Protection by melatonin in respiratory diseases: valuable information for the treatment of COVID-19
Melatonin in respiratory diseases
Abstract
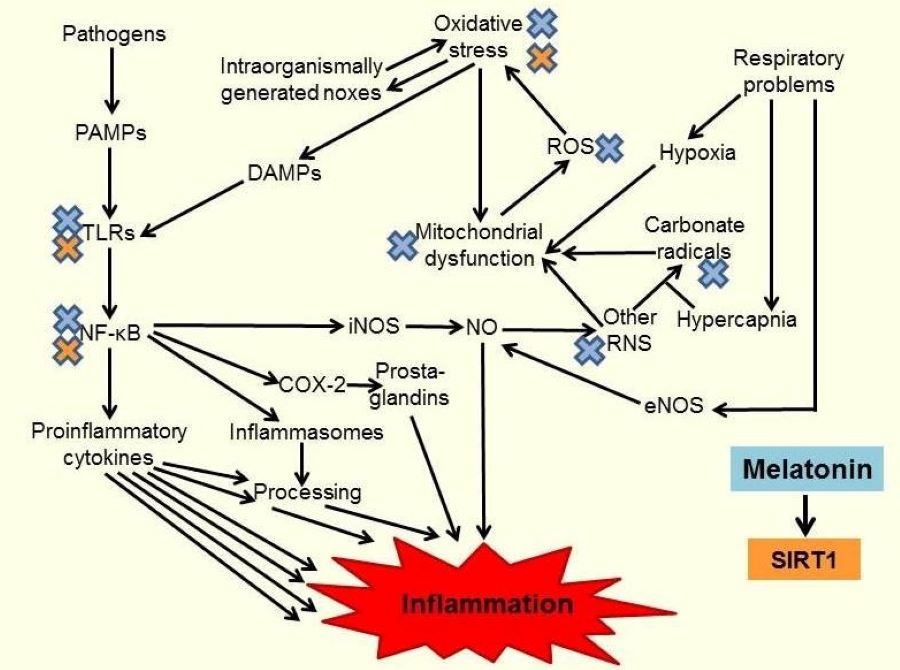
High mortality rates in severe progression of COVID-19 are predominantly caused by pulmonary failure due to high-grade airway inflammation. As investigations on the efficacy of melatonin in this disease are still in their beginning, it may be worth-while to recall the body of evidence on protective effects in other respiratory dysfunctions, which have been studied pre-clinically and clinically. In various diseases and corresponding animal models, melatonin has been shown to be protective, mainly because of its anti-inflammatory and antioxidant properties. This was documented in pathologies as different as allergic airway inflammation, toxicologically or radiation-induced acute lung injury, respiratory disorders such as COPD, obstructive sleep apnea, neonatal respiratory distress syndrome, bronchopulmonary dysplasia and asphyxia, impaired respiration in sepsis, idiopathic pulmonary fibrosis, and pulmonary hypertension. The prevailing outcome has been protection or amelioration by melatonin, in conjunction with reduced expression and release of proinflammatory cytokines, such as IL-1β, IL-2, IL-6, IL-8, and TNFα, which was often explained by interference with toll-like receptors, inhibition of NLRP3 inflammasome activation and suppression of NF-κB signaling. In several studies, these beneficial effects were partially related to the upregulation of sirtuin-1 (SIRT1) by melatonin. The body of knowledge on melatonin’s efficacy in respiratory diseases is encouraging for the use of this powerful agent in COVID-19.
References
2. Zhang R, et al. (2020) COVID-19: melatonin as a potential adjuvant treatment. Life Sci. [Epub ahead of print, Mar 23]:117583. doi: 10.1016/j.lfs.2020.117583.
3. Hardeland R. (2018) Melatonin and inflammation — Story of a double-edged blade. J. Pineal Res. 65: e12525.
4. Hardeland R. (2019) Aging, melatonin and the pro- and anti-inflammatory networks. Int. J. Mol. Sci. 20: 1223.
5. Wu HM, et al. (2019) Melatonin biosynthesis restored by CpG oligodeoxynucleotides attenuates allergic airway inflammation via regulating NLRP3 inflammasome. Life Sci. 239: 117067.
6. Wu HM, Zhao CC, Xie QM, Xu J, Fei GH. (2020) TLR2-melatonin feedback loop regulates the activation of NLRP3 inflammasome in murine allergic airway inflammation. Front. Immunol. 11: 172.
7. Chen CF, Wang D, Reiter RJ, Yeh DY. (2011) Oral melatonin attenuates lung inflammation and airway hyperreactivity induced by inhalation of aerosolized pancreatic fluid in rats. J. Pineal Res. 50: 46–53.
8. Kang J, et al. (2018) Exposure to a combination of formaldehyde and DINP aggravated asthma-like pathology through oxidative stress and NF-κB activation. Toxicology 404–405: 49–58.
9. Duan J, et al. (2018) Exposure to formaldehyde and diisononyl phthalate exacerbate neuroinflammation through NF-κB activation in a mouse asthma model. Ecotoxicol. Environ. Saf. 163: 356–364.
10. Peng Z, Zhang W, Qiao J, He B. (2018) Melatonin attenuates airway inflammation via SIRT1 dependent inhibition of NLRP3 inflammasome and IL-1β in rats with COPD. Int. Immunopharmacol. 62: 23–28.
11. Lee YD, et al. (2009) Melatonin attenuates lipopolysaccharide-induced acute lung inflammation in sleep-deprived mice. J. Pineal Res. 46: 53–57.
12. Ji Z, et al. (2018) Melatonin attenuates chronic cough mediated by oxidative stress via transient receptor potential melastatin-2 in guinea pigs exposed to particulate matter 2.5. Physiol. Res. 67: 293–305.
13. Taslidere E, Esrefoglu M, Elbe H, Cetin A, Ates B. (2014) Protective effects of melatonin and quercetin on experimental lung injury induced by carbon tetrachloride in rats. Exp. Lung Res. 40: 59–65.
14. Radovic M, et al. (2019) Melatonin treatment prevents carbon tetrachloride-induced acute lung injury in rats by mitigating tissue antioxidant capacity and inflammatory response. Bratisl. Lek. Listy 120: 527–531.
15. Han B, et al. (2019) Dietary melatonin attenuates chromium-induced lung injury via activating the Sirt1/Pgc-1α/Nrf2 pathway. Food Funct.10: 5555–5565.
16. Tang FR, Loke WK. (2012) Sulfur mustard and respiratory diseases. Crit. Rev. Toxicol. 42: 688–702.
17. Macit E, et al. (2913) The protective effect of melatonin and S-methylisothiourea treatments in nitrogen mustard induced lung toxicity in rats. Environ. Toxicol. Pharmacol. 36: 1283-1290.
18. Zhang L, He D, Shao Y, Xu D, Shen J. (2014) [Effect of melatonin on p38MAPKsignaling pathway in rats with phosgene-induced lung injury] (in Chinese). Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 32: 648–652.
19. Serin M, Gülbaş H, Gürses I, Erkal HS, Yücel N. (2007) The histopathological evaluation of the effectiveness of melatonin as a protectant against acute lung injury induced by radiation therapy in a rat model. Int. J. Radiat. Biol. 83:187–193.
20. Farhood B, et al. (2019) Mitigation of radiation-induced lung pneumonitis and fibrosis using metformin and melatonin: A histopathological study. Medicina (Kaunas) 55: E417.
21. Sun CK, et al. (2015) Systemic combined melatonin-mitochondria treatment improves acute respiratory distress syndrome in the rat. J. Pineal Res. 58:137–150.
22. Al-Rasheed NM, et al. (2017) Pulmonary prophylactic impact of melatonin and/or quercetin: A novel therapy for inflammatory hypoxic stress in rats. Acta Pharm. 67: 125–135.
23. Hung MW, et al. (2017) Melatonin attenuates pulmonary hypertension in chronically hypoxic rats. Int. J. Mol. Sci. 18: E1125.
24. Wang ML, et al. (2018) Melatonin attenuates lung ischaemia-reperfusion injury via inhibition of oxidative stress and inflammation. Interact. Cardiovasc. Thorac. Surg. 26: 761–767.
25. Huang SH, Cao XJ, Wei W. (2008) Melatonin decreases TLR3-mediated inflammatory factor expression via inhibition of NF-kappa B activation in respiratory syncytial virus-infected RAW264.7 macrophages. J. Pineal Res. 45: 93-100.
26. Rahman MA, et al. (2005) Serotonin and melatonin, neurohormones for homeostasis, as novel inhibitors of infections by the intracellular parasite chlamydia. J. Antimicrob. Chemother. 56: 861-868.
27. Hardeland R. (2017) Melatonin and the pathologies of weakened or dysregulated circadian oscillators. J. Pineal Res. 62: e12377.
28. Mayo JC, et al. (2017) Melatonin and sirtuins: A "not-so unexpected" relationship. J. Pineal Res. 62: e12391.
29. Hardeland R. (2018) Extended signaling by melatonin. Cell Cell. Life Sci. J. 3: 000123.
30. Hardeland R. (2018) Recent findings in melatonin research and their relevance to the CNS. Cent. Nerv. Syst. Agents Med. Chem. 18: 102–114.
31. He B, Zhang W, Qiao J, Peng Z, Chai X. (2019) Melatonin protects against COPD by attenuating apoptosis and endoplasmic reticulum stress via upregulating SIRT1 expression in rats. Can. J. Physiol. Pharmacol. 97: 386–391.
32. He B, Chen Q, Zhou D, Wang L, Liu Z. (2019) Role of reciprocal interaction between autophagy and endoplasmic reticulum stress in apoptosis of human bronchial epithelial cells induced by cigarette smoke extract. IUBMB Life 71: 66–80.
33. Kim GD, et al. (2012) Melatonin suppresses acrolein-induced IL-8 production in human pulmonary fibroblasts. J. Pineal Res. 52: 356–64.
34. Miłkowska-Dymanowska J, et al. (2017) Geroprotectors as a therapeutic strategy for COPD - where are we now? Clin. Interv. Aging 12: 1811–1817.
35. Shin IS, et al. (2014) Melatonin inhibits MUC5AC production via suppression of MAPK signaling in human airway epithelial cells. J. Pineal Res. 56: 398–407.
36. da Rosa DP, et al. (2015) Antioxidants inhibit the inflammatory and apoptotic processes in an intermittent hypoxia model of sleep apnea. Inflamm. Res. 64: 21–29.
37. Bertuglia S, Reiter RJ. (2009) Melatonin reduces microvascular damage and insulin resistance in hamsters due to chronic intermittent hypoxia. J. Pineal Res. 46: 307–313.
38. Bosco AD, et al. (2019) Melatonin effects on pulmonary tissue in the experimental model of Hepatopulmonary Syndrome. J. Bras. Pneumol. 45: e20170164.
39. Zhao X, et al. (2018) Melatonin protects against lung fibrosis by regulating the Hippo/YAP pathway. Int. J .Mol. Sci. 19: E1118.
40. Maarman GJ. (2017) Natural antioxidants as potential therapy, and a promising role for melatonin against pulmonary hypertension. Adv. Exp. Med. Biol. 967: 161–178.
41. Aguilar SA, et al. (2019) El tratamiento postnatal con melatonina modula la expresión de agentes prostanoides en pulmón de neonatos de oveja con hipertensión pulmonar. [in Spanish; shortened English title: Melatonin modulates the expression of pulmonary prostanoids]. Rev. Med. Chil. 147: 281–288.
42. Gonzalez-Candia A, et al. (2019) Antenatal melatonin modulates an enhanced antioxidant/pro-oxidant ratio in pulmonary hypertensive newborn sheep. Redox Biol. 22: 101128.
43. Gitto E, Pellegrino S, Gitto P, Barberi I, Reiter RJ. (2009) Oxidative stress of the newborn in the pre- and postnatal period and the clinical utility of melatonin. J. Pineal Res. 46: 128–139.
44. Aversa S, Pellegrino S, Barberi I, Reiter RJ, Gitto E. (2012) Potential utility of melatonin as an antioxidant during pregnancy and in the perinatal period. J. Matern. Fetal Neonatal. Med. 25: 207–221.
45. Srinivasan V, Mohamed M, Kato H. (2012) Melatonin in bacterial and viral infections with focus on sepsis: a review. Recent Pat. Endocr. Metab. Immune Drug Discov. 6: 30–39.
46. Chen YC, Tain YL, Sheen JM, Huang LT. (2012) Melatonin utility in neonates and children. J. Formos. Med, Assoc. 111: 57–66.
47. Poeggeler B. (2013) Melatonin replacement therapy in preterm infants: the impact of pharmacokinetics. Expert Rev. Clin. Pharmacol. 6: 367–368.
48. Gitto E, et al. (2013) Protective role of melatonin in neonatal diseases. Oxid. Med. Cell. Longev. 2013: 980374.
49. Poggi C, Dani C. (2014) Antioxidant strategies and respiratory disease of the preterm newborn: an update. Oxid. Med. Cell. Longev. 2014: 721043.
50. D'Angelo G, Marseglia L, Reiter RJ, Buonocore G, Gitto E. (2017) Melatonin and neonatal sepsis: a promising antioxidant adjuvant agent. Am. J. Perinatol. 34: 1382–1388.
51. Marseglia L, et al. (2019) Role of oxidative stress in neonatal respiratory distress syndrome. Free Radic. Biol. Med. 142: 132–137.
52. Fulia F, et al. (2001) Increased levels of malonaldehyde and nitrite/nitrate in the blood of asphyxiated newborns: reduction by melatonin. J. Pineal Res. 31: 343-349.
53. Gitto E, et al. (2004) Melatonin reduces oxidative stress in surgical neonates. J. Pediatr. Surg. 39:184-189.
54. Gitto E, et al. (2004) Oxidative and inflammatory parameters in respiratory distress syndrome of preterm newborns: beneficial effects of melatonin. Am. J. Perinatol. 21: 209–216.
55. Gitto E, et al. Early indicators of chronic lung disease in preterm infants with respiratory distress syndrome and their inhibition by melatonin. J. Pineal Res. 36: 250–255.
56. El-Gendy FM, El-Hawy MA, Hassan MG. (2018) Beneficial effect of melatonin in the treatment of neonatal sepsis. J. Matern. Fetal Neonatal. Med. 31: 2299–2303.
57. Henderson R, Kim S, Lee E. (2018) Use of melatonin as adjunctive therapy in neonatal sepsis: A systematic review and meta-analysis. Complement. Ther. Med. 39: 131–136.
58. Muñoz-Hoyos A, et al. (2007) Melatonin levels during the first week of life and their relation with the antioxidant response in the perinatal period. Neonatology 92: 209–216.
59. de Matos Cavalcante AG, et al. (2012) Melatonin reduces lung oxidative stress in patients with chronic obstructive pulmonary disease: a randomized, double-blind, placebo-controlled study. J. Pineal Res. 53: 238–244.
60. Barnaś M, Maskey-Warzęchowska M, Bielicki P, Kumor M, Chazan R. (2017) Diurnal and nocturnal serum melatonin concentrations after treatment with continuous positive airway pressure in patients with obstructive sleep apnea. Pol. Arch. Intern. Med. 127: 589–596.
61. Shilo L, et al. (2000) Effect of melatonin on sleep quality of COPD intensive care patients: a pilot study. Chronobiol. Int. 17: 71–76.
62. Hosseinzadeh A, et al. (2018) Idiopathic pulmonary fibrosis (IPF) signaling pathways and protective roles of melatonin. Life Sci. 201: 17–29.
63. Hosseinzadeh A, et al. (2018) Oxidative/nitrosative stress, autophagy and apoptosis as therapeutic targets of melatonin in idiopathic pulmonary fibrosis. Expert Opin. Ther. Targets 22: 1049–1061.
64. Cagnoni ML, Lombardi A, Cerinic MC, Dedola GL, Pignone A. (1995) Melatonin for treatment of chronic refractory sarcoidosis. Lancet 346: 1229-1230.
65. Pignone AM, et al. (2006) Melatonin is a safe and effective treatment for chronic pulmonary and extrapulmonary sarcoidosis. J. Pineal Res. 41: 95-100.
66. Hardeland R. (2009) New approaches in the management of insomnia: weighing the advantages of prolonged release melatonin and synthetic melatoninergic agonists. Neuropsychiatr. Dis. Treat. 5: 341–354.
67. Weishaupt JH, et al. (2006) Reduced oxidative damage in ALS by high-dose enteral melatonin treatment. J. Pineal Res. 41: 313-321.
68. Tan D-X, Chen L-D, Poeggeler B, Manchester LC, Reiter RJ. (1993) Melatonin: a potent, endogenous hydroxyl radical scavenger. Endocr. J. 1: 57–60.
69. Reiter RJ, et al. (1993) Antioxidant capacity of melatonin: a novel action not requiring a receptor. Neuro Endocrinol. Lett. 15: 103–116.
70. Tan D-X, et al. (2002) Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr. Top. Med. Chem. 2: 181–197.
71. Tan D-X, Manchester LC, Terron MP, Flores LJ, Reiter RJ. (2007) One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 42: 28–42.
72. Hardeland R, Poeggeler B, Niebergall R, Zelosko V. (2003) Oxidation of melatonin by carbonate radicals and chemiluminescence emitted during pyrrole ring cleavage. J. Pineal Res. 34: 17–25.
73. Hardeland R. (2017) The underrated carbonate radical (CO3•–) – Detoxification and reduced formation by melatonin. Biomed. J. Sci. Tech. Res. 1: 264.
74. Crespo E, et al. (1999) Melatonin inhibits expression of the inducible NO synthase II in liver and lung and prevents endotoxemia in lipopolysaccharide-induced multiple organ dysfunction syndrome in rats. FASEB J. 13:1537-1546.
75. Escames G, et al. (2006) Melatonin counteracts inducible mitochondrial nitric oxide synthase-dependent mitochondrial dysfunction in skeletal muscle of septic mice. J. Pineal Res. 40: 71–78.
76. García JA, et al. (2017) Contribution of inducible and neuronal nitric oxide synthases to mitochondrial damage and melatonin rescue in LPS-treated mice. J. Physiol. Biochem. 73: 235–244.
77. Acuña Castroviejo D, et al. (2002) Melatonin, mitochondrial homeostasis and mitochondrial-related diseases. Curr. Top. Med. Chem. 2: 133–151.
78. León J, et al. (2005) Melatonin mitigates mitochondrial malfunction. J. Pineal Res. 38:1–9.
79. Hardeland R. (2009) Melatonin, mitochondrial electron flux and leakage: recent findings and resolution of contradictory results. Adv. Stud. Biol. 1: 207–230.
80. Tan DX, Manchester LC, Qin L, Reiter RJ. (2016) Melatonin: A Mitochondrial Targeting Molecule Involving Mitochondrial Protection and Dynamics. Int. J. Mol. Sci. 17: E2124.
81. Reiter RJ, et al. (2017) Melatonin as a mitochondria-targeted antioxidant: one of evolution's best ideas. Cell. Mol. Life Sci. 74: 3863–3881.
82. Hardeland R. (2017) Melatonin and the electron transport chain. Cell. Mol. Life Sci. 74: 3883–3896.
83. Acuña-Castroviejo D, et al. Melatonin, clock genes and mitochondria in sepsis. Cell. Mol. Life Sci. 74: 3965–3987.
84. Wongprayoon P, Govitrapong P. (2017) Melatonin as a mitochondrial protector in neurodegenerative diseases. Cell. Mol. Life Sci. 74: 3999–4014.
85. Reiter RJ, et al. (2018) Mitochondria: central organelles for melatonin's antioxidant and anti-aging actions. Molecules 23: E509.
86. Pedreira RR, et al. (2008) Effects of melatonin in an experimental model of ventilator-induced lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 295: L820–L827.
87. Wu G-C, et al. (2020) Melatonin receptor agonist protects against acute lung injury induced by ventilator through up-regulation of IL-10 production. Respir. Res. 21: 65.
88. Ceraulo L, et al. (1999) Interactions of melatonin with membrane models: portioning of melatonin in AOT and lecithin reversed micelles. J. Pineal Res. 26: 108–112.
89. Bouhafs RKL, Jarstrand C. (2002) Effects of antioxidants on surfactant peroxidation by stimulated human polymorphonuclear leukocytes. Free Radic. Res. 36: 727–734.
90. Hardeland R. (2016) Deacceleration of brain aging by melatonin. In: Inflammation, Aging, and Oxidative Stress (Bondy SC, Campbell A, eds.), Humana Press, New York, pp. 345-376.
91. Xia Y, et al. (2019) Melatonin in macrophage biology: Current understanding and future perspectives. J. Pineal Res. 66: e12547.
92. Holder K. (2020) Dr. Neel treating 7 coronavirus patients; some with high fevers, severe cough, and it is working. https://devinenews.com/dr-neel-treating-7-coronavirus-patients-some-with-high-fevers-severe-cough-and-it-is-working/ Accessed April 16, 2020.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


