Rice N-acetylserotonin deacetylase regulates melatonin levels in transgenic rice
Functional role of N-acetylserotonin deacetylase
Abstract
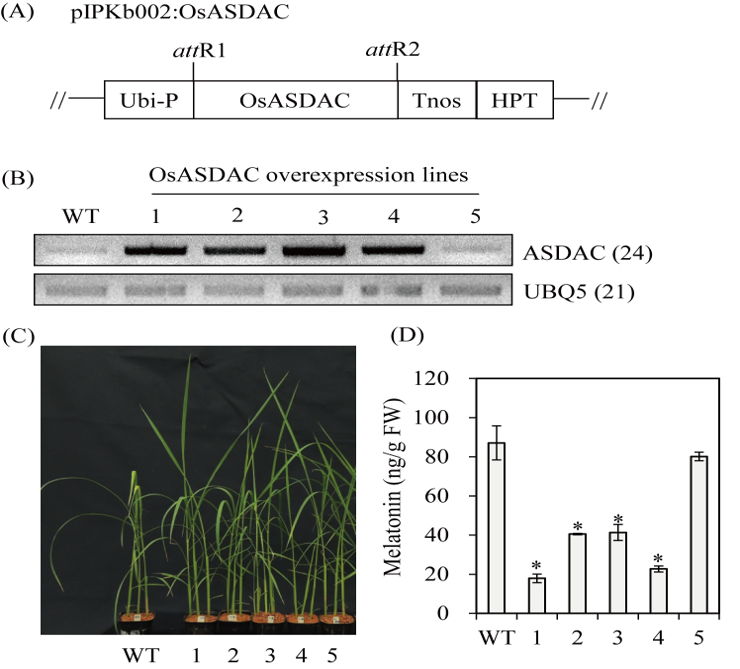
A reverse melatonin biosynthetic pathway was recently discovered in plants, by which N-acetylserotonin (NAS) is converted into serotonin by N-acetylserotonin deacetylase (ASDAC) rather than into melatonin by N-acetylserotonin O-methyltransferase (ASMT). In this study, we generated transgenic rice plants in which ASDAC was either suppressed or overexpressed to determine whether ASDAC is functionally involved in melatonin biosynthesis. ASDAC-suppressed rice showed increased levels of NAS, 5-methoxytryptamine (5-MT), and melatonin, whereas ASDAC-overexpressed rice exhibited less melatonin synthesis than observed in the wild type. This finding is strong evidence of the role of ASDAC in melatonin biosynthesis in rice. The increased levels of 5-MT, which is produced either by ASDAC from melatonin or by serotonin O-methyltransferase (SOMT) from serotonin in ASDAC-suppressed rice, was ascribed to enhanced SOMT enzyme activity rather than increased transcripts, such as ASMT or caffeic acid O-methyltransferase (COMT) encoding SOMT activity.
References
2. Arnao MB, Hernández-Ruiz J (2019) Melatonin: a new plant hormone and/or a plant master regulator. Trends Plant Sci. 24: 38-48.
3. Reina M, Castañeda-Arriaga R, Perez-Gonzalez A, Guzman-Lopez EG, Tan DX, Reiter RJ, Galano A (2018) A computer-assisted systematic search for melatonin derivatives with high potential as antioxidants. Melatonin Res. 1: 27-58.
4. Zhao D, Yu Y, Shen Y, Liu Q, Zhao Z, Sharma R, Reiter RJ (2019) Melatonin synthesis and function: evolutionary history in animals and plants. Front. Endocrinol. 10: 249.
5. Arnao MB, Hernández-Ruiz J (2019) Is phytomelatonin a new plant hormone? Agronomy 10: 95.
6. Mitra E, Bhattacharjee B, Pal PK, Ghosh AK, Mishra S, Chattopadhyay A, Bandyopadhyay D (2019) Melatonin protects against cadmium-induced oxidative damage in different tissues of rat: a mechanistic insight. Melatonin Res. 2 (2): 1-21.
7. Lee HY, Back K (2018) Melatonin plays a pivotal role in conferring tolerance against endoplasmic reticulum stress via mitogen-activated protein kinases and bZIP60 in Arabidopsis thaliana. Melatonin Res. 1: 93-107.
8. Potes Y, de Luxan-Delgado B, Rubio-González A, Reiter RJ, Coto-Montes A (2019) Dose-dependent beneficial effect of melatonin on obesity; interaction of melatonin and leptin. Melatonin Res. 2 (1): 1-8.
9. Gu Q, Chen Z, Yu X, Cui W, Pan J, Zhao G, Xu S, Wang R, Shen W (2017) Melatonin confers plant tolerance against cadmium stress via the decrease of cadmium accumulation and reestablishment of microRNA-mediated redox homeostasis. Plant Sci. 261: 28-37.
10. Lee HY, Back K (2017) Cadmium disrupts subcellular organelles, including chloroplasts, resulting in melatonin induction in plants. Molecules 22: 1791.
11. Hardeland R (2019) Melatonin in the evolution of plants and other phototrophs. Melatonin Res. 2 (3): 10-36.
12. Yu Y, Lv Y, Shi Y, Li T, Chen Y, Zhao D, Zhao Z (2018) The role of phyto-melatonin and related metabolites in response to stress. Molecules 23: 1887.
13. Liang C, Li A, Yu H, Li W, Liang C, Guo S, Zhang R, Chu C (2017) Melatonin regulates root architecture by modulating auxin response in rice. Front. Plant Sci. 8: 134.
14. Hwang OJ, Back K (2018) Melatonin is involved in skotomorphogenesis by regulating brassinosteroid biosynthesis in plants. J. Pineal Res. 65: e12495.
15. Yang J, Zhang C, Wang Z, Sun S, Zhan R, Zhao Y, Ma B, Ma F, Li M (2019) Melatonin-mediated sugar accumulation and growth inhibition in apple plants involves down-regulation of fructokinase 2 expression and activity. Front. Plant Sci. 10: 150.
16. Xu L, Yue Q, Bian F, Sun H, Zhai H, Yao Y (2017) Melatonin enhances phenolic accumulation partially via ethylene signaling and resulted in high antioxidant capacity in grape berries. Front. Plant Sci. 8: 1426.
17. Hong Y, Zhang Y, Sinumporn S, Yu N, Zhan X, Shen X, Chen D, Yu P, Wu W, Liu Q, Cao Z, Zhao C, Cheng S, Cao L (2018) Premature leaf senescence 3, encoding a methyltransferase, is required for melatonin biosynthesis in rice. Plant J. 95: 877-891.
18. Arnao MB, Hernández-Ruiz J (2018) Melatonin and its relationship to pant hormones. Ann. Bot. 121: 195-207.
19. Liu DD, Sun XS, Liu L, Shi HD, Chen SY, Zhao DK (2019) Overexpression of the melatonin synthesis-related gene SlCOMT1 improves the resistance of tomato to salt stress. Molecules 24: 1514.
20. Zhang Q, Liu X, Zhang Z, Liu N, Li D, Hu L (2019) Melatonin improved waterlogging tolerance in alfalfa (Medicago sativa) by reprogramming polyamine and ethylene metabolism. Front. Plant Sci. 10: 44.
21. Ahammed GJ, Xu W, Liu A, Chen S (2019) Endogenous melatonin deficiency aggravates high temperature-induced oxidative stress in Solanum lycopersicum L. Environ. Exp. Bot. 161: 303-311.
22. Wang Y, Reiter RJ, Chan Z (2018) Phytomelatonin: a universal abiotic stress regulator. J. Exp. Bot. 69: 963-974.
23. Arnao MB, Hernández-Ruiz J (2019) Role of melatonin to enhance phytoremediation capacity. Appl. Sci. 9: 5293.
24. Moustafa-Farag M, Almoneafy A, Mahmoud A, Elkelish A, Arnao MB, Li L, Ai S. (2020) Melatonin and its protective role against biotic stress impacts on plants. Biomolecules 10: 54.
25. Back K, Tan D-X, Reiter RJ (2016) Melatonin biosynthesis in plants: Multiple pathways catalyze tryptophan to melatonin in the cytoplasm or chloroplasts. J. Pineal Res. 61: 426-437.
26. Byeon Y, Back K (2015) Molecular cloning of melatonin 2-hydroxylase responsible for 2-hydroxymelatonin production in rice (Oryza sativa). J. Pineal Res. 58: 343-351.
27. Lee K, Zawadzka A, Czarnocki Z, Reiter RJ, Back K (2016) Molecular cloning of melatonin 3-hydroxylase and its production of cyclic 3-hydroxymelatonin in rice (Oryza sativa). J. Pineal Res. 61: 470-478.
28. Tan D-X, Manchester LC, Mascio P, Martinez GR, Prado FM, Reiter RJ (2007) Novel rhythms of N1-acetyl-N2-formyl-5-methoxykynuramine and its precursor melatonin in water hyacinth: importance for phytoremediation. FASEB J. 21: 1724–1729.
29. Lee HJ, Back K (2019) 2-Hydroxymelatonin confers tolerance against combined cold and drought stress in tobacco, tomato, and cucumber as a potent anti-stress compound in the evolution of land plants. Melatonin Res. 2 (2): 35-46.
30. Choi GH, Back K (2019) Cyclic 3-hydroxymelatonin exhibits diurnal rhythm and cyclic 3-hydroxymelatonin overproduction increases secondary tillers in rice by upregulating MOC1 expression. Melatonin Res. 2 (3):120-138.
31. Lee K, Lee HY, Back K (2018) Rice histone deacetylase 10 and Arabidopsis histone deacetylase 14 genes encode N-acetylserotonin deacetylase, which catalyzes conversion of N-acetylserotonin into serotonin, a reverse reaction for melatonin biosynthesis in plants. J Pineal Res. 64: e12460.
32. Kikuchi S, Satoh K, Nagata T, Kawagashira N, Doi K, Kishimoto N, Yazaki J, Ishikawa M, Yamada H, Ooka H, et al. (2003) Collection, mapping, and annotation of over 28,000 cDNA clones from japonica rice. Science 301: 376-379.
33. Wang Z, Chen C, Xu Y, Jiang R, Han Y, Xu Z, Chong K (2004) A practical vector for efficient knockdown of gene expression in rice (Oryza sativa L.). Plant Mol. Biol. Rep. 22: 409-417.
34. Himmelbach A, Zierold U, Hensel G, Riechen J, Douchkov D, Schweizer P, Kumlehn J (2007) A set of modular binary vectors for transformation of cereals. Plant Physiol. 145: 1192-1200.
35. Lee HJ, Lee SB, Chung JS, Han SU, Han O, Guh JO, Jeon JS, An G, Back K (2000) Transgenic rice plants expressing a Bacillus subtilis protoporphyrinogen oxidase gene are resistant to diphenyl ether herbicide oxyfluorfen. Plant Cell Physiol. 41: 743–749.
36. Byeon Y, Lee HY, Lee K, Back K (2014) Caffeic acid O-methyltransferase is involved in the synthesis of melatonin by methylating N-acetylserotonin in Arabidopsis. J. Pineal Res. 57: 219-227.
37. Tan D-X, Reiter RJ (2019) Mitochondria: the birth place, battle ground and site of melatonin metabolism in cells. Melatonin Res. 2 (1): 44-66.
38. Byeon Y, Lee HJ, Lee HY, Back K (2016) Cloning and functional characterization of the Arabidopsis N-acetylserotonin O-methyltransferase responsible for melatonin synthesis. J. Pineal Res. 60: 65-73.
39. Quintela T, Goncalves I, Silva M, et al. (2018) Choroid plexus is an additional source of melatonin in the brain. J. Pineal Res. 65: e12528.
40. Wei J, Li DX, Zhang JR, Shan C, Rengel Z, Song ZB, Chen Q (2018) Phytomelatonin receptor PMTR1-mediated signaling regulates stomatal closure in Arabidopsis thaliana. J. Pineal Res. 65: e12500.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


