Mitochondria and the Gut as crucial hubs for the interactions of melatonin with sirtuins, inflammation, butyrate, tryptophan metabolites, and alpha 7 nicotinic receptor across a host of medical conditions.
Melatonin, gut and mitochondria
Abstract
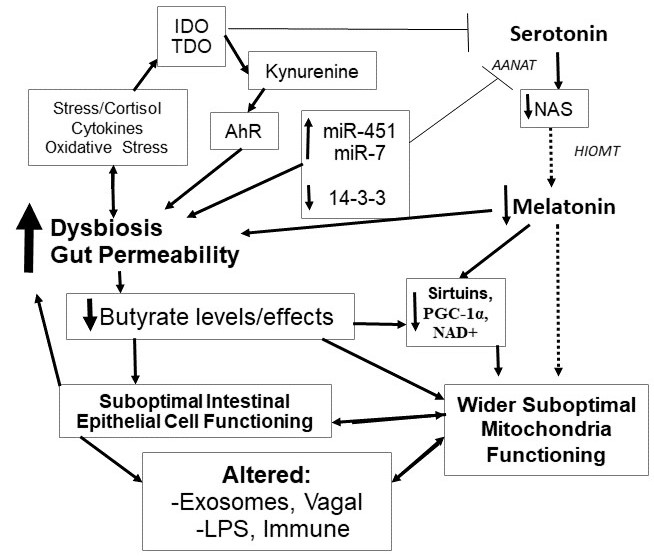
Two important hubs have emerged as cutting edge areas of research across a diverse array of medical conditions, the gut microbiome and mitochondria. This article highlights the role of melatonin in modulating changes in both the gut and mitochondria. The gut microbiome, especially via its production of the small chain fatty acid, butyate, can have a significant impact on immune inflammatory processes. Lower levels of butyrate producing bacteria can increase gut permeability, thereby increasing immune-inflammatory activity. Butyrate may also modulate immune and other cells via the regulation of the content of exosomes from intestinal epithelial cells. Butyrate also induces N-acetylserotonin and melatonin synthesis in the gut, suggesting that some of the effects of butyrate may be mediated via its induction of the melatonergic pathway. The induction of melatonin by butyrate may feed back on the microbiome via melatonin increasing gut bacteria swarming, as well as melatonin optimizing gut barrier and mitochondria functioning. As butyrate readily crosses into the circulation it is likely that the immune- and glia-dampening effects of butyrate also involve the induction of melatonin in these reactive cells. Butyrate also positively modulates mitochondria functioning, suggesting that butyrate, both directly and via melatonin, will have significant impacts on gut, immune, glia and other cells, via mitochondria regulation. Other factors that act to regulate melatonin, including dietary factors and stress, will therefore act to modulate many of butyrate's effects. The regulation of melatonin at these two important hubs has significant treatment and classification implications across a wide array of medical conditions. Overall, gut dysbiosis has a significant impact on central and systemic homeostasis, via decreased butyrate and melatonin driving suboptimal mitochondria functioning. This has implications for the pathoetiology and pathophysiology of a host of medical conditions associated with gut dysbiosis and decreased melatonin production.
References
2. de Melo LGP, Nunes SOV, Anderson G, et al. (2017) Shared metabolic and immune-inflammatory, oxidative and nitrosative stress pathways in the metabolic syndrome and mood disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 78: 34-50. doi: 10.1016/j.pnpbp.2017.04.027.
3. Wang Q, Xu R (2019) Data-driven multiple-level analysis of gut-microbiome-immune-joint interactions in rheumatoid arthritis. BMC. Genomics 20 (1): 124. doi: 10.1186/s12864-019-5510-y.
4. Kowalski K, Mulak A (2019) Brain-gut-microbiota axis in alzheimer's disease. J. Neurogastroenterol. Motil. 25 (1): 48-60. doi: 10.5056/jnm18087.
5. Martin-Subero M, Anderson G, Kanchanatawan B, Berk M, Maes M (2016) Comorbidity between depression and inflammatory bowel disease explained by immune-inflammatory, oxidative, and nitrosative stress; tryptophan catabolite; and gut-brain pathways. CNS. Spectr. 21 (2): 184-98. doi: 10.1017/S1092852915000449.
6. Breen DP, Halliday GM, Lang AE (2019) Gut-brain axis and the spread of α-synuclein pathology: Vagal highway or dead end? Mov. Disord. 34 (3): 307-316. doi: 10.1002/mds.27556.
7. Anderson G, Seo M, Berk M, Carvalho AF, Maes M (2016) Gut permeability and microbiota in parkinson's disease: role of depression, tryptophan catabolites, oxidative and nitrosative stress and melatonergic pathways. Curr. Pharm. Des. 222 (40): 6142-6151. doi: 10.2174/1381612822666160906161513.
8. Cleophas MCP, Ratter JM, Bekkering S, et al. (2019) Effects of oral butyrate supplementation on inflammatory potential of circulating peripheral blood mononuclear cells in healthy and obese males. Sci. Rep. 9 (1): 775. doi: 10.1038/s41598-018-37246-7.
9. Yang T, Rodriguez V, Malphurs WL, et al. (2018) Butyrate regulates inflammatory cytokine expression without affecting oxidative respiration in primary astrocytes from spontaneously hypertensive rats. Physiol. Rep. 6 (14): e13732. doi: 10.14814/phy2.13732.
10. Rose S, Bennuri SC, Davis JE, et al. (2018) Butyrate enhances mitochondrial function during oxidative stress in cell lines from boys with autism. Transl. Psychiatry 8 (1): 42. doi: 10.1038/s41398-017-0089-z.
11. Feng Y, Wang Y, Wang P, Huang Y, Wang F (2018) Short-Chain Fatty acids manifest stimulative and protective effects on intestinal barrier function through the inhibition of nlrp3 inflammasome and autophagy. Cell. Physiol. Biochem. 49 (1): 190-205. doi: 10.1159/000492853.
12. Ma S, Chen J, Feng J, et al. (2018) melatonin ameliorates the progression of atherosclerosis via mitophagy activation and NLRP3 inflammasome inhibition. Oxid. Med Cell. Longev. 2018: 9286458. doi: 10.1155/2018/9286458.
13. Jin CJ, Engstler AJ, Sellmann C, et al. (2016) Sodium butyrate protects mice from the development of the early signs of non-alcoholic fatty liver disease: role of melatonin and lipid peroxidation. Br. J. Nutr. 23: 1-12. doi: 10.1017/S0007114516004025.
14. Paulose JK, Wright JM, Patel AG, Cassone VM, et al. (2016) Human gut bacteria are sensitive to melatonin and express endogenous circadian rhythmicity. PloS One 11 (1): e0146643. doi: 10.1371/journal.pone.0146643.
15. Paulose JK, Cassone CV, Cassone VM (2019) Aging, melatonin biosynthesis, and circadian clockworks in the gastrointestinal system of the laboratory mouse. Physiol, Genomics 51 (1): 1-9. doi: 10.1152/physiolgenomics.00095.2018.
16. Milosevic I, Vujovic A, Barac A, et al. (2019) Gut-liver axis, gut microbiota, and its modulation in the management of liver diseases: a review of the literature. Int. J. Mol. Sci. 20 (2): pii: E395. doi: 10.3390/ijms20020395.
17. Anderson G (2018) Linking the biological underpinnings of depression: Role of mitochondria interactions with melatonin, inflammation, sirtuins, tryptophan catabolites, DNA repair and oxidative and nitrosative stress, with consequences for classification and cognition. Prog. Neuropsychopharmacol. Biol. Psychiatry 80 (Pt C): 255-266. doi: 10.1016/j.pnpbp.2017.04.022.
18. An Z, Su J (2018) Acinetobacter baumannii outer membrane protein 34 elicits NLRP3 inflammasome activation via mitochondria-derived reactive oxygen species in RAW264.7 macrophages. Microbes Infect. doi: 10.1016/j.micinf.2018.10.005.
19. Hu L, Zhang S, Wen H, et al. (2019) Melatonin decreases M1 polarization via attenuating mitochondrial oxidative damage depending on UCP2 pathway in prorenin-treated microglia. PloS One 14 (2): e0212138. doi: 10.1371/journal.pone.0212138.
20. Rose S, Bennuri SC, Davis JE, et al. (2018) Butyrate enhances mitochondrial function during oxidative stress in cell lines from boys with autism. Transl. Psychiatry 8 (1): 42. doi: 10.1038/s41398-017-0089-z.
21. Samczuk P, Hady HR, Adamska-Patruno E, et al. (2018) In-and-out molecular changes linked to the type 2 diabetes remission after bariatric surgery: an influence of gut microbes on mitochondria metabolism. Int. J. Mol. Sci. 19 (12): pii: E3744. doi: 10.3390/ijms19123744.
22. Saint-Georges-Chaumet Y, Edeas M (2016) Microbiota-mitochondria inter-talk: consequence for microbiota-host interaction. Pathog. Dis. 74 (1): ftv096.doi: 10.1093/femspd/ftv096.
23. Xing X, Jiang Z, Tang X, et al. (2016) Sodium butyrate protects against oxidative stress in HepG2 cells through modulating Nrf2 pathway and mitochondrial function. J. Physiol. Biochem. 73 (3): 405-414. doi: 10.1007/s13105-017-0568-y.
24. Shi W, Li L, Ding Y, et al. (2018) The critical role of epigallocatechin gallate in regulating mitochondrial metabolism. Future. Med. Chem. 10 (7): 795-809. doi: 10.4155/fmc-2017-0204.
25. Yu T, Dohl J, Elenberg F, Chen Y, Deuster P (2019) Curcumin induces concentration-dependent alterations in mitochondrial function through ROS in C2C12 mouse myoblasts. J. Cell. Physiol. 234 (5): 6371-6381. doi: 10.1002/jcp.27370.
26. Fakruddin M, Wei FY, Suzuki T, et al. (2018) Defective Mitochondrial tRNA Taurine Modification Activates Global Proteostress and Leads to Mitochondrial Disease. Cell. Rep. 22 (2): 482-496. doi: 10.1016/j.celrep.2017.12.051.
27. Ripps H, Shen W, (2012) Review: taurine: a "very essential" amino acid. Mol. Vis. 18: 2673-86. PMID: 23170060.
28. Yu H, Guo Z, Shen S, Shan W (2016) Effects of taurine on gut microbiota and metabolism in mice. Amino. Acids. 48 (7): 1601-17. doi: 10.1007/s00726-016-2219-y.
29. Jong CJ, Ito T, Prentice H, Wu JY, Schaffer SW, (2017) Role of mitochondria and endoplasmic reticulum in taurine-deficiency-mediated apoptosis. Nutrients 9 (8): pii: E795. doi: 10.3390/nu9080795.
30. Kawase T, Nagasawa M, Ikeda H, et al. (2017) Gut microbiota of mice putatively modifies amino acid metabolism in the host brain. Br. J. Nutr. 117 (6): 775-783. doi: 10.1017/S0007114517000678.
31. Sartori T, Galvão Dos Santos G, Nogueira-Pedro A, et al. (2018) Effects of glutamine, taurine and their association on inflammatory pathway markers in macrophages. Inflammopharmacology 26 (3): 829-838. doi: 10.1007/s10787-017-0406-4.
32. Akhalaya MY, Baizhumanov AA, Graevskaya EE (2006) Effects of taurine, carnosine, and casomorphine on functional activity of rat peritoneal mast cells. Bull. Exp. Biol. Med. 141 (3): 328-30. PMID: 17073151.
33. Che Y, Hou L, Sun F, et al. (2018) Taurine protects dopaminergic neurons in a mouse Parkinson's disease model through inhibition of microglial M1 polarization. Cell. Death. Dis. 9 (4): 435. doi: 10.1038/s41419-018-0468-2.
34. Choe KY, Olson JE, Bourque CW (2012) Taurine release by astrocytes modulates osmosensitive glycine receptor tone and excitability in the adult supraoptic nucleus. J. Neurosci. 32 (36): 12518-27. doi: 10.1523/JNEUROSCI.1380-12.2012.
35. Song NY, Shi HB, Li CY, Yin SK, (2012) Interaction between taurine and GABA(A)/glycine receptors in neurons of the rat anteroventral cochlear nucleus. Brain Res. 1472: 1-10. doi: 10.1016/j.brainres.2012.07.001.
36. Haque R, Alonso-Gómez AL, Chaurasia SS, Iuvone PM (2003) Diurnal regulation of arylalkylamine N-acetyltransferase activity in chicken retinal cells in vitro: analysis of culture conditions. Mol. Vis. 9: 52-9. PMID: 12629487.
37. Anderson G, Ojala J (2010) Alzheimer's and seizures: interleukin-18, indoleamine 2,3-dioxygenase and quinolinic Acid. Int. J. Tryptophan Res. 3: 169-73. doi: 10.4137/IJTR.S4603.
38. Hardeland R (2019) Aging, Melatonin, and the Pro- and Anti-Inflammatory Networks. Int. J. Mol. Sci. 220 (5): pii: E1223. doi: 10.3390/ijms20051223.
39. Muxel SM, Pires-Lapa MA, Monteiro AW, et al. (2012) NF-κB drives the synthesis of melatonin in RAW 264.7 macrophages by inducing the transcription of the arylalkylamine-N-acetyltransferase (AA-NAT) gene. PloS One 7 (12): e52010.
40. Lee JS, Cua DJ (2015) Melatonin lulling Th17 cells to sleep. Cell. 162 (6): 1212-1214.
41. Wu TH, Kuo HC, Lin IC, et al. (2014) Melatonin prevents neonatal dexamethasone induced programmed hypertension: histone deacetylase inhibition. J. Steroid. Biochem. Mol. Biol. 144 (B): 253-259.
42. Ansari Dezfouli M, Zahmatkesh M, Farahmandfar M, Khodagholi F (2019) Melatonin protective effect against amyloid β-induced neurotoxicity mediated by mitochondrial biogenesis; involvement of hippocampal Sirtuin-1 signaling pathway. Physiol. Behav. 204: 65-75.
43. Chen L, Sun M, Wu W, et al. (In press) Microbiota Metabolite Butyrate Differentially Regulates Th1 and Th17 Cells' Differentiation and Function in Induction of Colitis. Inflamm. Bowel. Dis. doi: 10.1093/ibd/izz046.
44. Ji J, Shu D, Zheng M, et al. (2016) Microbial metabolite butyrate facilitates M2 macrophage polarization and function. Sci. Rep. 6: 24838. doi: 10.1038/srep24838.
45. Endo H, Niioka M, Kobayashi N, Tanaka M, Watanabe T (2013) Butyrate-producing probiotics reduce nonalcoholic fatty liver disease progression in rats: new insight into the probiotics for the gut-liver axis. PloS One 8 (5): e63388. doi: 10.1371/journal.pone.0063388.
46. Zhang H, Du M, Yang Q, Zhu MJ, (2016) Butyrate suppresses murine mast cell proliferation and cytokine production through inhibiting histone deacetylase. J. Nutr. Biochem. 27: 299-306. doi: 10.1016/j.jnutbio.2015.09.020.
47. Schulthess J, Pandey S, Capitani M, et al. (2019) The short chain fatty acid butyrate imprints an antimicrobial program in macrophages. Immunity 50 (2): 432-445.e7. doi: 10.1016/j.immuni.2018.12.018.
48. Li HY, Leu YL, Wu YC, Wang SH (2019) Melatonin inhibits in vitro smooth muscle cell inflammation and proliferation and atherosclerosis in apolipoprotein e-deficient mice. J. Agric. Food Chem. 67 (7): 1889-1901. doi: 10.1021/acs.jafc.8b06217.
49. Ding K, Wang H, Xu J, et al. (2014) Melatonin reduced microglial activation and alleviated neuroinflammation induced neuron degeneration in experimental traumatic brain injury: Possible involvement of mTOR pathway. Neurochem. Int. 76: 23-31. doi: 10.1016/j.neuint.2014.06.015.
50. Markus RP, Ferreira ZS, Fernandes PA, Cecon E (2007) The immune-pineal axis: a shuttle between endocrine and paracrine melatonin sources. Neuroimmunomodulation 14 (3-4): 126-133. doi: 10.1159/000110635.
51. Tye H, Yu CH, Simms LA, et al. (2018) NLRP1 restricts butyrate producing commensals to exacerbate inflammatory bowel disease. Nat. Commun. 9 (1): 3728. doi: 10.1038/s41467-018-06125-0.
52. Al-Sadi R, Guo S, Dokladny K, et al. (2012) Mechanism of interleukin-1β induced-increase in mouse intestinal permeability in vivo. J. Interferon Cytokine Res. 32 (10): 474-84. doi: 10.1089/jir.2012.0031.
53. Sharma S, Wang J, Alqassim E, et al. (2019) Mitochondrial hypoxic stress induces widespread RNA editing by APOBEC3G in natural killer cells. Genome Biol. 20 (1): 37. doi: 10.1186/s13059-019-1651-1.
54. Liu PS, Ho PC (2018) Mitochondria: A master regulator in macrophage and T cell immunity. Mitochondrion. 41: 45-50. doi: 10.1016/j.mito.2017.11.002.
55. Tur J, Vico T, Lloberas J, Zorzano A, Celada A (2017) Macrophages and Mitochondria: A Critical Interplay Between Metabolism, Signaling, and the Functional Activity. Adv. Immunol. 133: 1-36. doi: 10.1016/bs.ai.2016.12.001.
56. Tan D-X, Reiter RJ, (2019) Mitochondria: the birth place, battle ground and the site of melatonin metabolism in cells. Melatonin. Res. 2 (1): 44-66; doi: 10.32794/mr11250011.
57. Huo X, Wang C, Yu Z, et al. (2017) Human transporters, PEPT1/2, facilitate melatonin transportation into mitochondria of cancer cells: An implication of the therapeutic potential. J. Pineal Res. 62 (4): e12390 doi: 10.1111/jpi.12390.
58. He C, Wang J, Zhang Z, et al. (2016) Mitochondria synthesize melatonin to ameliorate its function and improve mice oocyte's quality under in vitro conditions. Int. J. Mol. Sci. 17 (6): E939. doi: 10.3390/ijms17060939.
59. Aon MA, Cortassa S, Juhaszova M, Sollott SJ (2016) Mitochondrial health, the epigenome and healthspan. Clin. Sci. (Lond). 130 (15): 1285-305. doi: 10.1042/CS20160002.
60. García JJ, Piñol-Ripoll G, Martínez-Ballarín E, et al. (2011) Melatonin reduces membrane rigidity and oxidative damage in the brain of SAMP8 mice. Neurobiol. Aging 32 (11): 2045-2054. doi: 10.1016/j.neurobiolaging.2009.12.013.
61. Baran H, Staniek K, Bertignol-Spörr M, et al. (2016) Effects of various kynurenine metabolites on respiratory parameters of rat brain, liver and heart mitochondria. Int. J. Tryptophan Res. 9: 17-29. doi: 10.4137/IJTR.S37973.
62. Parada E, Buendia I, León R, et al. (2014) Neuroprotective effect of melatonin against ischemia is partially mediated by alpha-7 nicotinic receptor modulation and HO-1 overexpression. J. Pineal. Res. 56 (2): 204-212. doi: 10.1111/jpi.12113.
63. Alzarea S, Rahman S (2019) Alpha-7 nicotinic receptor allosteric modulator PNU120596 prevents lipopolysaccharide-induced anxiety, cognitive deficit and depression-like behaviors in mice. Behav. Brain Res. 366: 19-28. doi: 10.1016/j.bbr.2019.03.019.
64. Ren C, Li XH, Wang SB, et al. (2018) Activation of central alpha 7 nicotinic acetylcholine receptor reverses suppressed immune function of T lymphocytes and protects against sepsis lethality. Int. J. Biol. Sci. 14 (7): 748-759. doi: 10.7150/ijbs.2457.
65. Wang J, Li Z, Gao L, et al. (2018) The regulation effect of AMPK in immune related diseases. Sci. China Life. Sci. 61 (5): 523-533. doi: 10.1007/s11427-017-9169-6.
66. Dai SH, Chen T, Li X, et al. (2017) Sirt3 confers protection against neuronal ischemia by inducing autophagy: Involvement of the AMPK-mTOR pathway. Free Radic. Biol. Med. 108: 345-353. doi: 10.1016/j.freeradbiomed.2017.04.005.
67. Zhu H, Zhang L, Xu J, et al. (2018) AntogomiR-451 protects human gastric epithelial cells from ethanol via activating AMPK signaling Biochem. Biophys. Res. Commun. 497 (1): 339-346. doi: 10.1016/j.bbrc.2018.02.082.
68. Pagan C, Goubran-Botros H, Delorme R, et al. (2017) Disruption of melatonin synthesis is associated with impaired 14-3-3 and miR-451 levels in patients with autism spectrum disorders. Sci. Rep. 7 (1): 2096. doi: 10.1038/s41598-017-02152-x.
69. Liu D, Ma Z, Di S, et al. (2018) AMPK/PGC1α activation by melatonin attenuates acute doxorubicin cardiotoxicity via alleviating mitochondrial oxidative damage and apoptosis. Free Radic. Biol. Med. 129: 59-72. doi: 10.1016/j.freeradbiomed.2018.08.032.
70. Cui J, Li Z, Zhuang S, et al. (2018) Melatonin alleviates inflammation-induced apoptosis in human umbilical vein endothelial cells via suppression of Ca2+-XO-ROS-Drp1-mitochondrial fission axis by activation of AMPK/SERCA2a pathway. Cell Stress Chaperones 23 (2): 281-293. doi: 10.1007/s12192-017-0841-6.
71. Si X, Shang W, Zhou Z, et al. (2018) Gut microbiome-induced shift of acetate to butyrate positively manages dysbiosis in high fat diet. Mol. Nutr. Food Res. 62 (3): 1700670 doi: 10.1002/mnfr.201700670.
72. Li Q, Wu T, Liu R, Zhang M, Wang R (2017) Soluble dietary fiber reduces trimethylamine metabolism via gut microbiota and co-regulates host AMPK pathways. Mol. Nutr. Food Res. 61 (12): 1700473 doi: 10.1002/mnfr.201700473.
73. Mollica MP, Mattace Raso G, Cavaliere G, et al. (2017) Butyrate regulates liver mitochondrial function, efficiency, and dynamics in insulin-resistant obese mice. Diabetes 66 (5): 1405-1418. doi: 10.2337/db16-0924. doi: 10.2337/db16-0924.
74. Sun X, Yang Q, Rogers CJ, et al. (2017) AMPK improves gut epithelial differentiation and barrier function via regulating Cdx2 expression. Cell Death Differ. 24 (5): 819-831. doi: 10.1038/cdd.2017.14.
75. Kojima M, Costantini TW, Eliceiri BP, et al. (2018) Gut epithelial cell-derived exosomes trigger posttrauma immune dysfunction. J. Trauma Acute Care Surg. 84 (2): 257-264. doi: 10.1097/TA.0000000000001748.
76. Wang Y, Shen Y, Liu H, et al. (2019) Induction of inflammatory responses in splenocytes by exosomes released from intestinal epithelial cells following cryptosporidium parvum infection. Infect. Immun. 87 (4): e00705-18. doi: 10.1128/IAI.00705-18.
77. Liu F, Bu Z, Zhao F, Xiao D (2018) Increased T-helper 17 cell differentiation mediated by exosome-mediated microRNA-451 redistribution in gastric cancer infiltrated T cells. Cancer Sci. 109 (1): 65-73. doi: 10.1111/cas.13429.
78. Song C, Zhao J, Fu B, et al. (2017) Melatonin-mediated upregulation of Sirt3 attenuates sodium fluoride-induced hepatotoxicity by activating the MT1-PI3K/AKT-PGC-1α signaling pathway. Free Radic. Biol. Med. 112: 616-630. doi: 10.1016/j.freeradbiomed.2017.09.005.
79. St-Pierre J, Drori S, Uldry M, et al. (2006) Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 127 (2): 397–408. doi: 10.1016/j.cell.2006.09.024.
80. Kwon S, Seok S, Yau P, et al. (2017) Obesity and aging diminish sirtuin 1 (SIRT1)-mediated deacetylation of SIRT3, leading to hyperacetylation and decreased activity and stability of SIRT3. J. Biol. Chem. 292 (42): 17312-17323. doi: 10.1074/jbc.M117.778720.
81. Govender J, Loos B, Marais E, Engelbrecht AM (2018) Melatonin improves cardiac and mitochondrial function during doxorubicin-induced cardiotoxicity: A possible role for peroxisome proliferator-activated receptor gamma coactivator 1-alpha and sirtuin activity? Toxicol. Appl. Pharmacol. 358: 86-101. doi: 10.1016/j.taap.2018.06.031.
82. Bonomini F, Favero G, Rodella LF, Moghadasian MH, Rezzani R (2018) Melatonin modulation of sirtuin-1 attenuates liver injury in a hypercholesterolemic mouse model. Biomed. Res. Int. 2018: 7968452. doi: 10.1155/2018/7968452.
83. Wellman AS, Metukuri MR, Kazgan N, et al. (2017) Intestinal epithelial sirtuin 1 regulates intestinal inflammation during aging in mice by altering the intestinal microbiota. Gastroenterology 153 (3): 772-786. doi: 10.1053/j.gastro.2017.05.022.
84. Zhang Y, Wang XL, Zhou M, et al. (2018) Crosstalk between gut microbiota and Sirtuin-3 in colonic inflammation and tumorigenesis. Exp. Mol. Med. 50 (4): 21. doi: 10.1038/s12276-017-0002-0.
85. Yang L, Liu C, Zhao W, et al (2018) Impaired autophagy in intestinal epithelial cells alters gut microbiota and host immune responses. Appl. Environ. Microbiol. 84 (18): e00880-18. doi: 10.1128/AEM.00880-18.
86. Khader A, Yang WL, Godwin A, et al (2016) Sirtuin 1 stimulation attenuates ischemic liver injury and enhances mitochondrial recovery and autophagy. Crit. Care. Med. 44 (8): e651-63. doi: 10.1097/CCM.0000000000001637.
87. Sun CK, Chen CH, Chang CL, et al. (2017) Melatonin treatment enhances therapeutic effects of exosomes against acute liver ischemia-reperfusion injury. Am. J. Transl. Res. 9 (4): 1543-1560. PMID: 28469765
88. Liu Z, Gan L, Zhang T, Ren Q, Sun C (2018) Melatonin alleviates adipose inflammation through elevating α-ketoglutarate and diverting adipose-derived exosomes to macrophages in mice. J. Pineal Res. 64 (1): e12455 doi: 10.1111/jpi.12455.
89. Rong B, Feng R, Liu C, Wu Q, Sun C, (In press) Reduced delivery of epididymal adipocyte-derived exosomal resistin is essential for melatonin ameliorating hepatic steatosis in mice. J. Pineal Res. doi: 10.1111/jpi.12561.
90. Romano S, Salustri E, Ruscitti P, et al. (2018) Cardiovascular and metabolic comorbidities in rheumatoid arthritis. Curr. Rheumatol. Rep. 20 (12): 81. doi: 10.1007/s11926-018-0790-9.
91. Perumpail BJ, Khan MA, Yoo ER, et al. (2017) Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J. Gastroenterol. 23 (47): 8263-8276. doi: 10.3748/wjg.v23.i47.8263.
92. Anderson G and Maes M (2015) Co-enzyme Q10, depression and depression associated conditions. Chap 8; 147-170 in Book: Co-Enzyme Q10: from fact to fiction. Nova; Ed: Hargreaves IP. ISBN: 9781634828222.
93. Anderson G, Maes M (2016) Alpha 7 nicotinic receptor agonist modulatory interactions with melatonin: relevance not only to cognition, but to wider neuropsychiatric and immune inflammatory disorders. In press. Chap 4; page 186-202; in bk: Frontiers in Clinical Drug Research-Central Nervous System. Bentham Press. doi: 10.2174/97816810818921160201.
94. Anderson G, Maes M (2013) Schizophrenia is primed for an increased expression of depression through activation of immuno-inflammatory, oxidative and nitrosative stress, and tryptophan catabolite pathways. Prog. Neuropsychopharmacol. Biol. Psychiatry 42: 101-14. doi: 10.1016/j.pnpbp.2012.07.016.
95. Meyer ML, Lin FC, Jaensch A, et al. (2019) Multi-state models of transitions in depression and anxiety symptom severity and cardiovascular events in patients with coronary heart disease. PloS One 14 (3): e0213334. doi: 10.1371/journal.pone.0213334.
96. Slyepchenko A, Maes M, Machado-Veira R, et al. (2016) Intestinal dysbiosis, gut hyperpermeability and bacterial translocation: missing links between depression, obesity and type 2 diabetes? Curr. Pharm. Des. 22 (40): 6087-6106 doi: 10.2174/1381612822666160922165706.
97. Løge-Hagen JS, Sæle A, Juhl C, et al. (2019) Prevalence of depressive disorder among patients with fibromyalgia: systematic review and meta-analysis. J. Affect. Disord. 245: 1098-1105. doi: 10.1016/j.jad.2018.12.001.
98. Rodriguez M, Wootla B, Anderson G (2016) Multiple Sclerosis, Gut Microbiota and Permeability: Role of Tryptophan Catabolites, Depression and the Driving Down of Local Melatonin. Curr. Pharm. Des. 22 (40): 6134 - 6141 doi: 10.2174/1381612822666160915160520.
99. Anderson G, Maes M (2015) Melatonin: A Natural Homeostatic Regulator – Interactions with Immune Inflammation and Trytophan Catabolite Pathways in the Modulation of Migraine and Endometriosis. J. Nat. Prod. Res. Updates 1: 7-17. doi: 10.1111/j.1600-079X.2007.00547.x.
100. Ostadmohammadi V, Jamilian M, Bahmani F, Asemi Z, (2019) Vitamin D and probiotic co-supplementation affects mental health, hormonal, inflammatory and oxidative stress parameters in women with polycystic ovary syndrome. J. Ovarian. Res. 12 (1): 5. doi: 10.1186/s13048-019-0480-x.
101. Anderson G, Maes M (2015) Melatonin in neurodegenerative, psychiatric and systemic inflammatory disorders. Chap 8 in the Book: Indoleamines: Sources, Role in Biological Processes and Health Effects. Ed. Angel Catala. Nova Press. ISBN-10: 1634820975.
102. Butler MI, Sandhu K, Cryan JF, Dinan TG (2019) From isoniazid to psychobiotics: the gut microbiome as a new antidepressant target. Br. J. Hosp. Med. (Lond). 80 (3): 139-145. doi: 10.12968/hmed.2019.80.3.139.
103. Allen J, Romay-Tallon R, Brymer KJ, Caruncho HJ, Kalynchuk LE (2018) Mitochondria and mood: mitochondrial dysfunction as a key player in the manifestation of depression. Front Neurosci. 12: 386. doi: 10.3389/fnins.2018.00386.
104. Shabani A, Foroozanfard F, Kavossian E, et al. (2019) Effects of melatonin administration on mental health parameters, metabolic and genetic profiles in women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. J. Affect. Disord. 250: 51-56. doi: 10.1016/j.jad.2019.02.066.
105. Gao T, Wang Z, Dong Y, et al. (2019) Role of melatonin in sleep deprivation-induced intestinal barrier dysfunction in mice. J. Pineal Res. e12574.
106. Saxena A, Lopes F, McKay DM (2018) Reduced intestinal epithelial mitochondrial function enhances in vitro interleukin-8 production in response to commensal Escherichia coli. Inflamm. Res. 67 (10): 829-837. doi: 10.1007/s00011-018-1172-5.
107. Wang A, Keita ÅV, Phan V, et al. (2014) Targeting mitochondria-derived reactive oxygen species to reduce epithelial barrier dysfunction and colitis. Am. J. Pathol. 184 (9): 2516-27. doi: 10.1016/j.ajpath.2014.05.019.
108. Sommansson A, Nylander O, Sjöblom M (2013) Melatonin decreases duodenal epithelial paracellular permeability via a nicotinic receptor-dependent pathway in rats in vivo. J. Pineal Res. 54 (3): 282-91. doi: 10.1111/jpi.12013.
109. Markus RP, Silva CL, Franco DG, Barbosa EM Jr, Ferreira ZS (2010) Is modulation of nicotinic acetylcholine receptors by melatonin relevant for therapy with cholinergic drugs? Pharmacol. Ther. 126 (3): 251-262. doi: 10.1016/j.pharmthera.2010.02.009.
110. Ferry G, Ubeaud C, Lambert PH, et al. (2005) Molecular evidence that melatonin is enzymatically oxidized in a different manner than tryptophan: investigations with both indoleamine 2,3-dioxygenase and myeloperoxidase. Biochem. J. 388 (1): 205-215. doi:10.1042/BJ20042075.
111. Suofu Y, Li W, Jean-Alphonse FG, et al. (2017) Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc. Natl. Acad. Sci. USA. 114 (38): E7997-E8006. doi: 10.1073/pnas.1705768114.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


