
157. Liu Y-J, Zhuang J, Zhu H-Y, Shen Y-X, Tan Z-L, Zhou J-N (2007) Cultured rat cortical astrocytes synthesize melatonin: absence of a diurnal rhythm. J. Pineal Res. 43 (3): 232–238. DOI: 10.1111/j.1600-079X.2007.00466.x.
158. Meng X, Li Y, Li S, Zhou Y, Gan R-Y, Xu D-P, Li H-B (2017) Dietary sources and bioactivities of melatonin. Nutrients 9 (4): 367. DOI: 10.3390/nu9040367.
159. Dubocovich ML, Markowska M (2005) Functional MT1 and MT2 melatonin receptors in mammals. Endocrine 27 (2): 101–110. DOI: 10.1385/ENDO:27:2:101.
160. Lacoste B, Angeloni D, Dominguez-Lopez S, Calderoni S, Mauro A, Fraschini F, Descarries L, Gobbi G (2015) Anatomical and cellular localization of melatonin MT 1 and MT 2 receptors in the adult rat brain. J. Pineal Res. 58 (4): 397–417. DOI: 10.1111/jpi.12224.
161. Mailliet F, Ferry G, Vella F, Berger S, Cogé F, Chomarat P, Mallet C, Guénin S-P, Guillaumet G, Viaud-Massuard M-C, Yous S, Delagrange P, Boutin JA (2005) Characterization of the melatoninergic MT3 binding site on the NRH:quinone oxidoreductase 2 enzyme. Biochem. Pharmacol. 71 (1–2): 74–88. DOI: 10.1016/j.bcp.2005.09.030.
162. Suofu Y, Li W, Jean-Alphonse FG, Jia J, Khattar NK, Li J et al. (2017) Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc. Natl. Acad. Sci. 114 (38): E7997–E8006. DOI: 10.1073/pnas.1705768114.
163. Mohan N, Sadeghi K, Reiter RJ, Meltz ML (1995) The neurohormone melatonin inhibits cytokine, mitogen and ionizing radiation induced NF-kappa B. Biochem. Mol. Biol. Int. 37 (6): 1063–1070.
164. Mayo JC, Sainz RM, Tan DX, Hardeland R, Leon J, Rodriguez C, Reiter RJ (2005) Anti-inflammatory actions of melatonin and its metabolites, N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) and N1-acetyl-5-methoxykynuramine (AMK), in macrophages. J. Neuroimmunol. 165 (1–2): 139–149. DOI: 10.1016/j.jneuroim.2005.05.002.
165. Calabrese V, Boyd-Kimball D, Scapagnini G, Butterfield DA (2004) Nitric oxide and cellular stress response in brain aging and neurodegenerative disorders: the role of vitagenes. In Vivo 18 (3): 245–267.
166. Calabrese V, Scapagnini G, Giuffrida Stella AM, Bates TE, Clark JB (2001) Mitochondrial involvement in brain function and dysfunction: relevance to aging, neurodegenerative disorders and longevity. Neurochem. Res. 26 (6): 739–764. DOI: 10.1023/a:1010955807739.
167. Rosenbluth J (1980) Central myelin in the mouse mutant shiverer. J. Comp. Neurol. 194 (3): 639–648. DOI: 10.1002/cne.901940310.
168. Rubio-González A, Reiter RJ, De Luxán-Delgado B, Potes Y, Caballero B, Boga JA, Solano JJ, Vega-Naredo I, Coto-Montes A (2020) Pleiotropic role of melatonin in brain mitochondria of obese mice. Melatonin Res. 3 (4): 538–557. DOI: 10.32794/MR11250078.
169. Laposky AD, Bass J, Kohsaka A, Turek FW (2008) Sleep and circadian rhythms: Key components in the regulation of energy metabolism. FEBS Lett. 582 (1): 142–151. DOI: 10.1016/j.febslet.2007.06.079.
170. Jenwitheesuk A, Nopparat C, Mukda S, Wongchitrat P, Govitrapong P (2014) Melatonin regulates aging and neurodegeneration through energy metabolism, epigenetics, autophagy and circadian rhythm pathways. Int. J. Mol. Sci. 15 (9): 16848–16884. DOI: 10.3390/ijms150916848.
171. Shen S, Liao Q, Wong YK, Chen X, Yang C, Xu C, Sun J, Wang J (2022) The role of melatonin in the treatment of type 2 diabetes mellitus and Alzheimer’s disease. Int. J. Biol. Sci. 18 (3): 983–994. DOI: 10.7150/ijbs.66871.
172. Hölscher C (2019) Insulin signaling impairment in the brain as a risk factor in Alzheimer’s disease. Front. Aging Neurosci. 11 (APR). DOI: 10.3389/fnagi.2019.00088.
173. Shin Y, Choi SH, Kim E, Bylykbashi E, Kim JA, Chung S, Kim DY, Kamm RD, Tanzi RE (2019) Blood–brain barrier dysfunction in a 3D in vitro model of Alzheimer’s disease. Adv. Sci. 6 (20): 1900962. DOI: 10.1002/advs.201900962.
174. Banks W (2005) Blood-brain barrier transport of cytokines: A mechanism for neuropathology. Curr. Pharm. Des. 11 (8): 973–984. DOI: 10.2174/1381612053381684.
175. Alluri H, Wilson RL, Anasooya Shaji C, Wiggins-Dohlvik K, Patel S, Liu Y, Peng X, Beeram MR, Davis ML, Huang JH, Tharakan B (2016) Melatonin preserves blood-brain barrier integrity and permeability via matrix metalloproteinase-9 inhibition. Thomas B, editor. PLoS One 11 (5): e0154427. DOI: 10.1371/journal.pone.0154427.
176. Frölich L, Blum-Degen D, Bernstein H-G, Engelsberger S, Humrich J, Laufer S, Muschner D, Thalheimer A, Türk A, Hoyer S, Zöchling R, Boissl KW, Jellinger K, Riederer P (1998) Brain insulin and insulin receptors in aging and sporadic Alzheimer’s disease. J. Neural Transm. 105 (4): 423. DOI: 10.1007/s007020050068.
177. Heras-Sandoval D, Pérez-Rojas JM, Hernández-Damián J, Pedraza-Chaverri J (2014) The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell. Signal. 26 (12): 2694–2701. DOI: 10.1016/j.cellsig.2014.08.019.
178. Ferrario CR, Reagan LP (2018) Insulin-mediated synaptic plasticity in the CNS: Anatomical, functional and temporal contexts. Neuropharmacology 136 (Pt B): 182–191. DOI: 10.1016/j.neuropharm.2017.12.001.
179. Peschke E, Frese T, Chankiewitz E, Peschke D, Preiss U, Schneyer U, Spessert R, Muhlbauer E (2006) Diabetic Goto Kakizaki rats as well as type 2 diabetic patients show a decreased diurnal serum melatonin level and an increased pancreatic melatonin-receptor status. J. Pineal Res. 40 (2): 135–143. DOI: 10.1111/j.1600-079X.2005.00287.x.
180. Peschke E, Stumpf I, Bazwinsky I, Litvak L, Dralle H, Mühlbauer E (2007) Melatonin and type 2 diabetes ? a possible link? J. Pineal Res. 42 (4): 350–358. DOI: 10.1111/j.1600-079X.2007.00426.x.
181. Mirick DK, Davis S (2008) Melatonin as a biomarker of circadian dysregulation. Cancer Epidemiol. Biomarkers Prev. 17 (12): 3306–3313. DOI: 10.1158/1055-9965.EPI-08-0605.
182. Cagnacci A, Arangino S, Renzi A, Paoletti AM, Melis GB, Cagnacci P, Volpe A (2001) Influence of melatonin administration on glucose tolerance and insulin sensitivity of postmenopausal women. Clin. Endocrinol. (Oxf). 54 (3): 339–346. DOI: 10.1046/j.1365-2265.2001.01232.x.
183. Boden G, Ruiz J, Urbain JL, Chen X (1996) Evidence for a circadian rhythm of insulin secretion. Am. J. Physiol. Metab. 271 (2): E246–E252. DOI: 10.1152/ajpendo.1996.271.2.E246.
184. Peschke E (2008) Melatonin, endocrine pancreas and diabetes. J. Pineal Res. 44 (1): 26–40. DOI: 10.1111/j.1600-079X.2007.00519.x.
185. Rasmussen DD, Mitton DR, Larsen SA, Yellon SM (2001) Aging-dependent changes in the effect of daily melatonin supplementation on rat metabolic and behavioral responses. J. Pineal Res. 31 (1): 89–94. DOI: 10.1034/j.1600-079X.2001.310113.x.
186. Champney TH, Steger RW, Christie DS, Reiter RJ (1985) Alterations in components of the pineal melatonin synthetic pathway by acute insulin stress in the rat and Syrian hamster. Brain Res. 338 (1): 25–32. DOI: 10.1016/0006-8993(85)90244-6.
187. Champney TH, Brainard GC, Richardson BA, Reiter RJ (1983) Experimentally-induced diabetes reduces nocturnal pineal melatonin content in the Syrian hamster. Comp. Biochem. Physiol. Part A Physiol. 76 (1): 199–201. DOI: 10.1016/0300-9629(83)90314-6.
188. Champney TH, Holtorf AP, Craft CM, Reiter RJ (1986) Hormonal modulation of pineal melatonin synthesis in rats and syrian hamsters: Effects of streptozotocin-induced diabetes and insulin injections. Comp. Biochem. Physiol. Part A Physiol. 83 (2): 391–395. DOI: 10.1016/0300-9629(86)90594-3.
189. Rahman MM, Kwon H-S, Kim M-J, Go H-K, Oak M-H, Kim D-H (2017) Melatonin supplementation plus exercise behavior ameliorate insulin resistance, hypertension and fatigue in a rat model of type 2 diabetes mellitus. Biomed. Pharmacother. 92 : 606–614. DOI: 10.1016/j.biopha.2017.05.035.
190. Conti A, Maestroni GJM (1996) Role of the pineal gland and melatonin in the development of autoimmune diabetes in non-obese diabetic mice. J. Pineal Res. 20 (3): 164–172. DOI: 10.1111/j.1600-079X.1996.tb00253.x.
191. Sun H, Wang X, Chen J, Gusdon AM, Song K, Li L, Qu S (2018) Melatonin treatment improves insulin resistance and pigmentation in obese patients with Acanthosis Nigricans. Int. J. Endocrinol. 2018 (2304746): 1–7. DOI: 10.1155/2018/2304746.
192. Oliveira AC de, Andreotti S, Sertie RAL, Campana AB, de Proença ARG, Vasconcelos RP, Oliveira KA de, Coelho-de-Souza AN, Donato-Junior J, Lima FB (2018) Combined treatment with melatonin and insulin improves glycemic control, white adipose tissue metabolism and reproductive axis of diabetic male rats. Life Sci. 199 : 158–166. DOI: 10.1016/j.lfs.2018.02.040.
193. Sartori C, Dessen P, Mathieu C, Monney A, Bloch J, Nicod P, Scherrer U, Duplain H (2009) Melatonin improves glucose homeostasis and endothelial vascular function in high-fat diet-fed insulin-resistant mice. Endocrinology 150 (12): 5311–5317. DOI: 10.1210/en.2009-0425.
194. Kadhim HM, Ismail SH, Hussein KI, Bakir IH, Sahib AS, Khalaf BH, Hussain SA-R (2006) Effects of melatonin and zinc on lipid profile and renal function in type 2 diabetic patients poorly controlled with metformin. J. Pineal Res. 41 (2): 189–193. DOI: 10.1111/j.1600-079X.2006.00353.x.
195. Biessels GJ, Kappelle AC, Bravenboer B, Erkelens DW, Gispen WH (1994) Cerebral function in diabetes mellitus. Diabetologia 37 (7): 643–650. DOI: 10.1007/BF00417687.
196. Li Z-G, Zhang W, Grunberger G, Sima AAF (2002) Hippocampal neuronal apoptosis in type 1 diabetes. Brain Res. 946 (2): 221–231. DOI: 10.1016/S0006-8993(02)02887-1.
197. Li Z, Zhang W, Sima AAF (2005) The role of impaired insulin/IGF action in primary diabetic encephalopathy. Brain Res. 1037 (1–2): 12–24. DOI: 10.1016/j.brainres.2004.11.063.
198. Sima AAF (2004) Diabetes underlies common neurological disorders. Ann. Neurol. 56 (4): 459–461. DOI: 10.1002/ana.20249.
199. Ristow M (2004) Neurodegenerative disorders associated with diabetes mellitus. J. Mol. Med. 82 (8): 510–529. DOI: 10.1007/s00109-004-0552-1.
200. Northam EA, Rankins D, Cameron FJ (2006) Therapy Insight: the impact of type 1 diabetes on brain development and function. Nat. Clin. Pract. Neurol. 2 (2): 78–86. DOI: 10.1038/ncpneuro0097.
201. Baydas G, Canatan H, Turkoglu A (2002) Comparative analysis of the protective effects of melatonin and vitamin E on streptozocin-induced diabetes mellitus. J. Pineal Res. 32 (4): 225–230. DOI: 10.1034/j.1600-079X.2002.01856.x.
202. Baydas G, Nedzvetskii VS, Tuzcu M, Yasar A, Kirichenko S V. (2003) Increase of glial fibrillary acidic protein and S-100B in hippocampus and cortex of diabetic rats: effects of vitamin E. Eur. J. Pharmacol. 462 (1–3): 67–71. DOI: 10.1016/S0014-2999(03)01294-9.
203. Baydas G, Donder E, Kiliboz M, Sonkaya E, Tuzcu M, Yasar A, Nedzvetskii VS (2004) Neuroprotection by -lipoic acid in streptozotocin-induced diabetes. Biochem. 69 (9): 1001–1005. DOI: 10.1023/B:BIRY.0000043542.39691.95.
204. Poeggeler B, Saarela S, Reiter RJ, Tan DX, Chen ID, Manchester lc BI (2006) Melatonin- a highly potent endogenous radical scavenger and electron donor: new aspects of the oxidation chemistry of this indole accessed in vitro. Ann. N. Y. Acad. Sci. 738 (1): 419–420. DOI: 10.1111/j.1749-6632.1994.tb21831.x.
205. Reiter RJ, Tan DX, Manchester LC, Qi W (2001) Biochemical reactivity of melatonin with reactive oxygen and nitrogen species: A review of the evidence. Cell Biochem. Biophys. 34 (2): 237–256. DOI: 10.1385/CBB:34:2:237.
206. Baydas G, Reiter RJ, Yasar A, Tuzcu M, Akdemir I, Nedzvetskii VS (2003) Melatonin reduces glial reactivity in the hippocampus, cortex, and cerebellum of streptozotocin-induced diabetic rats. Free Radic. Biol. Med. 35 (7): 797–804. DOI: 10.1016/S0891-5849(03)00408-8.
207. Baydas G, Nedzvetskii VS, Kirichenko S V., Nerush PA (2008) Astrogliosis in the hippocampus and cortex and cognitive deficits in rats with streptozotocin-induced diabetes: Effects of melatonin. Neurophysiology 40 (2): 91–97. DOI: 10.1007/s11062-008-9026-3.
208. Hajam YA, Rai S, Ghosh H, Basheer M (2020) Combined administration of exogenous melatonin and insulin ameliorates streptozotocin induced toxic alteration on hematological parameters in diabetic male Wistar rats. Toxicol. Reports 7 : 353–359. DOI: 10.1016/j.toxrep.2020.01.020.
209. Kahya MC, Naziroʇlu M, Çiʇ B (2015) Melatonin and selenium reduce plasma cytokine and brain oxidative stress levels in diabetic rats. Brain Inj. 29 (12): 1490–1496. DOI: 10.3109/02699052.2015.1053526.
210. Gürpınar T, Ekerbiçer N, Uysal N, Barut T, Tarakçı F, Tuglu MI (2012) The effects of the melatonin treatment on the oxidative stress and apoptosis in diabetic eye and brain. Sci. World J. 2012 : 1–5. DOI: 10.1100/2012/498489.
211. Jangra A, Datusalia AK, Khandwe S, Sharma SS (2013) Amelioration of diabetes-induced neurobehavioral and neurochemical changes by melatonin and nicotinamide: Implication of oxidative stress–PARP pathway. Pharmacol. Biochem. Behav. 114–115 : 43–51. DOI: 10.1016/j.pbb.2013.10.021.
212. Negi G, Kumar A, Kaundal RK, Gulati A, Sharma SS (2010) Functional and biochemical evidence indicating beneficial effect of Melatonin and Nicotinamide alone and in combination in experimental diabetic neuropathy. Neuropharmacology 58 (3): 585–592. DOI: 10.1016/j.neuropharm.2009.11.018.
213. Oliveira-Abreu K, Cipolla-Neto J, Leal-Cardoso JH (2021) Effects of melatonin on diabetic neuropathy and retinopathy. Int. J. Mol. Sci. 23 (1): 100. DOI: 10.3390/ijms23010100.
214. Scott JN, Clark AW, Zochodne DW (1999) Neurofilament and tubulin gene expression in progressive experimental diabetes. Brain 122 (11): 2109–2118. DOI: 10.1093/brain/122.11.2109.
215. Lupachyk S, Watcho P, Stavniichuk R, Shevalye H, Obrosova IG (2013) Endoplasmic reticulum stress plays a key role in the pathogenesis of diabetic peripheral neuropathy. Diabetes 62 (3): 944–952. DOI: 10.2337/db12-0716.
216. Ma J, Pan P, Anyika M, Blagg BSJ, Dobrowsky RT (2015) Modulating molecular chaperones improves mitochondrial bioenergetics and decreases the inflammatory transcriptome in diabetic sensory neurons. ACS Chem. Neurosci. 6 (9): 1637–1648. DOI: 10.1021/acschemneuro.5b00165.
217. Ilnytska O, Lyzogubov V V., Stevens MJ, Drel VR, Mashtalir N, Pacher P, Yorek MA, Obrosova IG (2006) Poly(ADP-ribose) polymerase inhibition alleviates experimental diabetic sensory neuropathy. Diabetes 55 (6): 1686–1694. DOI: 10.2337/db06-0067.
218. Feldman EL, Nave K-A, Jensen TS, Bennett DLH (2017) New horizons in diabetic neuropathy: mechanisms, bioenergetics, and pain. Neuron 93 (6): 1296–1313. DOI: 10.1016/j.neuron.2017.02.005.
219. Hosseini A, Samadi M, Baeeri M, Rahimifard M, Haghi-Aminjan H (2022) The neuroprotective effects of melatonin against diabetic neuropathy: A systematic review of non-clinical studies. Front. Pharmacol. 13 (984499): 1–14. DOI: 10.3389/fphar.2022.984499.
220. Metwally MMM, Ebraheim LLM, Galal AAA (2018) Potential therapeutic role of melatonin on STZ-induced diabetic central neuropathy: A biochemical, histopathological, immunohistochemical and ultrastructural study. Acta Histochem. 120 (8): 828–836. DOI: 10.1016/j.acthis.2018.09.008.
221. Negi G, Kumar A, Sharma SS (2010) Melatonin modulates neuroinflammation and oxidative stress in experimental diabetic neuropathy: effects on NF-κB and Nrf2 cascades. J. Pineal Res. 50 (2): no-no. DOI: 10.1111/j.1600-079X.2010.00821.x.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


