The phytomelatonin receptor (PMRT1) Arabidopsis Cand2 is not a bona fide G protein–coupled melatonin receptor
Functional role of Cand2
Abstract
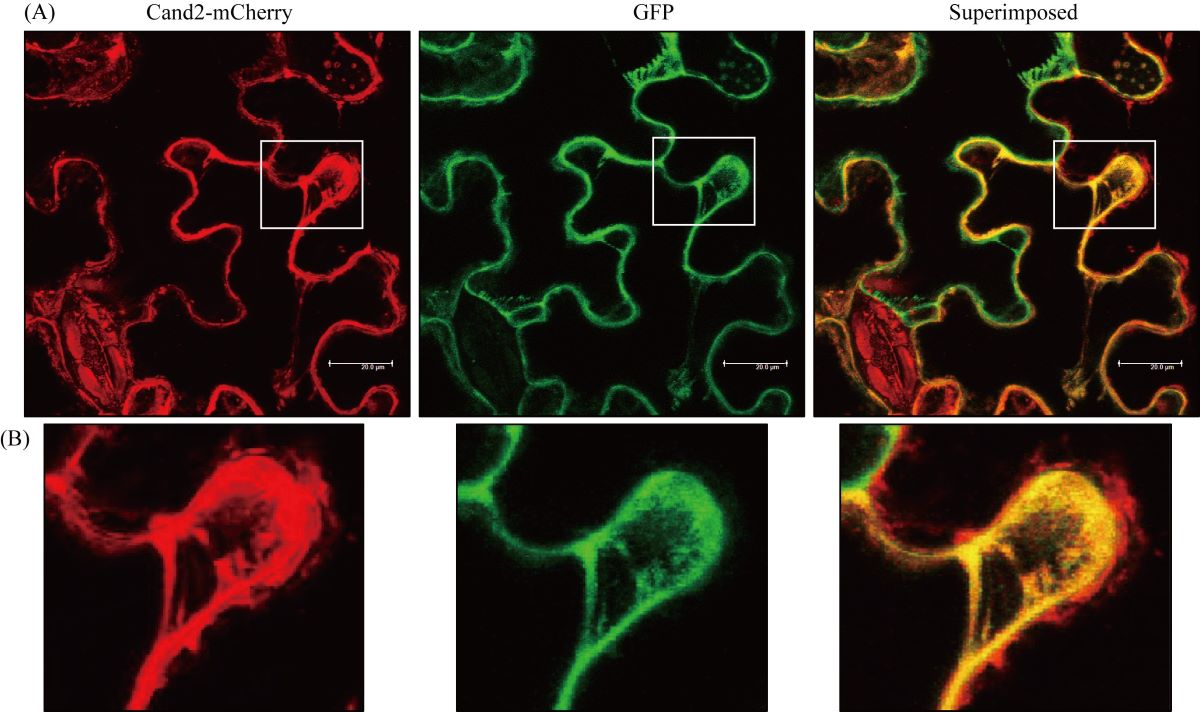
It was recently suggested that the protein Cand2 acts as a G protein–coupled receptor (GPCR) for melatonin in Arabidopsis by mediating stomatal closure via H2O2 production and Ca2+ influx. Here, we examined whether Cand2 is indeed a melatonin receptor. Contrary to previous reports, confocal microscopy analyses indicated that Cand2 protein is localized in the cytoplasm rather than the plasma membrane. The role of Cand2 was further investigated in genetic analyses using two Arabidopsis cand2 knockout mutant lines, SALK_071302 (cand2-1) and SALK_068848 (cand2-2). We found that melatonin-mediated mitogen-activated protein kinase (MAPK) activation was not abolished in the cand2 mutant lines, nor did melatonin-mediated defense gene induction (e.g., GST1) change relative to that in the wild type Col-0. Following ER stress, the two cand2 mutant lines were identical to Col-0 in terms of defense gene induction, ion leakage, and ROS levels. Two G protein mutants, gpa1 (Gα mutant) and agb1 (Gβ mutant), also exhibited no disturbance in melatonin-mediated defense gene induction or melatonin-mediated MAPK activation. Collectively, our data indicate that Cand2 is neither a phytomelatonin receptor localized in the plasma membrane nor is it involved in the melatonin-mediated defense signaling pathway via G protein components. However, it remains unclear how melatonin-mediated MAPK activation was slightly decreased in the mutant cand2-2 without affecting downstream defense gene induction. Also, it cannot rule out the possibility that Cand2 may be a melatonin binding protein and that its binding may result in a decrease of free melatonin level in plants.
References
2. Tan D-X, Reiter RJ (2019) Mitochondria: the birth place, battle ground and site of melatonin metabolism in cells. Melatonin Res. 2 (1): 44-66.
3. de Mendoza A, Sebé-Pedrós A, Ruiz-Trillo I (2014). The evolution of the DPCR signaling system in eukaryotes: modularity, conservation, and the transition to metazoan multicellularity. Genome Biol. Evol. 6: 606-619.
4. Urano D, Chen JG, Botella JR, Jones AM (2013) Heterotrimeric G protein signaling in the plant kingdom. Open Biol. 3: 120186.
5. Urano D, Jones AM (2013) “Round up the usual suspects”: a comment on nonexistent plant G protein-coupled receptors. Plant Physiol. 161: 1097-1102.
6. Wei J, Li DX, Zhang JR, Shan C, Rengel Z, Song ZB, Chen Q (2018) Phytomelatonin receptor PMTR1-mediated signaling regulates stomatal closure in Arabidopsis thaliana. J. Pineal Res. 65: e12500.
7. Lee HY, Back K. (2016) Mitogen-activated protein kinase pathways are required for melatonin-mediated defense responses in plants. J. Pineal Res. 60: 327-335.
8. Lee HY, Back K (2018) Melatonin plays a pivotal role in conferring tolerance against endoplasmic reticulum stress via mitogen-activated protein kinases and bZIP60 in Arabidopsis thaliana. Melatonin Res. 1: 93-107.
9. Chinchilla,D, Bauer Z, Regenass M, Boller T, Felixa G. (2006) The Arabidopsis receptor kinase FLS2 binds flg22 and determines the specificity of flagellin perception. Plant Cell 18: 465-476.
10. Arnao MB, Hernández-Ruiz J (2019) Melatonin and reactive oxygen and nitrogen species: a model for the plant redox network. Melatonin Res. 2 (3): 152-168.
11. Hardeland R (2019) Melatonin in the evolution of plants and other phototrophs. Melatonin Res. 2 (3): 10-36.
12. Pal PK, Bhattacharjee B, Chattopadhyay A, Bandyopadhyay D. (2019) Pleiotropic roles of melatonin against oxidative stress mediated tissue injury in the gastrointestinal tract: An overview. Melatonin Res. 2 (2): 158-184.
13. Arnao MB, Hernández-Ruiz J (2019b) Melatonin as a chemical substance or as phytomelatonin rich-extracts for use as plant protector and/or biostimulant in accordance with EC legislation. Agronomy 9, 570.
14. Choi GH, Back K (2019) Cyclic 3-hydroxymelatonin exhibits diurnal rhythm and cyclic 3-hydroxymelatonin overproduction increases secondary tillers in rice by upregulating MOC1 expression. Melatonin Res. 2 (3): 120-138.
15. Yu Y, Lv Y, Shi Y, Li T, Chen Y, Zhao D, Zhao Z (2018) The role of phyto-melatonin and related metabolites in response to stress. Molecules 23: 1887.
16. Lee K, Hwang OJ, Back K. (2020) Rice N-acetylserotonin deacetylase regulates melatonin levels in transgenic rice. Melatonin Res. 3 (1): 32-42.
17. Galano A, Reiter RJ. (2018) Melatonin and its metabolites vs oxidative stress: From individual actions to collective protection. J. Pineal Res. 65: e12514.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


