Melatonin is a potential therapeutic molecule for oxidative stress induced red blood cell (RBC) injury : A review
Melatonin protects RBC from oxidative stress
Abstract
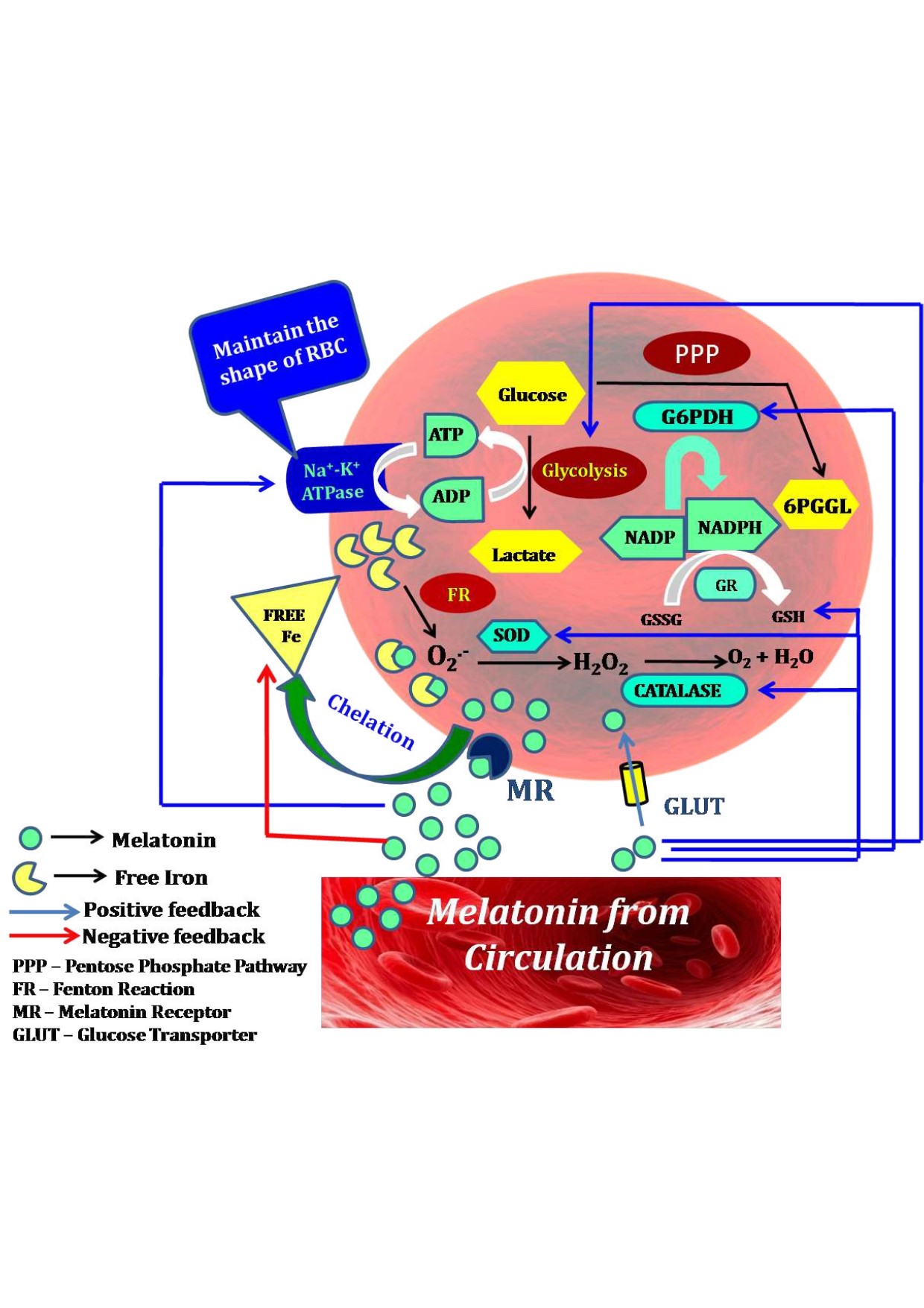
Red blood cells (RBCs) or erythrocytes are highly vulnerable to oxidative stress due to their absence of nuclei and mitochondria, presence of iron containing heme and high amounts of fatty acids in their uniquely constructed lipid bilayer membrane. The principal function of RBCs is to carry oxygen to tissues. Thus, RBCs have to pass through the micro-capillaries in which it requires these cells exhibits high structural deformability and great elasticity. The intact cytoskeletal architecture and proper membrane fluidity of RBCs are crucial for their deformability. Many factors can jeopardize the structural and functional harmony of RBCs. One of them is ROS which causes RBC oxidative injuries manifested by hemolytic anaemia such as occurring in β-thalassemia. Melatonin, as a potent free radical scavenger and antioxidant, can effectively protect against RBC oxidative injuries. In addition, melatonin chelates the free iron and upregulates gene expression of antioxidant enzymes of RBCs. Melatonin is synthesized and highly accumulated in RBCs to exhibit the on-site protection. All of these indicate that melatonin is a best molecule to preserve the structural and functional intactness of RBCs. This review tries to update the current development in the field and suggests the potential utility of melatonin on the RBC related disorders.
References
2. Johnson RM, Goyette JrG, Ravindranath Y, Ho YS (2005) Hemoglobin autoxidation and regulation of endogenous H2O2 levels in erythrocytes. Free Radic. Biol. Med. 39 (11):1407-1417. DOI:10.1016/j.freeradbiomed.2005.07.002.
3. Danielczok JG, Terriac E, Hertz L, Petkova-Kirova P, Lautenschläger F, Laschke MW, Kaestner L (2017). Red blood cell passage of small capillaries is associated with transient Ca2+-mediated adaptations. Front. Physiol. 8: 979. DOI: 10.3389/fphys.2017.00979.DOI: 10.3389/fphys.2017.00979.
4. Pandey KB, Rizvi SI (2011) Biomarkers of oxidative stress in red blood cells. Biomedical Papers of the Medical Faculty of Palacky University in Olomouc. 155 (2): 131-136. DOI: 10.5507/bp.2011.027.
5. Arbos KA, Claro LM, Borges L, Santos CA, Weffort-Santos AM (2008) Human erythrocytes as a system for evaluating the antioxidant capacity of vegetable extracts. Nutr. Res. 28 (7): 457-463. DOI: 10.1016/j.nutres.2008.04.004.
6. Siems WG, Sommerburg O, Grune T (2000) Erythrocyte free radical and energy metabolism. Clin. Nephrol. 53: S9-17.
7. Jensen FB (2009) The dual roles of red blood cells in tissue oxygen delivery: oxygen carriers and regulators of local blood flow. J. Exp. Biol. 212 (21): 3387-3393. DOI: 10.1242/jeb.023697.
8. Davies KJ (1995) Oxidative stress: the paradox of aerobic life. In Biochemical Society Symposia. 61: 1-31. DOI: 10.1042/bss0610001.
9. Mohanty J, Nagababu E, Rifkind JM (2014) Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Front physiol. 5: 1-6. DOI: 10.3389/fphys.2014.00084.
10. Shukla P, Yadav NK, Singh P, Bansode FW, Singh RK (2012) Phenylhydrazine induced toxicity: a review on its haematotoxicity. Int. J. Basic Appl. Med. Sci. 2 (2): 86-91.
11. Bryszewska M, Zavodnik IB, Niekurzak A, Szosland K (1995) Oxidative processes in red blood cells from normal and diabetic individuals. Biochem. Mol. Biol. Int. 37 (2): 345-354.
12. Çimen MB (2008) Free radical metabolism in human erythrocytes. Clin. Chim. Acta. 390 (1-2): 1-11. DOI: 10.1016/j.cca.2007.12.025.
13. Eder HA, Finch C, McKee RW (1949) Congenital methemoglobinemia. A clinical and biochemical study of a case. J. Clinic. Invest. 28 (2): 265-272. DOI: 10.1172/JCI102067.
14. Edwards CJ, Fuller J (1996) Oxidative stress in erythrocytes. Comp. Haematol. Int. 6 (1): 24-31.
15. Pantaleo A, Ferru E, Pau MC, Khadjavi A, Mandili G, Mattè A, Spano A, De Franceschi L, Pippia P, Turrini F (2015) Band 3 erythrocyte membrane protein acts as redox stress sensor leading to its phosphorylation by p72 Syk. Oxid. Med. Cell. Longev. 2016: 1- 11. Article ID 6051093. https://doi.org/10.1155/2016/6051093.
16. Lang K, Lang P, Bauer C, Duranton C, Wieder T, Huber S, Lang F (2005) Mechanisms of suicidal erythrocyte death. Cell Physiol. Biochem. 15 (5): 195-202. DOI: 10.1159/000086406.
17. Kehrer JP (1993) Free radicals as mediators of tissue injury and disease. Critic. Rev. Toxicol. 23 (1): 21-48. DOI: 10.3109/10408449309104073.
18. Wink DA, Mitchell JB (1998) Chemical biology of nitric oxide: insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic. Biol. Medic. 25 (4-5): 434-456. DOI: 10.1016/s0891-5849(98)00092-6.
19. Radi R, Peluffo G, Alvarez MN, Naviliat M, Cayota A (2001). Unraveling peroxynitrite formation in biological systems. Free Radic. Biol. Medic. 30 (5): 463-488. DOI: 10.1016/s0891-5849(00)00373-7.
20. Matte A, Low PS, Turrini F, Bertoldi M, Campanella ME, Spano D, Pantaleo A, Siciliano A Franceschi LD (2010) Peroxiredoxin-2 expression is increased in β-thalassemic mouse red cells but is displaced from the membrane as a marker of oxidative stress. Free Radic. Biol. Medic. 49 (3): 457-466. DOI: 10.1016/j. freeradbiomed.2010.05.003.
21. Rana RB, Jilani K, Shahid M, Riaz M, Ranjha MH, Bibi I, Asghar A, Irfan M (2019) Atorvastatin induced erythrocytes membrane blebbing. Dose-Response. 17 (3). DOI: 10.1177/1559325819869076.
22. Yang Q, Noviana M, Zhao Y, Chen D, Wang X (2019) Effect of curcumin extract against oxidative stress on both structure and deformation capability of red blood cell. J. Biomec.7: 109301. DOI: 10.1016/j.jbiomech.2019.07.045.
23. Van den Berg JJ, Den Kamp JAO, Lubin BH, Roelofsen B, Kuypers FA (1992) Kinetics and site specificity of hydroperoxide-induced oxidative damage in red blood cells. Free Radic. Biol. Medic. 12 (6): 487-498. DOI: 10.1016/0891-5849(92)90102-m.
24. Revin VV, Gromova NV, Revina ES, Samonova AY, Tychkov AY, Bochkareva SS, Moskovkin AA, Kuzmenko TP (2019) The influence of oxidative stress and natural antioxidants on morphometric parameters of red blood cells, the hemoglobin oxygen binding capacity, and the activity of antioxidant enzymes. Biomed. Res. Int. 2019: 1-12. DOI: 10.1155/2019/2109269.
25. Morabito R, Remigante A, Di Pietro ML, Giannetto A, La Spada G, Marino A (2017) SO4= uptake and catalase role in preconditioning after H2O2-induced oxidative stress in human erythrocytes. Pflügers Arch-Eur.J. Physiol. 469 (2): 235-250. DOI:10.1007/s00424-016-1927-1.
26. Silveira CR, Junior AV, Corcini CD, Soares SL, Anciuti AN, Kütter MT, Martínez PE (2019) Effects of Bisphenol A on redox balance in red blood and sperm cells and spermatic quality in zebrafish Danio rerio. Ecotoxicol. 28 (8): 913- 922. DOI: 10.1007/s10646-019-02091-5.
27. Bhuyan AAM, Bissinger R, Cao H, Lang F (2016) Triggering of suicidal erythrocyte death by bexarotene. Cell. Physiol. Biochem. 40 (5): 1239-1251. DOI: 10.1159/000453178.
28. Fink M, Bhuyan AAM, Nürnberg B, Faggio C, Lang F (2019) Triggering of eryptosis, the suicidal erythrocyte death, by phenoxodiol. N-S Arch. Pharmacol. 392 (10): 1311- 1318.DOI: 10.1007/s00210-019-01681-8.
29. Silva MC, Madeira VM, Almeida LM, Custódio JB (2000) Hemolysis of human erythrocytes induced by tamoxifen is related to disruption of membrane structure. Biochim. Biophys. Acta 1464 (1): 49-61. DOI: 10.1016/s0005-2736(99)00237-0.
30. Rhoads DL, Wei L, Lin ET, Rezvani A, Way EL (1986) Opioids and rat erythrocyte deformability. in: NIDA Res. Monogr. 75: 121-124.
31. Banday UZ, Swaleh SB, Usmani N (2019) Insights into the heavy metal-induced immunotoxic and genotoxic alterations as health indicators of Clarias gariepinusinhabiting a rivulet. Ecotox. Environ. Safe. 183: 109584. DOI: 10.1016/j.ecoenv. 2019.109584.
32. Tortora F, Notariale R, Maresca V, Good KV, Sorbo S, Basile A, Piscopo M, Manna C (2019) Phenol-rich Feijoa sellowiana (pineapple guava) extracts protect human red blood cells from mercury-induced cellular toxicity. Antioxidants 8 (7): 220. DOI: 10.3390/antiox8070220.
33. Ahmad S, Mahmood R (2019) Mercury chloride toxicity in human erythrocytes: enhanced generation of ROS and RNS, hemoglobin oxidation, impaired antioxidant power, and inhibition of plasma membrane redox system. Environ. Sci. Pollut. Res. Int. 26 (6): 5645-5657. DOI: 10.1007/s11356-018-04062-5.
34. Omanwar S, Ravi K, Fahim M (2011) Persistence of EDHF pathway and impairment of the nitric oxide pathway after chronic mercury chloride exposure in rats: mechanisms of endothelial dysfunction. Human Exp. Toxicol. 30 (11): 1777-1784. DOI: 10.1177/0960327110391389.
35. Harisa GI, Mariee AD, Abo‐Salem OM, Attiaa SM (2014) Erythrocyte nitric oxide synthase as a surrogate marker for mercury‐induced vascular damage: The modulatory effects of naringin. Environ. Toxicol. 29 (11): 1314-1322.DOI: 10.1002/tox.21862.
36. Espín S, Martínez-López E, León-Ortega M, Martínez JE, García-Fernández AJ (2014) Oxidative stress biomarkers in Eurasian eagle owls (Bubo bubo) in three different scenarios of heavy metal exposure. Environ. Res. 131: 134-144. DOI: 10.1016/j.envres.2014.03.015.
37. Valko M, Morris H, Cronin MTD (2005) Metals, toxicity and oxidative stress. Curr. Medic. Chem. 12 (10): 1161-1208. DOI: 10.2174/0929867053764635.
38. Sharma B, Singh S, Siddiqi NJ (2014) Biomedical implications of heavy metals induced imbalances in redox systems. Biomed. Res. Int. 2014: 640754. DOI: 10.1155/2014/640754.
39. Gürer H, Özgünes H, Neal R, Spitz DR, Erçal N (1998) Antioxidant effects of N-acetylcysteine and succimer in red blood cells from lead-exposed rats. Toxicology. 128 (3): 181-189. DOI: 10.1016/s0300-483x(98)00074-2.
40. Conterato GM, Bulcão RP, Sobieski R, Moro AM, Charão MF, de Freitas FA, Almeida FL, Moreira APL, Roehrs M, Tonello R, Grotto D, Barbosa Jr F, Garcia SC, Emanuelli T, Batista BL (2013) Blood thioredoxin reductase activity, oxidative stress and hematological parameters in painters and battery workers: relationship with lead and cadmium levels in blood. J. Appl. Toxicol. 33 (2): 142-150. DOI: 10.1002/jat.1731.
41. Singh N, Rani P, Gupta M, Tandan N (2013) Role of green tea on cadmium toxicity on haematological profile of albino rats. Am. J. Phytomed. Clin. Ther. 1 (5): 537-542.
42. Hamden K, Carreau S, Ellouz F, Masmoudi H, Feki EI (2009) Improvement effect of green tea on hepatic dysfunction, lipid peroxidation and antioxidant defence depletion induced by cadmium. Afr. J. Biotechnol. 8 (17): 4233-4238. DOI: S0716-97602008000300009.
43. Witeska M (2005) Stress in fish-hematological and immunological effects of heavy metals. Electron. J. Ichthyol. 1 (1): 35-41.
44. Gavrić JP, Prokić MD, Anđelković MZ, Despotović SG, Gavrilović BR, Borković-Mitić SS, Radovanović TB, Tomović LM, Pavlović SZ, Saičić ZS (2015) Effects of metals on blood oxidative stress biomarkers and acetylcholinesterase activity in dice snakes (Natrix tessellata) from Serbia. Arch. Biol. Sci. (Belgrade). 67 (1): 303-315. DOI: https://doi.org/10.2298/ABS141203047G.
45. Gantzer J (2019) Heavy Metal Body Burden – Current Day Glimpse at Xenobiotic Lead. Acta Sci. Neuro. 2 (7): 67-71.
46. Halliwell B, Gutteridge J (1984) Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 219 (1): 1. DOI: 10.1042/bj2190001.
47. Hunaiti A, Soud M, Khalil A (1995) Lead concentration and the level of glutathione, glutathione S-transferase, reductase and peroxidase in the blood of some occupational workers from Irbid City, Jordan. Sci. Total Environ. 170 (1-2): 95-100. DOI: 10.1016/0048-9697(95)04606-2.
48. Sugawara E, Nakamura K, Miyake T, Fukumura A, Seki Y (1991) Lipid peroxidation and concentration of glutathione in erythrocytes from workers exposed to lead. Br. J. Ind. Med. 48 (4): 239-242. DOI: 10.1136/oem.48.4.239.
49. Al-Mustafa AH (2006) In vitro study involving the comparative effect of heavy metal ions on antioxidant enzymes activity and lipid peroxide levels in human erytrocytes. Pak. J. Biol. Sci. 9 (14): 2586-2592.
50. Karaulov AV, Renieri EA, Smolyagin AI, Mikhaylova IV, Stadnikov AA, Begun DN, Tsarouhas K, Buha Djordjevic A, Hartung T, Tsatsakis A (2019) Long-term effects of chromium on morphological and immunological parameters of Wistar rats. Food Chem. Toxicol. 133: 110748. DOI: 10.1016/j.fct.2019.110748.
51. Clemens MR, Waller HD (1987) Lipid peroxidation in erythrocytes. Chem. Phys. Lipids. 45 (2-4): 251-268. DOI: 10.1016/0009-3084(87)90068-5.
52. Ribarov SR, Benov LC, Benchev IC (1981) The effect of lead on hemoglobin-catalyzed lipid peroxidation. Biochim. Biophys. Acta 664 (3): 453-459. DOI: 10.1016/0005-2760(81)90123-5.
53. Roche H, Boge G (1993) Effects of Cu, Zn and Cr salts on antioxidant enzyme activities in vitro of red blood cells of a marine fish Dicentrarchus labrax. Toxicol. in Vitro 7 (5): 623-629. DOI: 10.1016/0887-2333(93)90096-n.
54. Pryor WA, Stone K (1993) Oxidants in cigarette smoke radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Ann. N. Y. Acad. Sci. 686 (1): 12-27. DOI: 10.1111/j.1749-6632.1993.tb39148.x.
55. Fernandes AC, Filipe PM, Manso CF (1992) Protective effects of a 21-aminosteroid against copper-induced erythrocyte and plasma lipid peroxidation. Eur. J. Pharmacol. 220 (2-3): 211-216.DOI: 10.1016/0014-2999(92)90750-x.
56. Codandabany U (2000) Erythrocyte lipid peroxidation and antioxidants in cigarette smokers. Cell. Biochem. Funct. 18 (2): 99-102. DOI: 10.1002/(SICI)1099-0844(200006)18:2<99: AID-CBF855>3.0.CO;2-F.
57. Haest CWM, Kamp D, Plasa G, Deuticke B (1977) Intra-and intermolecular cross-linking of membrane proteins in intact erythrocytes and ghosts by SH-oxidizing agents. Biochim. Biophys. Acta. 469 (2): 226-230. DOI: 10.1016/0005-2736(77)90186-9.
58. Asgary S, Naderi GH, Ghannady A (2005) Effects of cigarette smoke, nicotine and cotinine on red blood cell hemolysis and their-SH capacity. Exp. Clin. Cardiol. 10 (2): 116- 119.
59. Mehlhorn RJ (2000) Increased vulnerability of human erythrocytes to hydroperoxide damage after exposure to cigarette smoke or 1-chloro-2, 4-dinitrobenzene in vitro. Nicotine Tob. Res. 2 (2): 141-148. DOI: 10.1080/713688126.
60. Diederich L, Suvorava T, Sansone R, Keller I V, Stevenson TC, Barbarino F, Sutton TR, Kramer CM, Lückstädt W, Isakson BE, Gohlke, H, Feelisch M, Kelm M, Cortese-Krott MM (2018) On the effects of reactive oxygen species and nitric oxide on red blood cell deformability. Front. Physiol. 9: 332. DOI: 10.3389/ fphys.2018.00332.
61. Metta S, Uppala S, Basalingappa DR, Badeti SR, Guntin SS (2015) Impact of smoking on erythrocyte indices and oxidative stress in acute myocardial infarction. J. Dr. NTR University of Health Sciences 4 (3): 159- 164. DOI: 10.4103/2277-8632.165400.
62. Khan MI, Bukhari MH, Akhtar MS, Brar S (2014) Effect of smoking on red blood cells count, hemoglobin concentration and red cell indices. PJMHS 8: 361-364.
63. Bain, BJ, Rothwell M, Feher MD, Robinson R, Brow J, Sever PS (1992) Acute changes in haematological parameters on cessation of smoking. J. R. Soc. Med. 85 (2): 80- 82.
64. Suwazono Y, Dochi M, Oishi M, Tanaka K, Morimoto H, Sakata K (2010) Longitudinal effect of smoking cessation on physical and laboratory findings. Am. J. Prev. Med. 38 (2): 192-200.DOI: 10.1016/j.amepre.2009.09.040.
65. Milman N, Pedersen AN (2009) Blood hemoglobin concentrations are higher in smokers and heavy alcohol consumers than in non-smokers and abstainers—should we adjust the reference range? Ann, Hematol. 88 (7): 687. DOI: 10.1007/s00277-008-0647-9.
66. Attanzio A, Frazzitta A, Vasto S, Tesoriere L, Pintaudi AM, Livrea MA, Cilla A, Allegra M (2019) Increased eryptosis in smokers is associated with the antioxidant status and C-reactive protein levels. Toxicology. 411: 43-48. DOI: 10.1016/ j.tox.2018.10.019.
67. Pretorius E, du Plooy JN, Soma P, Keyser I, Buys AV (2013) Smoking and fluidity of erythrocyte membranes: A high resolution scanning electron and atomic force microscopy investigation. Nitric Oxide. 35: 42-46. DOI: 10.1016/j.niox.2013.08.003.
68. Church DF, Pryor WA (1985) Free-radical chemistry of cigarette smoke and its toxicological implications. Environ. Health Perspect. 64: 111-126. DOI: 10.1289/ehp.8564111.
69. Padmavathi P, Reddy VD, Maturu P, Varadacharyulu N (2010) Smoking-induced alterations in platelet membrane fluidity and Na+/K+-ATPase activity in chronic cigarette smokers. J. Atheroscler. Thromb. 17 (6): 619-627. DOI: 10.5551/jat.2857.
70. Padmavathi P, Reddy VD, Narendra M, Varadacharyulu N (2009) Bidis—hand-rolled, Indian cigarettes: Induced biochemical changes in plasma and red cell membranes of human male volunteers. Clinic. Biochem. 42 (10-11): 1041-1045. DOI: 10.1016/j.clinbiochem.2009.03.005.
71. Padmavathi P, Reddy VD, Kavitha G, Paramahamsa M, Varadacharyulu N (2010) Chronic cigarette smoking alters erythrocyte membrane lipid composition and properties in male human volunteers. Nitric oxide 23 (3): 181-186. DOI: 10.1016/j.niox.2010.05.287.
72. Bergmark E (1997) Hemoglobin adducts of acrylamide and acrylonitrile in laboratory workers, smokers and nonsmokers. Chem. Res. Toxicol. 10 (1): 78-84. DOI: 10.1021/tx960113p.
73. Abdelhalim MAK, Moussa SA (2010) Biochemical changes of hemoglobin and osmotic fragility of red blood cells in high fat diet rabbits. Pak. J. Biol. Sci. 13 (2): 73-77.
74. Kempaiah RK, Srinivasan K (2004) Influence of dietary curcumin, capsaicin and garlic on the antioxidant status of red blood cells and the liver in high-fat-fed rats. Ann. Nutr. Metab. 48 (5): 314-320. DOI: 10.1159/000081198.
75. Wu Z, Jin F, Wang L, Zhao Y, Jiang Y, Li J, Tu P, Zheng J (2019) Antioxidant effects of baoyuan decoction on dysfunctional erythrocytes in high-fat diet-induced hyperlipidemic ApoE-/-mice. Oxid. Med. Cell Longev. 2019. DOI: 10.1155/2019/5172480.
76. Kanakaraj P, Singh M (1989) Influence of hypercholesterolemia on morphological and rheological characteristics of erythrocytes. Atherosclerosis 76 (2-3): 209-218. DOI: 10.1016/0021-9150(89)90105-6.
77. Unruh D, Srinivasan R, Benson T, Haigh S, Coyle D, Batra N, Franco RS (2015) Red blood cell dysfunction induced by high-fat diet: potential implications for obesity-related atherosclerosis. Circulation 132 (20): 1898-1908. DOI: 10.1161/ CIRCULATIONAHA.
115.017313.
78. Vatsala TM, Singh M (1979) In vitro changes and recovery of erythrocytes shape in induced atherogenesis in rabbits. Curr. Sci. 48 (18): 797-799.
79. Cooper RA, Arner EC, Wiley JS, Shattil SJ (1975) Modification of red cell membrane structure by cholesterol-rich lipid dispersions. A model for the primary spur cell defect. J. Clinic. Invest. 55 (1): 115-126. DOI: 10.1172/JCI107901.
80. Murphy JR (1965) Erythrocyte Metabolism. VI. Cell shape and the location of cholesterol in the erythrocyte membrane. J. Lab. Clin. Med. 65: 756.
81. Vatsala TM, Singh M (1980) Changes in shape of erythrocytes in rabbits on atherogenic diet and onion extracts. Atherosclerosis 36 (1): 39-45. DOI: 10.1016/0021-9150(80)90196-3.
82. Potthoff S, Horn P, Stegbauer J, Rump LC, Kelm, M, Cortese-Krott, MM (2010) Hypercholesterolemia decrease NO levels in Red Blood Cells via oxidative stress: PP429. J. Hypertension 28: e179. DOI: 10.1097/01.hjh. 0000378754.86228.83.
83. Bulle S, Reddy VD, Padmavathi P, Maturu P, Puvvada, PK, Nallanchakravarthula, V (2016) Association between alcohol-induced erythrocyte membrane alterations and hemolysis in chronic alcoholics. J. Clin. Biochem. Nutr. 60 (1): 63- 69. DOI: 10.3164/jcbn.16-16.
84. Reddy KR, Reddy VD, Padmavathi P, Kavitha G, Saradamma B, Varadacharyulu N C (2013). Gender differences in alcohol-induced oxidative stress and altered membrane properties in erythrocytes of rats. Indian J. Biochem. Biophys. 50 (1): 32-39.
85. Doyle J, Cooper JS (2018) Physiology, Carbon Dioxide Transport. In: StatPearls [Internet]. StatPearls Publishing.
86. Sosnowska B, Huras B, Nowacka-Krukowska H, Bukowska B (2013) Oxidative damage to human red blood cells treated with chlorfenvinphos, an organophosphate insecticide (in vitro). Biologia. 68 (4): 773-778. DOI: 10.2478/s11756-013-0200-8.
87. Stasiuk M, Kijanka G, Kozubek A (2009) Transformations of erythrocytes shape and its regulation. Postepy. Biochem. 27: 425-433.
88. Mansour SA, Mossa ATH (2009) Lipid peroxidation and oxidative stress in rat erythrocytes induced by chlorpyrifos and the protective effect of zinc. Pestic. Biochem. Physiol. 93 (1): 34-39. https://doi.org/10.1016/j.pestbp.2008.09.004.
89. Saxena R, Garg P (2010) Vitamin E provides protection against in vitro oxidative stress due to pesticide (Chlorpyrifos and Endosulfan) in goat RBC. Bull Biosci. 1: 1-6.
90. Kale M, Rathore N, John S, Bhatnagar D (1999) Lipid peroxidative damage on pyrethroid exposure and alterations in antioxidant status in rat erythrocytes: a possible involvement of reactive oxygen species. Toxicology lett. 105 (3): 197-205. DOI: 10.1016/s0378-4274(98)00399-3.
91. Ogut S, Gultekin F, Nesimi Kisioglu A, Kucukoner E (2011) Oxidative stress in the blood of farm workers following intensive pesticide exposure. Toxicol. Ind. Health 27 (9): 820-825. DOI: 10.1177/0748233711399311.
92. Soltaninejad K, Abdollahi M (2009) Current opinion on the science of organophosphate pesticides and toxic stress: a systematic review. Med. Sci. Monit. 15 (3): RA75-RA90.
93. Bhatti GK, Bhatti JS, Kiran R, Sandhir R (2011) Biochemical and morphological perturbations in rat erythrocytes exposed to ethion: protective effect of vitamin E. Cell Mol. Biol. (Noisy-le-grand). 57 (1): 70-79.
94. Shadnia S, Azizi E, Hosseini R, Khoei S, Fouladdel S, Pajoumand A, Abdollahi, M. (2005) Evaluation of oxidative stress and genotoxicity in organophosphorus insecticide formulators. Human Exp. Toxicol. 24 (9): 439-445. DOI: 10.1191/0960327105ht549oa.
95. Gultekin F, Ozturk M, Akdogan M (2000) The effect of organophosphate insecticide chlorpyrifos-ethyl on lipid peroxidation and antioxidant enzymes (in vitro). Arch. Toxicol. 74 (9): 533-538. DOI: 10.1007/s002040000167.
96. Singh M, Sandhir R, Kiran R (2004) In vitro effects of organophosphate pesticides on rat erythrocytes. Indian J. Exp. Biol. 42: 292-296.
97. Bukowska B, Huras B, Jarosiewicz M, Witaszewska J, Słowińska M, Mokra K, Zakrzewski J, Michałowicz J (2018) The effect of two bromfenvinphos impurities: BDCEE and β-ketophosphonate on oxidative stress induction, acetylcholinesterase activity, and viability of human red blood cells. J. Environ. Sci. Health. A Tox. Hazard. Subst. Environ. Eng. 53 (10): 931-937. DOI: 10.1080/10934529.2018.1462908.
98. John S, Kale M, Rathore N, Bhatnagar D (2001) Protective effect of vitamin E in dimethoate and malathion induced oxidative stress in rat erythrocytes. J. Nutr. Biochem. 12 (9): 500-504. DOI: 10.1016/s0955-2863(01)00160-7.
99. Kumar SS, Sikka HC, Saxena J, Zweig G (1975) Membrane damage in human erythrocytes caused by captan and captafol. Pesticide Biochem. Physiol. 5 (4): 338-347. https://doi.org/10.1016/0048-3575(75)90053-X.
100. Thapar A, Sandhir R, Kiran R (2002) Acephate induced oxidative damage in erythrocytes. Indian J. Exp. Biol. 40: 963-966.
101. Altuntas I, Delibas N, Sutcu R (2002) The effects of organophosphate insecticide methidathion on lipid peroxidation and anti-oxidant enzymes in rat erythrocytes: role of vitamins E and C. Human Exp. Toxicol. 21 (12): 681-685. DOI: 10.1191/ 0960327102ht304oa.
102. Lippi G, Sanchis-Gomar F (2019) Epidemiological, biological and clinical update on exercise-induced hemolysis. Ann. Transl. Med. 7 (12): 270. DOI: 10.21037/atm.2019.05.41.
103. Siquier JC, Muñoz DM, Grijota FP, Bartolomé IS, Robles MG, Montero JA, Maynar MM (2019) Influence of soccer training on parameters of oxidative stress in erythrocytes. Nutr. Hosp. 36 (4): 926-930. DOI: 10.20960/nh.02381.
104. Xiong Y, Xiong Y, Wang Y, Zhao Y, Li Y, Ren Y, Wang R, Zhao M, Hao Y, Liu H Wang, X (2018) Exhaustive-exercise-induced oxidative stress alteration of erythrocyte oxygen release capacity. Can. J. Physiol. Pharmacol. 96 (9): 953-962. DOI: 10.1139/cjpp-2017-0691.
105. Gwozdzinski K, Pieniazek A, Tabaczar S, Jegier A, Brzeszczynska J (2017) Investigation of oxidative stress parameters in different lifespan erythrocyte fractions in young untrained men after acute exercise. Exp. Physiol. 102 (2): 190-201. DOI: 10.1113/EP085930.
106. Medeiros-Lima DJM, Mendes-Ribeiro AC, Brunini TMC, Martins MA, Mury WV, Freire RA, Monteiro WD, Farinatti PT, Matsuura C (2017) Erythrocyte nitric oxide availability and oxidative stress following exercise. Clin. Hemorheol. Microcirc. 65 (3): 219-228. DOI: 10.3233/CH-16162.
107. Xiong Y, Xiong Y, Zhou S, Yu Z, Zhao D, Wang Z, Li Y, Yan J, Cai Y, Zhang W (2016) Inhibition of glutathione synthesis induced by exhaustive running exercise via the decreased influx rate of l-cysteine in rat erythrocytes. Cell. Physiol. Biochem. 40 (6): 1410-1421. DOI: 10.1159/000453193.
108. Higgs DR, Engel JD, Stamatoyannopoulos G (2012) Thalassemia. Lancet 379 (9813): 373-383. DOI: 10.1016/S0140-6736(11)60283-3.
109. Taher AT, Weatherall DJ, Cappellini MD (2018) Thalassemia. Lancet 391:155–167. DOI: 10.1016/S0140-6736(17)31822-6.
110. Chaichompoo P, Qillah A, Sirankapracha P, Kaewchuchuen J, Rimthong P, Paiboonsukwong K, Fucharoen S, Svasti S, Worawichawong S (2019) Abnormal red blood cell morphological changes in thalassemia associated with iron overload and oxidative stress. J. Clin. Path. 72 (8): 520-524. http://dx.doi.org/10.1136/jclinpath-2019-205775.
111. Hebbel RP (1991) Beyond hemoglobin polymerization: the red blood cell membrane and sickle disease pathophysiology. Blood 77 (2): 214-237.
112. Yuan J, Bunyaratvej A, Fucharoen S, Fung C, Shinar E, Schrier SL (1995) The instability of the membrane skeleton in thalassemic red blood cells. Blood 86 (10): 3945-3950.
113. Schrier SL (2002) Pathophysiology of thalassemia. Curr. Opin. Hematol. 9 (2): 123-126. DOI: 10.1097/00062752-200203000-00007.
114. Leecharoenkiat K, Lithanatudom P, Sornjai W, Smith DR (2016) Iron dysregulation in beta-thalassemia. Asian Pac. J. Trop. Med. 9 (11): 1035-1043. https://doi.org/10.1016/j.apjtm.2016.07.035.DOI: 10.1016/j.apjtm.2016.07.035.
115. Rachmilewitz EA, Weizer‐Stern ORLY, Adamsky K, Amariglio N, Rechavi G, Breda L, Cabantchik ZI (2005) Role of iron in inducing oxidative stress in thalassemia: can it be prevented by inhibition of absorption and by antioxidants? Ann. N. Y. Acad. Sci. 1054 (1): 118-123. DOI: 10.1196/annals.1345.014.
116. Dissayabutra T, Tosukhowong P, Seksan P (2005) The benefits of vitamin C and vitamin E in children with beta-thalassemia with high oxidative stress. J. Med. Assoc. Thai. 88 (Suppl 4): S317-S321.
117. Prus E, Fibach E (2010) Effect of iron chelators on labile iron and oxidative status of thalassaemic erythroid cells. Acta Haematol. 123 (1): 14-20. https://doi.org/10.1159/ 000258958.
118. Advani R, Rubin E, Mohandas NA, Schrier SL (1992) Oxidative red blood cell membrane injury in the pathophysiology of severe mouse beta-thalassemia. Blood 79 (4): 1064-1067.
119. Advani R, Sorenson S, Shinar E, Lande W, Rachmilewitz E, Schrier SL (1992) Characterization and comparison of the red blood cell membrane damage in severe human alpha-and beta-thalassemia. Blood 79 (4): 1058-1063.
120. Kassab-Chekir A, Laradi S, Ferchichi S, Khelil AH, Feki M, Amri F, Selmi H, Bejaoui M, Miled A (2003) Oxidant, antioxidant status and metabolic data in patients with beta-thalassemia. Clin. Chim. Acta. 338 (1-2): 79-86. DOI: 10.1016/j.cccn.2003.07.010.
121. Aziz BN, Ali WK, Al-Kataan MA (2009) Lipid peroxidation and antioxidant status in β-thalassemic patients: effect of iron overload. Iraqi J. Pharm. Sci. 18 (2): 8-14. DOI: 10.4103/ejh.ejh_41_16.
122. Kalpravidh RW, Tangjaidee T, Hatairaktham S, Charoensakdi R, Panichkul N, Siritanaratkul N, Fucharoen S (2013) Glutathione redox system in β-thalassemia/Hb E patients. Sci. World J. 2013: 543973. http://dx.doi.org/10.1155/2013/543973.
123. Cheng M L, Ho HY, Tseng HC, Lee CH, Shih LY, Chiu DTY (2005) Antioxidant deficit and enhanced susceptibility to oxidative damage in individuals with different forms of α‐thalassemia. Br. J. Haematol. 128 (1): 119-127. DOI: 10.1111/j.1365-2141.2004.05257.x.
124. Chiou SS, Tsao CJ, Tsai SM, Wu YR, Liao YM, Lin PC, Tsai LY (2014) Metabolic pathways related to oxidative stress in patients with hemoglobin H disease and iron overload. J. Clin. Lab. Anal. 28 (4): 261-268. DOI: 10.1002/jcla.21676.
125. Korkina L, De Luca C, Deeva I, Perrotta S, Nobili B, Passi S, Puddu P (2000) L1 effects on reactive oxygen (ROS) and nitrogen species (RNS) release, hemoglobin oxidation, low molecular weight antioxidants, and antioxidant enzyme activities in red and white blood cells of thalassemic patients. Transfus. Sci. 23 (3): 253-254. DOI: 10.1016/s0955-3886(00)00099-0.
126. Yuan J, Bunyaratvej A, Fucharoen S, Fung C, Shinar E, Schrier SL (1995) The instability of the membrane skeleton in thalassemic red blood cells. Blood 86 (10): 3945-3950.
127. Naithani R, Chandra J, Bhattacharjee J, Verma P, Narayan S (2006) Peroxidative stress and antioxidant enzymes in children with β‐thalassemia major. Pediatr. Blood Cancer 46 (7): 780-785. DOI: 10.1002/pbc.20669.
128. Ozment CP, Turi JL (2009) Iron overload following red blood cell transfusion and its impact on disease severity. Biochim. Biophys. Acta. 1790 (7): 694-701. DOI: 10.1016/j.bbagen.2008.09.010.
129. Diez-Ewald M, Layrisse M, Ojeda A (1968) Mechanisms of hemolysis in iron deficiency anemia. Further studies. Blood 32 (6): 884-894.
130. Huser HJ, Rieber EE, Berman AR (1967) Experimental evidence of excess hemolysis in the course of chronic iron deficiency anemia. J. Lab. Clin. Med. 69 (3): 405-414.
131. Yip R, Mohandas N, Clark MR, Jain S, Shohet SB, Dallman PR (1983) Red cell membrane stiffness in iron deficiency. Blood 62 (1): 99-106.
132. Tillmann W, Schröter W (1980) Deformability of erythrocytes in iron deficiency anemia. Blut. 40 (3): 179-186. DOI: 10.1007/bf01008575.
133. Vayá A, Simó M, Santaolaria M, Todolí J, Aznar J (2005) Red blood cell deformability in iron deficiency anaemia. Clin. Hemorheol. Microcirc. 33 (1): 75-80.
134. Kempe DS, Lang PA, Duranton C, Akel A, Lang KS, Huber SM, Wieder T, Lang F (2006) Enhanced programmed cell death of iron-deficient erythrocytes. FASEB J. 20 (2): 368-370. DOI: 10.1096/fj.05-4872fje.
135. Lang F, Lang KS, Lang PA, Huber SM, Wieder T (2006) Mechanisms and significance of eryptosis. Antioxid. Redox Signal 8 (7-8): 1183-1192. DOI: 10.1089/ars.2006.8.1183.
136. Pfafferott C, Meiselman HJ, Hochstein P (1982) The effect of malonyldialdehyde on erythrocyte deformability. Blood 59 (1): 12-15.
137. Damonte G, Guida L, Sdraffa A, Benatti U, Melloni E, Forteleoni G, Meloni T, Carafoli E, De Flora A (1992) Mechanisms of perturbation of erythrocyte calcium homeostasis in favism. Cell Calcium 13 (10): 649-658. DOI: 10.1016/0143-4160(92)90075-4.
138. Rodvien R, Gillum A, Weintraub LR (1974) Decreased glutathione peroxidase activity secondary to severe iron deficiency: A possible mechanism responsible for the shortened life span of the iron-deficient red cell. Blood 43 (2): 281-289.
139. Jain SK, Yip R, Hoesch RM, Pramanik AK, Dallman PR, Shohet SB (1983) Evidence of peroxidative damage to the erythrocyte membrane in iron deficiency. Am. J. Clinic. Nutri. 37 (1): 26-30. DOI: 10.1093/ajcn/37.1.26.
140. Moshynska OV, Tretiak NN, Anoshina MY, Yagovdic, MV (2001) Hemoglobin-induced lipid peroxidation in anemia. Lik. Sprava. 4: 39-43.
141. Kumerova A, Lece A, Skesters A, Silova A, Petuhovs V (1998) Anaemia and antioxidant defence of the red blood cells. Mater. Med. Pol. 30 (1-2): 12-15.
142. Cellerino R, Guidi G, Perona G (1976) Plasma iron and erythrocytic glutathione peroxidase activity: a possible mechanism for oxidative hemolysis in iron deficiency anaemia. Scand. J. Haemat. 17 (2): 111-116.
143. Bartal M, Mazor D, Dvilansky A, Meyerstein N (1993) Iron deficiency anemia: recovery from in vitro oxidative stress. Acta haematol. 90 (2): 94-98.DOI: 10.1159/000204383.
144. Melhorn DK, Gross S, Lake GA, Leu JA (1971) The hydrogen peroxide fragility test and serum tocopherol level in anemias of various etiologies. Blood 37 (4): 438-446.
145. Nagababu E, Chrest FJ, Rifkind JM (2003) Hydrogen-peroxide-induced heme degradation in red blood cells: the protective roles of catalase and glutathione peroxidase. Biochim. Biophys. Acta. 1620 (1-3): 211-217. DOI: 10.1016/s0304-4165(02)00537-8.
146. Nagababu E, Gulyani S, Earley CJ, Cutler RG, Mattson MP, Rifkind JM (2008) Iron-deficiency anaemia enhances red blood cell oxidative stress. Free Radic. Res. 42 (9): 824-829. DOI: 10.1080/10715760802459879.
147. Hébert PC, der Linden Van P, Biro G, Hu LQ (2004) Physiologic aspects of anemia. Crit. Care Clin. 20 (2): 187-212.DOI: 10.1016/j.ccc.2004.01.001.
148. Hare GM (2004) Anaemia and the brain. Curr. Opin. Anaesthesiol. 17 (5): 363-369.
149. Barnham KJ, Masters CL, Bush AI (2004) Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 3 (3): 205-214. DOI: 10.1038/nrd1330.
150. Liu Z, Zhou T, Ziegler AC, Dimitrion P, Zuo L (2017) Oxidative stress in neurodegenerative diseases: from molecular mechanisms to clinical applications. Oxid. Med. Cell Longev. 2017: 1- 11. DOI: 10.1155/2017/2525967.
151. Albers DS, Beal MF (2000) Mitochondrial dysfunction and oxidative stress in aging and neurodegenerative disease. J. Neural. Transm. Suppl. 59: 133-154. DOI: 10.1007/978-3-7091-6781-6_16.
152. Dias V, Junn E, Mouradian MM (2013) The role of oxidative stress in Parkinson's disease. J. Parkinsons Dis. 3 (4): 461-491. DOI: 10.3233/JPD-130230.
153. Mar RJ, Lovell MA, Markesbery WR, Uchida K, Mattson MP (1997) A role for 4-hydroxynonenal in disruption of ion homeostasis and neuronal death by amyloid β-peptide. J. Neurochem. 68: 255-264. DOI: 10.1046/j.1471-4159.1997.68010255.x.
154. Bosman GJCGM, Bartholomeus IGP, De Man AJM, Van Kalmthout PJC, De Grip WJ (1991) Erythrocyte membrane characteristics indicate abnormal cellular aging in patients with Alzheimer's disease. Neurobiol. Aging 12 (1): 13-18. DOI: 10.1016/ 0197-4580(91)90033-g.
155. Passow H (1986) Molecular aspects of band 3 protein-mediated anion transport across the red blood cell membrane. Rev. Physiol. Biochem. Pharmacol. 103: 61-203. DOI: 10.1007/3540153330_2.
156. Bosman GJ, Kay MM (1988) Erythrocyte aging: a comparison of model systems for simulating cellular aging in vitro. Blood Cells 14 (1): 19-46.
157. Kay MM, Bosman GJ, Shapiro SS, Bendich A, Bassel PS (1986) Oxidation as a possible mechanism of cellular aging: vitamin E deficiency causes premature aging and IgG binding to erythrocytes. Proc. Natl. Acad. Sci. USA. 83 (8): 2463-2467. DOI: 10.1073/pnas.83.8.2463.
158. Kay MM, Bosman G, Notter M, Coleman P (1988) Life and death of neurons: the role of senescent cell antigen. Ann. NY. Acad. Sci. 521 (1): 155-169. DOI: 10.1111/j.1749-6632.1988.tb35274.x.
159. Markesbery WR, Leung PK, Butterfield DA (1980) Spin label and biochemical studies of erythrocyte membranes in Alzheimer's disease. J. Neurol. Sci. 45 (2-3): 323-330. DOI: 10.1016/0022-510x(80)90175-6.
160. Mattson MP, Begley JG, Mark RJ, Furukawa K (1997) Aβ25–35 induces rapid lysis of red blood cells: contrast with Aβ1–42 and examination of underlying mechanisms. Brain Res. 771 (1): 147-153. https://doi.org/10.1016/S0006-8993(97)00824-X.
161. Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S, Chiba S, Atwood CS, Petersen RB, Chiba S (2001) Oxidative damage is the earliest event in Alzheimer disease. J. Neuropathol. Exp. Neurol. 60 (8): 759-767. DOI: 10.1093/jnen/60.8.759.
162. Seubert P, Vigo-Pelfrey C, Esch F, Lee M, Dovey H, Davis D, Sinha S, Schlossmacher M, Whaley J, McCormack, R. (1992). Isolation and quantification of soluble Alzheimer's β-peptide from biological fluids. Nature 359 (6393): 325.DOI: 10.1038/359325a0.
163. De la Torre JC (2002) Vascular basis of Alzheimer's pathogenesis. Ann. NY. Acad. Sci. 977 (1): 196-215. DOI: 10.1111/j.1749-6632.2002.tb04817.x.
164. Kay MM (1984) Localization of senescent cell antigen on band 3. Proc. Nat. Acad. Sci. 81 (18): 5753-5757. DOI: 10.1073/pnas.81.18.5753.
165. Clementi ME, Giardina B, Colucci D, Galtieri A, Misiti F (2007) Amyloid-beta peptide affects the oxygen dependence of erythrocyte metabolism: a role for caspase 3. Int. J. Biochem. Cell Biol. 39 (4): 727-735. DOI: 10.1016/j.biocel.2006.11.013.
166. Mandal D, Baudin-Creuza V, Bhattacharyya A, Pathak S, Delaunay J, Kundu M, Basu J (2003) Caspase 3-mediated proteolysis of the N-terminal cytoplasmic domain of the human erythroid anion exchanger 1 (band 3). J. Biol. Chem. 278 (52): 52551-52558. DOI: 10.1074/jbc.M306914200.
167. Nicolay J, Gatz S, Liebig G, Gulbins E, Lang F (2007) Amyloid induced suicidal erythrocyte death. Cell. Physiol. Biochem.19 (1-4): 175-184. DOI: 10.1159/ 000099205.
168. Skoumalová A, Hort J (2012) Blood markers of oxidative stress in Alzheimer's disease. J. Cell Mol. Med. 16 (10): 2291-2300.DOI: 10.1111/j.1582-4934.2012. 01585.x.
169. Carelli-Alinovi C, Ficarra S, Russo AM, Giunta E, Barreca D, Galtieri A, Misiti F, Tellone E (2016) Involvement of acetylcholinesterase and protein kinase C in the protective effect of caffeine against β-amyloid-induced alterations in red blood cells. Biochimie. 121: 52-59.DOI: 10.1016/j.biochi.2015.11.022.
170. Gilca M, Lixandru D, Gaman L, Vîrgolici B, Atanasiu V, Stoian I (2014) Erythrocyte membrane stability to hydrogen peroxide is decreased in Alzheimer disease. Alzheimer Dis. Assoc. Disord. 28 (4): 358-363. DOI: 10.1097/WAD. 0000000000000026.
171. Serra JA, Dominguez RO, De Lustig ES, Guareschi EM, Famulari AL, Bartolome E L, Marschoff ER (2001) Parkinson's disease is associated with oxidative stress: comparison of peripheral antioxidant profiles in living Parkinson's, Alzheimer's and vascular dementia patients. J. Neural. Transm. (Vienna). 108 (10): 1135-1148. DOI: 10.1007/s007020170003.
172. Serra JA, Famulari AL, Kohan S, Marschoff ER, Dominguez RO, de Lustig ES (1994) Copper-zinc superoxide dismutase activity in red blood cells in probable Alzheimer's patients and their first-degree relatives. J. Neurol. Sci. 122 (2): 179-188. DOI: 10.1016/0022-510x(94)90297-6.
173. Carelli-Alinovi C, Misiti F (2017) Erythrocytes as potential link between diabetes and Alzheimer’s disease. Front. Aging Neurosci. 9 (276): 1- 11. DOI: 10.3389/fnagi.2017.00276.
174. de Farias CC, Maes M, Bonifácio KL, Bortolasci CC, de Souza Nogueira A, Brinholi, FF, Matsumoto AK, do Nascimento MA, de Melo LB, Nixdorf SL, Lavado EL, Moreira EG, Lavado EL (2016) Highly specific changes in antioxidant levels and lipid peroxidation in Parkinson’s disease and its progression: disease and staging biomarkers and new drug targets. Neurosci. Lett. 617: 66-71. DOI: 10.1016/ j.neulet.2016.02.011.
175. Puspita L, Chung SY, Shim JW (2017) Oxidative stress and cellular pathologies in Parkinson’s disease. Mol. Brain 10 (53): 1- 12. DOI: 10.1186/s13041-017-0340-9.
176. Mastroberardino PG, Hoffman EK, Horowitz MP, Betarbet R, Taylor G, Cheng D, Na HM, Gutekunst CA, Gearing M, Trojanowski JQ, Anderson M, Chu CT, Peng J, Anderson, M (2009) A novel transferrin/TfR2-mediated mitochondrial iron transport system is disrupted in Parkinson's disease. Neurobiol. Dis. 34 (3): 417-431. DOI: 10.1016/j.nbd.2009.02.009.
177. Isaya G (2014) Mitochondrial iron-sulfur cluster dysfunction in neurodegenerative disease. Front. Pharmacol. 5 (29): 1- 7. DOI: 10.3389/fphar.2014.00029.
178. Du XX, Xu HM, Jiang H, Song N, Wang J, Xie JX (2012) Curcumin protects nigral dopaminergic neurons by iron-chelation in the 6-hydroxydopamine rat model of Parkinson’s disease. Neurosci. Bull. 28 (3): 253-258. DOI: 10.1007/s12264-012-1238-2.
179. Pretorius E, Swanepoel AC, Buys AV, Vermeulen N, Duim W, Kell DB (2014) Eryptosis as a marker of Parkinson's disease. Aging (Albany NY). 6 (10): 788- 819. DOI: 10.18632/aging.100695.
180. Rizvi SI, Srivastava N (2010) Erythrocyte plasma membrane redox system in first degree relatives of type 2 diabetic patients. Int. J. Diabetes Mel. 2 (2): 119-121. https://doi.org/10.1016/j.ijdm.2010.05.005.
181. Pescarmona GP, Bosia A, Ghigo D (1982) Shortened red cell life span in diabetes: mechanism of hemolysis. In: Adv. Red Cell Biol. Weatherall DJ, Fiorelli G, Gorini S, (Eds.) New York, Raven, 391-397.
182. Peterson CM, Jones RL, Koenig RJ, Melvin ET, Lehrman ML (1977) Reversible hematologic sequelae of diabetes mellitus. Ann. Intern. Med. 86 (4): 425-429. DOI: 10.7326/0003-4819-86-4-425.
183. Wali RK, Jaffe S, Kuma, D, Kalra VK (1988) Alterations in organization of phospholipids in erythrocytes as factor in adherence to endothelial cells in diabetes mellitus. Diabetes 37 (1): 104-111. DOI: 10.2337/diab.37.1.104.
184. Schmid-Schönbein H, Volger E (1976) Red-cell aggregation and red-cell deformability in diabetes. Diabetes 25 (2 SUPPL): 897-902.
185. Satoh M, Imaizumi K, Bessho T, Shiga T (1984) Increased erythrocyte aggregation in diabetes mellitus and its relationship to glycosylated hemoglobin and retinopathy. Diabetologia 27 (5): 517-521. DOI: 10.1007/bf00290387.
186. Jones RL, Peterson CM (1981) Hematologic alterations in diabetes mellitus. Am. J. Med. 70 (2): 339-352. DOI: 10.1016/0002-9343(81)90771-3.
187. Nicolay J, Schneider J, Niemoeller O, Artunc F, Portero-Otin M, Haik Jr G, Thornalley PJ, Schleicher E, Wieder T, Lang F (2006) Stimulation of suicidal erythrocyte death by methylglyoxal. Cell Physiol. Biochem. 18 (4-5): 223-232. DOI: 10.1159/000097669.
188. Buys AV, Van Rooy MJ, Soma P, Van Papendorp D, Lipinski B, Pretorius E (2013) Changes in red blood cell membrane structure in type 2 diabetes: a scanning electron and atomic force microscopy study. Cardiovasc. Diabetol. 12 (1): 25. DOI: 10.1186/ 1475-2840-12-25.
189. Fava S, Azzopardi J, Ellard S, Hattersley AT (2001) ACE gene polymorphism as a prognostic indicator in patients with type 2 diabetes and established renal disease. Diabetes Care 24 (12): 2115-2120. DOI: 10.2337/diacare.24.12.2115.
190. Angelousi A, Larger E (2015) Anaemia, a common but often unrecognized risk in diabetic patients: a review. Diabetes Metab. 41 (1): 18-27. DOI: 10.1016/j.diabet.2014.06.001.
191. Astor BC, Muntner P, Levin A, Eustace JA, Coresh J (2002) Association of kidney function with anemia: the third national health and nutrition examination survey (1988-1994). Arch. Intern. Med. 162 (12), 1401-1408. DOI: 10.1001/archinte. 162.12.1401.
192. Dincer Y, Akcay T, Alademir Z, Ilkova H (2002) Effect of oxidative stress on glutathione pathway in red blood cells from patients with insulin-dependent diabetes mellitus. Metabolism 51 (10): 1360-1362. DOI: 10.1053/meta.2002.35192.
193. Efferth T, Schwarzl SM, Smith J, Osieka R (2006) Role of glucose-6-phosphate dehydrogenase for oxidative stress and apoptosis. Cell Death Differ. 13 (3): 527-528. https://doi.org/10.1038/sj.cdd.4401807.
194. Vanella A, Campisi A, Castorina C, Sorrenti V, Attaguile G, Samperi P, Azzia N, Di Giacomo C, Schiliro G (1991) Antioxidant enzymatic systems and oxidative stress in erythrocytes with G6PD deficiency: effect of deferoxamine. Pharmacol. Res. 24 (1): 25-31. DOI: 10.1016/1043-6618(91)90061-2.
195. Beutler E (1983) The metabolic basis of inherited disease. Stanbury JB, Wyngaarden DJ, Fiorelli G, Gorini S, (Eds.) 5: 1629- 1653. McGraw-Hill Book Company, New York.
196. Fico A, Paglialunga F, Cigliano L, Abrescia P, Verde P, Martini G, Iaccarino I, Filosa S (2004) Glucose-6-phosphate dehydrogenase plays a crucial role in protection from redox-stress-induced apoptosis. Cell Death Differ. 11 (8): 823-831. DOI: 10.1038/sj.cdd.4401420.
197. Pandolfi PP, Sonati F, Rivi R, Mason P, Grosveld F, Luzzatto L (1995) Targeted disruption of the housekeeping gene encoding glucose 6-phosphate dehydrogenase (G6PD): G6PD is dispensable for pentose synthesis but essential for defense against oxidative stress. EMBO J. 14 (21): 5209-5215.
198. Revin VV, Gromova NV, Revina ES, Samonova AY, Tychkov AY, Bochkarev SS, Moskovkin AA, Kuzmenko TP (2019) The influence of oxidative stress and natural antioxidants on morphometric parameters of red blood cells, the hemoglobin oxygen binding capacity, and the activity of antioxidant enzymes. Biomed. Res. Int. 2019: 2109269 DOI: 10.1155/2019/2109269.
199. Castenmiller JJ, Lauridsen ST, Dragsted LO, Hof KHVH, Linssen JP, West CE (1999) β-Carotene does not change markers of enzymatic and nonenzymatic antioxidant activity in human blood. J. Nutri. 129 (12): 2162-2169. DOI: 10.1093/jn/129.12.2162.
200. Mira L, Fernandez MT, Santos M, Rocha R, Florêncio MH, Jennings KR (2002) Interactions of flavonoids with iron and copper ions: a mechanism for their antioxidant activity. Free Radic. Res. 36 (11): 1199-1208. DOI: 10.1080/1071576021000016463.
201. Verma SP, Wallach DF (1975) Carotenoids as a Raman-active probes of erythrocyte membrane structure. Biochim. Biophys. Acta 401 (2): 168-176.
202. Wang H, Yang YJ, Qian HY, Zhang Q, Xu H, Li JJ (2012) Resveratrol in cardiovascular disease: what is known from current research? Heart Fail. Rev. 17 (3): 437-448. DOI: 10.1007/s10741-011-9260-4.
203. Kiruthiga PV, Shafreen RB, Pandian SK, Devi KP (2007) Silymarin protection against major reactive oxygen species released by environmental toxins: exogenous H2O2 exposure in erythrocytes. Basic Clinic. Pharmacol. Toxicol. 100 (6): 414-419. DOI: 10.1111/j.1742-7843.2007.00069.x.
204. Biswas S, Bhattacharyya J, Dutta AG (2005) Oxidant induced injury of erythrocyte—Role of green tea leaf and ascorbic acid. Mol. Cell. Biochem. 276 (1-2): 205-210. DOI: 10.1007/s11010-005-4062-4.
205. Brown KM, Morrice PC, Arthur JR, Duthie GG (1996) Effects of vitamin E supplementation on erythrocyte antioxidant defence mechanisms of smoking and non-smoking men. Clinic. Sci. 91(1): 107-111. DOI: 10.1042/cs0910107.
206. Paul S, Ghosh AK, Ghosh D, Dutta M, Mitra E, Dey, M, Bhowmick D, Das T, Firdaus SB, Mishra S, Bandyopadhyay D, Chattopadhyay A (2014) Aqueous bark extract of Terminalia arjuna protects against phenylhydrazine induced oxidative damage in goat red blood cell membrane protein, phospholipid asymmetry and structural morphology: a flow cytometric and biochemical analysis. J. Pharm. Res. 8 (12): 1790-1804.
207. Asgary S, Naderi GH, Askari N (2005) Protective effect of flavonoids against red blood cell hemolysis by free radicals. Exp. Clinic. Cardiol. 10 (2): 88-90.
208. Kucherenko YV, Bernhardt I (2015) Natural antioxidants improve red blood cell “survival” in non-leukoreduced blood samples. Cell Physiol. Biochem. 35 (5): 2055-2068.DOI: 10.1159/000374012.
209. Ajibade TO, Oyagbemi AA, Durotoye LA, Omóbòwálé TO, Asenuga ER, Olayemi FO (2017) Modulatory effects of melatonin and vitamin C on oxidative stress-mediated hemolytic anaemia and associated cardiovascular dysfunctions in rats. J. Complement. Integr. Med. 14 (1). DOI: 10.1515/jcim-2015-0082.
210. Yanpanitch OU, Hatairaktham S, Charoensakdi R, Panichkul N, Fucharoen S, Srichairatanakool S, Kalpravidh RW (2015) Treatment of β-thalassemia/hemoglobin E with antioxidant cocktails results in decreased oxidative stress, increased hemoglobin concentration, and improvement of the hypercoagulable state. Oxi. Med. Cell. Long. 2015: 537954. DOI: 10.1155/2015/537954.
211. Menon N, Sparks J, Omoruyi FO (2016) Oxidative stress parameters and erythrocyte membrane adenosine triphosphatase activities in streptozotocin-induced diabetic rats administered aqueous preparation of Kalanchoe Pinnata leaves. Pharmacognosy Res. 8 (2): 85-88. DOI: 10.4103/0974-8490.172656.
212. Straface E, Rivabene R, Masella R, Santulli M, Paganelli R, Malorni W (2002) Structural changes of the erythrocyte as a marker of non-insulin-dependent diabetes: protective effects of N-acetylcysteine. Biochem. Biophys. Res. Commun. 290 (5): 1393-1398. DOI: 10.1006/bbrc.2002.6340.
213. Lerner AB, Case JD, Takahashi Y, Lee TH, Mori W (1958) Isolation of melatonin, the pineal gland factor that lightens melanocyte S1. J. Am. Chem. Soc. 80 (10): 2587-2587. https://doi.org/10.1021/ja01543a060.
214. Tan DX (1993) Melatonin: a potent, endogenous hydroxyl radical scavenger. Endocr. J. 1:57-60.
215. Manchester LC, Poeggeler B, Alvares FL, Ogden GB, Reiter RJ (1995) Melatonin immunoreactivity in the photosynthetic prokaryote Rhodospirillum rubrum: implications for an ancient antioxidant system. Cell. Mol. Biol. Res. 41 (5): 391-395.
216. Tan DX, Hardeland R, Manchester LC, Paredes SD, Korkmaz A, Sainz RM, Mayo JC, Fuentes-Broto L, Reiter RJ (2010) The changing biological roles of melatonin during evolution: from an antioxidant to signals of darkness, sexual selection and fitness. Biol. Rev. 85 (3): 607-623. DOI: 10.1111/j.1469-185X.2009.00118.x.
217. Galano A, Tan DX, Reiter RJ (2011) Melatonin as a natural ally against oxidative stress: a physicochemical examination. J pineal Res. 51 (1): 1-6. DOI: 10.1111/j.1600-079X.2011.00916.x.
218. Stasica P, Ulanski P, Rosiak JM (1998) Melatonin as a hydroxyl radical scavenger. J. Pineal Res. 25 (1): 65-66. DOI: 10.1111/j.1600-079x.1998.tb00387.x.
219. Ebelt H, Peschke D, Brömme HJ, Mörke W, Blume R, Peschke E (2000) Influence of melatonin on free radical‐induced changes in rat pancreatic beta‐cells in vitro. J. Pineal Res. 28 (2): 65-72. DOI: 10.1034/j.1600-079x.2001.280201.x.
220. Bandyopadhyay D, Biswas K, Bandyopadhyay U, Reiter RJ, Banerjee RK (2000) Melatonin protects against stress‐induced gastric lesions by scavenging the hydroxyl radical. J. Pineal Res. 29 (3): 143-151. DOI: 10.1034/j.1600-079x.2000.290303.x.
221. Brömme HJ, Mörke W, Peschke E, Ebelt H, Peschke D (2000) Scavenging effect of melatonin on hydroxyl radicals generated by alloxan. J. Pineal Res. 29 (4): 201-208. DOI: 10.1034/j.1600-0633.2002.290402.x.
222. Scaiano JC (1995) Exploratory laser flash photolysis study of free radical reactions and magnetic field effects in melatonin chemistry. J. Pineal Res. 19 (4): 189-195. DOI: 10.1111/j.1600-079x.1995.tb00188.x.
223. Reiter RJ, Acuña‐Castroviejo D, Tan DX, Burkhardt S (2001) Free radical‐mediated molecular damage: mechanisms for the protective actions of melatonin in the central nervous system. Ann. N. Y. Acad. Sci. 939 (1): 200-215.
224. Mayo JC, Tan DX, Sainz RM, Lopez-Burillo S, Reiter RJ (2003) Oxidative damage to catalase induced by peroxyl radicals: functional protection by melatonin and other antioxidants. Free Radic. Res. 37 (5): 543-553. DOI: 10.1080/1071576031000083206.
225. Livrea MA, Tesoriere L, D'Arpa D, Morreale M (1997) Reaction of melatonin with lipoperoxyl radicals in phospholipid bilayers. Free Radic. Biol. Med. 23 (5): 706-711. DOI: 10.1016/s0891-5849(97)00018-x.
226. Escames G, Guerrero JM, Reiter RJ, Garcia JJ, Munoz-Hoyos A, Ortiz GG, Oh CS (1997) Melatonin and vitamin E limit nitric oxide-induced lipid peroxidation in rat brain homogenates. Neurosci. Lett. 230 (3): 147-150.DOI: 10.1016/s0304-3940(97)00498-9.
227. Siu AW, Ortiz GG, Benitez-King G, To CH, Reiter RJ (2004) Effects of melatonin on the nitric oxide treated retina. Br. J. Ophthalmol. 88 (8): 1078-1081. http://dx.doi.org/ 10.1136/bjo.2003.037879.
228. Cagnoli CM, Atabay C, Kharlamova E, Manev H (1995) Melatonin protects neurons from singlet oxygen‐induced apoptosis. J. Pineal Res. 18 (4): 222-226. DOI: 10.1111/j.1600-079x.1995.tb00163.x.
229. Matuszak Z, Bilska MA, Reszka KJ, Chignell CF, Bilski P (2003) Interaction of singlet molecular oxygen with melatonin and related indoles. Photochem. Photobiol. 78 (5): 449-455. DOI: 10.1562/0031-8655(2003)078<0449:iosmow>2.0.co;2.
230. Reiter R, Tan D, Rosales-Corral S, Galano A, Zhou X, Xu B (2018) Mitochondria: central organelles for melatonin′s antioxidant and anti-aging actions. Molecules 23 (2): pii: E509.DOI: 10.3390/molecules23020509.
231. Venegas C, García JA, Escames G, Ortiz F, López A, Doerrier C, García-Corzo L, López LC, Reiter RJ, Acuña-Castroviejo D (2012) Extrapineal melatonin: analysis of its subcellular distribution and daily fluctuations. J. Pineal Res. 52 (2): 217-227. DOI: 10.1111/j.1600-079X.2011.00931.x.
232. Reiter RJ, Rosales-Corral S, Tan DX, Jou MJ, Galano A, Xu B (2017) Melatonin as a mitochondria-targeted antioxidant: one of evolution’s best ideas. Cell. Mol. Life Sci. 74 (21): 3863-3881. DOI: 10.1007/s00018-017-2609-7.
233. Van der Bliek AM, Sedensky MM, Morgan PG (2017) Cell biology of the mitochondrion. Genetics 207 (3): 843-871. DOI: 10.1534/genetics.117.300262.
234. Tan DX, Reiter RJ (2019) Mitochondria: the birth place, battle ground and the site of melatonin metabolism in cells. Melatonin Res. 2 (1): 44-66. DOI:https://doi.org/https://doi.org/10.32794/mr11250011.
235. Karbownik M, Reiter RJ (2000) Antioxidative effects of melatonin in protection against cellular damage caused by ionizing radiation. Proc. Soc. Exp. Biol. Med. 225 (1): 9-22. DOI: 10.1046/j.1525-1373.2000.22502.x.
236. Melchiorri D, Reiter RJ, Attia AM, Hara M, Burgos A, Nistico G (1994) Potent protective effect of melatonin on in vivo paraquat-induced oxidative damage in rats. Life Sci. 56 (2): 83-89. DOI: 10.1016/0024-3205(94)00417-q.
237. Tan DX, Reiter RJ, Chen LD, Poeggeler B, Manchester LC, Barlow-Walden LR (1994) Both physiological and pharmacological levels of melatonin reduce DNA adduct formation induced by the carcinogen safrole. Carcinogenesis 15 (2): 215-218. DOI: 10.1093/carcin/15.2.215.
238. Tan DX, Manchester LC, Burkhardt S, Sainz RM, Mayo JC, Kohen R, Shohami E, Huo YS, Hardeland R, Reiter RJ (2001) N 1-acetyl-N 2-formyl-5-methoxykynuramine, a biogenic amine and melatonin metabolite, functions as a potent antioxidant. FASEB J. 15 (12): 2294-2296. DOI: 10.1096/fj.01-0309fje.
239. León J, Escames G, Rodríguez MI, López LC, Tapias V, Entrena A, Camacho E, Carrión MD, Gallo MA, Espinosa A, Tan DX, Reiter RJ, Acuña-Castroviejo D (2006) Inhibition of neuronal nitric oxide synthase activity by N1-acetyl-5-methoxykynuramine, a brain metabolite of melatonin. J. Neurochem. 98 (6): 2023-2033. DOI: 10.1111/j.1471-4159.2006.04029.x.
240. Rosen J, Than NN, Koch D, Poeggeler B, Laatsch H, Hardeland R (2006) Interactions of melatoninand its metabolites with the ABTS cation radical: extension of the radical scavenger cascade and formation of a novel class of oxidation products, C2‐substituted 3-indolinones. J. Pineal Res. 41 (4): 374-381. DOI: 10.1111/j.1600-079X.2006.00379.x.
241. Manda K, Ueno M, Anzai K (2007) AFMK, a melatonin metabolite, attenuates X-ray-induced oxidative damage to DNA, proteins and lipids in mice. J. Pineal Res. 42 (4): 386-393. DOI: 10.1111/j.1600-079X.2007.00432.x.
242. Galano A, Tan DX, Reiter RJ (2013) On the free radical scavenging activities of melatonin's metabolites, AFMK and AMK. J. Pineal Res. 54 (3): 245-257. DOI: 10.1111/jpi.12010.
243. Blanchard-Fillion B, Servy C, Ducrocq C (2001) 1-Nitrosomelatonin is a spontaneous NO-releasing compound. Free Radic. Res. 35 (6): 857-866. DOI: 10.1080/ 10715760100301351.
244. Pandi-Perumal SR, Srinivasan V, Maestroni GJ, Cardinali DP, Poeggeler B, Hardeland R (2006) Melatonin: nature's most versatile biological signal? FEBS J. 273 (13): 2813-2838. DOI: 10.1111/j.1742-4658.2006.05322.x.
245. Antolín I, Rodríguez C, Saínz RM, Mayo JC, Uría H, Kotler ML, Rodríguez-Colunga MJ, Tolivia D, Menéndez-Peláez A (1996) Neurohormone melatonin prevents cell damage: effect on gene expression for antioxidant enzymes. FASEB J. 10 (8): 882-890. DOI: 10.1096/fasebj.10.8.8666165.
246. Mayo JC, Sainz RM, Antoli I, Herrera F, Martin V, Rodriguez C (2002) Melatonin regulation of antioxidant enzyme gene expression. Cell Mol. Life Sci. 59 (10): 1706-1713. DOI: 10.1007/pl00012498.
247. Rodriguez C, Mayo JC, Sainz RM, Antolín I, Herrera F, Martín V, Reiter RJ (2004) Regulation of antioxidant enzymes: a significant role for melatonin. J. Pineal Res. 36 (1): 1-9. DOI: 10.1046/j.1600-079x.2003.00092.x.
248. Konturek SJ, Konturek PC, Brzozowska I, Pawlik M, Sliwowski Z, Cześnikiewicz-Guzik M, Kwiecień S, Brzozowski T, Bubenik GA, Pawlik WW (2007) Localization and biological activities of melatonin. J. Physiol. Pharmacol. 58 (3): 381-405.
249. Hevia D, González-Menéndez P, Quiros-González I, Miar A, Rodríguez-García A, Tan DX, Reiter RJ, Mayo JC, Sainz RM (2015) Melatonin uptake through glucose transporters: a new target for melatonin inhibition of cancer. J. Pineal Res. 58 (2): 234-250. DOI: 10.1111/jpi.12210.
250. Kaya H, Delibas N, Serteser M, Ulukaya E, Özkaya O (1999) The effect of melatonin on lipid peroxidation during radiotherapy in female rats. Strahlenther Onkol. 175 (6): 285-288. DOI: 10.1007/bf02743581.
251. Nordlund JJ, Lerner AB (1977) The effects of oral melatonin on skin color and on the release of pituitary hormones. J. Clinic. Endocrinol. Metab. 45 (4): 768-774. DOI: 10.1210/jcem-45-4-768.
252. Galano A, Medina ME, Tan DX, Reiter RJ (2015) Melatonin and its metabolites as copper chelating agents and their role in inhibiting oxidative stress: a physicochemical analysis. J. Pineal Res. 58 (1): 107-116. DOI: 10.1111/jpi.12196.
253. Stankov B, Reiter RJ (1990) Melatonin receptors: current status, facts, and hypotheses. Life Sci. 46 (14): 971-982.DOI: 10.1016/0024-3205(90)90020-r.
254. Dubocovich ML, Delagrange P, Krause DN, Sugden D, Cardinali DP, Olcese J (2010) International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol. Rev. 62 (3): 343-380. DOI: 10.1124/pr.110.002832.
255. Urata Y, Honma S, Goto S, Todoroki S, Iida T, Cho S, Honma K, Kondo T (1999) Melatonin induces γ-glutamylcysteine synthetase mediated by activator protein-1 in human vascular endothelial cells. Free Radic. Biol. Med. 27 (7-8): 838-847. DOI: 10.1016/s0891-5849(99)00131-8.
256. Ghosh AK, Naaz S, Bhattacharjee B, Ghosal N, Chattopadhyay A, Roy S, Reiter RJ, Bandyopadhyay D (2017) Mechanism of melatonin protection against copper-ascorbate-induced oxidative damage in vitro through isothermal titration calorimetry. Life Sci. 180: 123-136. DOI: 10.1016/j.lfs.2017.05.022.
257. Tan DX, Manchester LC, Reiter RJ, Qi W, Kim SJ, El-Sokkary GH (1998) Ischemia/reperfusion-induced arrhythmias in the isolated rat heart: prevention by melatonin. J. Pineal Res. 25 (3): 184-191. DOI: 10.1111/j.1600-079X. 1998.tb00558.x.
258. Mukherjee D, Roy SG, Bandyopadhyay A, Chattopadhyay A, Basu A, Mitra E, Ghosh AK, Reiter RJ, Bandyopadhyay D (2010) Melatonin protects against isoproterenol-induced myocardial injury in the rat: antioxidative mechanisms. J. Pineal Res. 48 (3): 251-262. DOI: 10.1111/j.1600-079X.2010.00749.x.
259. Li XJ, Zhang LM, Gu J, Zhang AZ, Sun FY (1997) Melatonin decreases production of hydroxyl radical during cerebral ischemia–reperfusion. Acta Pharmacol. Sin. 18 (5): 394-396.
260. Lochner A, Marais E, Huisamen B (2018) Melatonin and cardioprotection against ischaemia/reperfusion injury: What's new? A review. J. Pineal Res. 65 (1): e12490.DOI: 10.1111/jpi.12490.
261. Lagneux C, Joyeux M, Demenge P, Ribuot C, Godin-Ribuot D (2000) Protective effects of melatonin against ischemia-reperfusion injury in the isolated rat heart. Life Sci. 66 (6): 503–509.DOI: 10.1016/s0024-3205(99)00620-7.
262. Konturek PC, Konturek SJ, Majka J Zembala M, Hahn EG (1997) Melatonin affords protection against gastric lesions induced by ischemia–reperfusion possibly due to its antioxidant and mucosal microcirculatory effects. Eur. J. Pharmacol. 322 (1): 73–77. DOI: 10.1016/s0014-2999(97)00051-4.
263. Brzozowski T, Konturek PC, Konturek SJ, Pajdo R, Bielanski W, Brzozowska I, Stachura J, Hahn EG (1997) The role of melatonin and L-tryptophan in prevention of acute gastric lesions induced by stress, ethanol, ischemia, and aspirin. J. Pineal Res. 23 (2): 79-89. DOI: 10.1111/j.1600-079x.1997.tb00339.x.
264. Mukherjee D, Ghosh AK, Bandyopadhyay A, Basu A, Datta S, Pattari SK, Reiter RJ, Bandyopadhyay D (2012) Melatonin protects against isoproterenol-induced alterations in cardiac mitochondrial energy-metabolizing enzymes, apoptotic proteins, and assists in complete recovery from myocardial injury in rats. J. Pineal Res. 53 (2): 166-179. DOI: 10.1111/j.1600-079X.2012.00984.x.
265. Ghosh A, Bose G, Dey T, Pal PK, Mishra S, Ghosh AK, Chattopadhyay A, Bandyopadhyay D (2019) Melatonin protects against cardiac damage induced by a combination of high fat diet and isoproterenol exacerbated oxidative stress in male Wistar rats. Melatonin Res. 2 (1): 9-31. DOI: 10.32794/mr11250009.
266. Karamitri A, Jockers R (2019) Melatonin in type 2 diabetes mellitus and obesity. Nat. Rev. Endocrinol. 15 (2): 105-125. DOI: 10.1038/s41574-018-0130-1.
267. Dantas Ferreira RF, Raingard H, Dumont S, Schuster Klein C, Guardiola Lemaitre B, Pevet P, Challet E (2018) Melatonin potentiates the effects of metformin on glucose metabolism and food intake in high fat fed rats. Endocrinol. Diabetes Metab. 1 (4): e00039. DOI: org/10.1002/edm2.39.
268. Zhang M, Lin J, Wang, Cheng Z, Hu J, Wang T, Man W, Yin T, Guo W, Gao E, Reiter RJ, Wang H, Sun D (2017) Melatonin protects against diabetic cardiomyopathy through Mst1/Sirt3 signaling. J. Pineal Res. 63 (2): DOI:10.1111/jpi.12418.
269. Pal PK, Bhattacharjee B, Chattopadhyay A, Bandyopadhyay D (2019) Melatonin as an armament against non-steroidal anti-inflammatory drug (NSAID) induced gastric injury: An overview. Melatonin Res. 2(1): 115-137. DOI: 10.32794/mr11250015.
270. Konturek PC, Konturek SJ, Celinski K, Slomka M, Cichoz-Lach H, Bielanski W, Reiter RJ (2010) Role of melatonin in mucosal gastroprotection against aspirin-induced gastric lesions in humans. J. Pineal Res. 48: 318–323. DOI: 10.1111/j.1600-079x.2010.00755.x.
271. Pal PK, Bhattacharjee B, Ghosh A, Chattopadhyay A, Bandyopadhyay D (2018) Adrenaline induced disruption of endogenous melatonergic system, antioxidant and inflammatory responses in the gastrointestinal tissues of male Wistar rat: an in vitro study. Melatonin Res. 1: 109–131.DOI: 10.32794/mr11250007.
272. Konturek PC, Konturek SJ, Burnat G, Brzozowski T, Brzozowska I, Reiter RJ (2008) Dynamic physiological and molecular changes in gastric ulcer healing achieved by melatonin and its precursor L-tryptophan in rats. J. Pineal Res. 45: 180–190. DOI: 10.1111/j.1600-079X.2008.00574.x.
273. Majumder R, Datta M, Pal PK, Bhattacharjee B, Chattopadhyay A, Bandyopadhyay D (2019) Protective mechanisms of melatonin on caprine spleen injury induced by cadmium (Cd): an in vitro study. Melatonin Res. 2 (3): 57-75. DOI: 10.32794/mr11250031.
274. Pappolla MA, Sos M, Omar RA, Bick RJ, Hickson-Bick DL, Reiter RJ, Efthimiopoulos S, Robakis NK (1997) Melatonin prevents death of neuroblastoma cells exposed to the Alzheimer amyloid peptide. J. Neurosci. 17 (5): 1683-1690.
275. Daniels WM, van Rensburg SJ, van Zyl JM, Taljaard JJ (1998) Melatonin prevents beta-amyloid-induced lipid peroxidation. J. Pineal Res. 24 (2): 78-82.DOI: 10.1111/j.1600-079x.1998.tb00370.x.
276. Dong W, Huang F, Fan W, Cheng S, Chen Y, Zhang W, Shi H, He H (2010) Differential effects of melatonin on amyloid-beta peptide 25-35-induced mitochondrial dysfunction in hippocampal neurons at different stages of culture J. Pineal Res. 48 (2): 117-125. DOI: 10.1111/j.1600-079X.2009.00734.x.
277. Pappolla MA, Chyan YJ, Poeggeler B, Bozner P, Ghiso J, LeDoux SP, Wilson GL (1999) Alzheimer beta protein mediated oxidative damage of mitochondrial DNA: prevention by melatonin. J. Pineal Res. 27 (4): 226-229. DOI: 10.1111/j.1600 079x.1999.tb00619.x.
278. Reiter RJ, Tan DX, Kim SJ, Manchester LC, Qi W, Garcia JJ, Cabrera JC, El-Sokkary G, Rouvier-Garay V (1999) Augmentation of Indices of Oxidative Damage in Life-long Melatonin-deficient Rats. Mech. Ageing Dev. 110 (3): 157–173. DOI: 10.1016/s0047-6374(99)00058-5.
279. Pablos MI, Agapito MT, Gutierrez R, Recio JM, Reiter RJ, Barlow-Walden L, Acuña-Castroviejo D, Menendez-Pelaez A (1995) Melatonin stimulates the activity of the detoxifying enzyme glutathione peroxidase in several tissues of chicks. J. Pineal Res. 19 (3): 111-115. DOI: 10.1111/j.1600-079x.1995.tb00178.x.
280. Rosengarten H, Meller E, Friedhoff AJ (1972) In vitro enzymatic formation of melatonin by human erythrocytes. Res. Comm. Chem. Pathol. Pharmacol. 4 (2): 457- 465.
281. Tesoriere L, D'Arpa D, Conti S, Giaccone V, Pintaudi AM, Livrea MA (1999) Melatonin protects human red blood cells from oxidative hemolysis: new insights into the radical-scavenging activity. J. Pineal Res. 27 (2): 95-105. DOI: 10.1111/j.1600-079x.1999.tb00602.x.
282. Tesoriere L, Allegra M, D'Arpa D, Butera D, Livrea MA (2001) Reaction of melatonin with hemoglobin-derived oxoferryl radicals and inhibition of the hydroperoxide-induced hemoglobin denaturation in red blood cells. J. Pineal Res. 31 (2): 114-119. DOI: 10.1034/j.1600-079x.2001.310204.x.
283. Allegra M, Gentile C, Tesoriere L, Livrea MA (2002) Protective effect of melatonin against cytotoxic actions of malondialdehyde: an in vitro study on human erythrocytes. J. Pineal Res. 32 (3): 187-93. DOI: 10.1034/j.1600-079x.2002.1o852.x.
284. Paul S, Naaz S, Ghosh A, Mishra S, Chattopadhyay A and Bandyopadhyay D (2018) Melatonin chelates iron and binds directly with phenylhydrazine to provide protection against phenylhydrazine induced oxidative damage in red blood cells along with its antioxidant mechanisms: an in vitro study. Melatonin Res. 1 (1): 11-20. DOI: https: //doi.org/https://doi.org/10.32794/mr11250001.
285. Czuczejko J, Sielski Ł, Woźniak B, Woźniak A, Szewczyk-Golec K (2019) Melatonin supplementation improves oxidative and inflammatory state in the blood of professional athletes during the preparatory period for competitions. Free Radic. Res. 53 (2): 198-209. DOI: 10.1080/10715762.2018.1563688.
286. Ochoa JJ, Díaz-Castro J, Kajarabille N, García C, Guisado IM, De TC, Guisado R (2011) Melatonin supplementation ameliorates oxidative stress and inflammatory signaling induced by strenuous exercise in adult human males. J. Pineal Res. 51 (4): 373-80.DOI: 10.1111/j.1600-079X.2011.00899.x.
287. Ozcelik I (2014) Beneficial Effects of Melatonin on Oxidative Damage Observed During Whole Blood Storage. Int. J. Haematol. Oncol. 2 (24): 89-96. DOI: 10.4999/uhod.13089.
288.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


