COVID-19 pathophysiology: interactions of gut microbiome, melatonin, vitamin D, stress, kynurenine and the alpha 7 nicotinic receptor: Treatment implications
COVID-19 pathophysiology and Treatment
Abstract
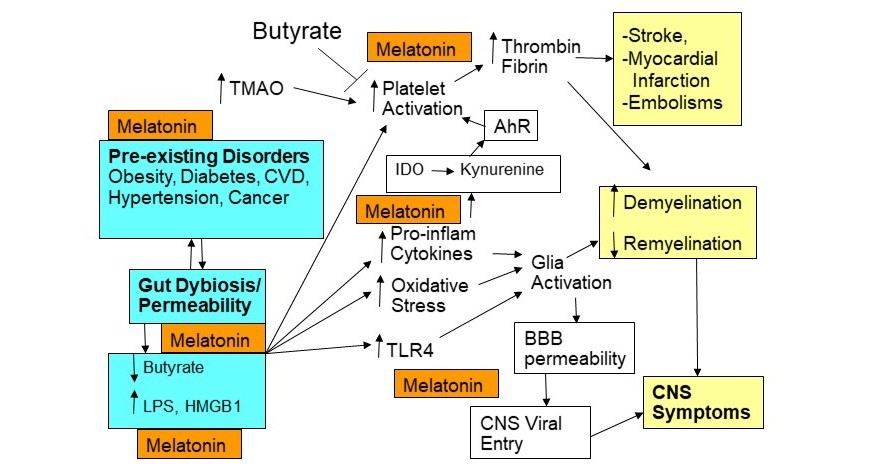
As data emerges on the pathophysiological underpinnings of severe acute respiratory syndrome coronavirus (SARS-CoV)-2, it is clear that there are considerable variations in its susceptibility and severity/fatality, which give indications as to its pathophysiology and treatment. SARS-CoV-2 modulatory factors include age, vitamin D levels, cigarette smoking, gender and ethnicity as well as premorbid medical conditions, including diabetes, cancer, obesity, cardiovascular disease, and immune-compromised conditions. A complex picture is emerging, with an array of systemic physiological processes interacting including circadian, immune, intestinal, CNS and coagulation factors. This article reviews data on SARS-CoV-2 pathoetiology and pathophysiology. It is proposed that a decrease in pineal and systemic melatonin is an important driver of SARS-CoV-2 susceptibility and severity, with the loss of pineal melatonin's induction of the alpha 7 nicotinic acetylcholine receptor (α7nAChR) in pulmonary epithelial cells and immune cells being a powerful regulator of susceptibility and severity, respectively. Stress, including discrimination stress, and decreased vitamin D also regulate SARS-CoV-2, including via gut dysbiosis and permeability, with a resultant decrease in the short-chain fatty acid, butyrate, and increase in circulating lipopolysaccharide. Stress and cytokine induction of the kynurenine pathways, leads to aryl hydrocarbon receptor activation, which primes platelets for heightened activity, coagulation and thrombin production, thereby driving elevations in thrombin that underpin many SARS-CoV-2 fatalities. On the basis of these pathophysiological changes, prophylactic and symptomatic treatments are proposed, including the use of melatonin and α7nAChR agonism.
References
2. Cohen S, Tyrrell DA, Smith AP (1991) Psychological stress and susceptibility to the common cold. N. Engl. J. Med. 325 (9): 606-612.
3. Vanuytsel T, van Wanrooy S, Vanheel H, Vanormelingen C, Verschueren S, Houben E, et al. (2014) Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut 63 (8): 1293-1299. doi: 10.1136/gutjnl-2013-305690.
4. Anderson G, Maes M (2020) Gut dysbiosis dysregulates central and systemic homeostasis via suboptimal mitochondrial function: assessment, treatment and classification implications. Curr. Top. Med. Chem. 20 (7): 524-539. doi: 10.2174/1568026620666200131094445.
5. Anderson G, Reiter RJ (2020) Melatonin: Roles in influenza, Covid-19 and other viral infections. Rev. Med. Virol. 21: e2109. doi:org/10.1002/rmv.2109.
6. Mika A, Rumian N, Loughridge AB, Fleshner M (2016) Exercise and prebiotics produce stress resistance: converging impacts on stress-protective and butyrate-producing gut bacteria. Int. Rev. Neurobiol. 131: 165-191. doi: 10.1016/bs.irn.2016.08.004.
7. Jin CJ, Engstler AJ, Sellmann C, Ziegenhardt D, Landmann M, Kanuri G, et al. (2016) Sodium butyrate protects mice from the development of the early signs of non-alcoholic fatty liver disease: role of melatonin and lipid peroxidation. Br. J. Nutr. 23: 1-12. doi:10.1017/S0007114516004025.
8. Chen Y, Sun H, Bai Y, Zhi F (2019) Gut dysbiosis-derived exosomes trigger hepatic steatosis by transiting HMGB1 from intestinal to liver in mice. Biochem. Biophys. Res. Commun. 509 (3): 767-772. doi: 10.1016/j.bbrc.2018.12.180.
9. Shi Z, Gewirtz AT (2018) Together Forever: Bacterial-viral interactions in infection and immunity. Viruses 10 (3): pii: E122. doi: 10.3390/v10030122.
10. Perrin-Cocon L, Aublin-Gex A, Sestito SE, Shirey KA, Patel MC, André P, et al. (2017) TLR4 antagonist FP7 inhibits LPS-induced cytokine production and glycolytic reprogramming in dendritic cells, and protects mice from lethal influenza infection. Sci. Rep. 7: 40791. doi: 10.1038/srep40791.
11. Locke T (2020) Three studies give insight into BAME COVID-19 risks. Medscape May07.2020.
12. He L, Liu T, Shi Y, Tian F, Hu H, Deb DK, et al. (2018) Gut epithelial vitamin D receptor regulates microbiota-dependent mucosal inflammation by suppressing intestinal epithelial cell apoptosis. Endocrinology 159 (2): 967-979. doi: 10.1210/en.2017-00748.
13. Telcian AG, Zdrenghea MT, Edwards MR, Laza-Stanca V, Mallia P, Johnston SL, et al. (2017) Vitamin D increases the antiviral activity of bronchial epithelial cells in vitro. Antiviral Res. 137: 93-101. doi: 10.1016/j.antiviral.2016.11.004.
14. Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL, et al. (2020) Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 Infections and Deaths. Nutrients 12 (4): pii: E988. doi: 10.3390/nu12040988.
15. Sawalha AH, Zhao M, Coit P, Lu Q (2020) Epigenetic dysregulation of ACE2 and interferon-regulated genes might suggest increased COVID-19 susceptibility and severity in lupus patients. Clin. Immunol. 215:108410. doi: 10.1016/j.clim.2020.108410.
16. Vavougios GD (2020) A data-driven hypothesis on the epigenetic dysregulation of host metabolism by SARS coronaviral infection: Potential implications for the SARS-CoV-2 modus operandi. Med. Hypotheses 140: 109759. doi: 10.1016/j.mehy.2020.109759.
17. Yagi K, Ishii M, Namkoong H, Fujii H, Asami T, Suzuki S, et al. (2016) Histone deacetylase inhibition protects mice against lethal postinfluenza pneumococcal infection. Crit. Care Med. 44 (10): e980-7. doi: 10.1097/CCM.0000000000001821.
18. Anderson G (2019) Integrating pathophysiology in migraine: role of the gut microbiome and melatonin. Curr. Pharm. Des. 25 (33): 3550-3562. doi: 10.2174/1381612825666190920114611.
19. Markus RP, Fernandes PA, Kinker GS, da Silveira Cruz-Machado S, Marçola M (2018) Immune-pineal axis - acute inflammatory responses coordinate melatonin synthesis by pinealocytes and phagocytes. Br. J. Pharmacol. 175 (16): 3239-3250. doi: 10.1111/bph.14083.
20. Sengupta S, Tang SY, Devine JC, Anderson ST, Nayak S, Zhang SL, et al. (2019) Circadian control of lung inflammation in influenza infection. Nat. Commun. 10 (1): 4107. doi: 10.1038/s41467-019-11400-9.
21. Meira E Cruz M, Miyazawa M, Gozal D (2020) Putative contributions of circadian clock and sleep in the context of SARS-CoV-2 infection. Eur. Respir. J. 55 (6): pii: 2001023. doi: 10.1183/13993003.01023-2020.
22. Markus RP, Silva CL, Franco DG, Barbosa EM Jr, Ferreira ZS. (2010) Is modulation of nicotinic acetylcholine receptors by melatonin relevant for therapy with cholinergic drugs? Pharmacol. Ther. 126 (3): 251-62. doi: 10.1016/j.pharmthera.2010.02.009.
23. Anderson G, Maes M (2017) Interactions of tryptophan and its catabolites with melatonin and the alpha 7 nicotinic receptor in central nervous system and psychiatric disorders: role of the aryl hydrocarbon receptor and direct mitochondria regulation. Int. J. Tryptophan Res. 10: 1178646917691738. doi: 10.1177/1178646917691738.
24. Gahring LC, Myers EJ, Dunn DM, Weiss RB, Rogers SW (2017) Nicotinic alpha 7 receptor expression and modulation of the lung epithelial response to lipopolysaccharide. PLoS One 12 (4): e0175367. doi: 10.1371/journal.pone.0175367.
25. Propper RE. (2020) Does cigarette smoking protect against SARS-CoV-2 infection? Nicotine Tob. Res. pii: ntaa073. doi: 10.1093/ntr/ntaa073.
26. Ren C, Li XH, Wang SB, Wang LX, Dong N, Wu Y, et al. (2018) Activation of central alpha 7 nicotinic acetylcholine receptor reverses suppressed immune function of T lymphocytes and protects against sepsis lethality. Int. J. Biol. Sci. 14 (7): 748-759. doi: 10.7150/ijbs.24576.
27. Cecon E, Chen M, Marçola M, Fernandes PA, Jockers R, Markus RP (2015) Amyloid β peptide directly impairs pineal gland melatonin synthesis and melatonin receptor signaling through the ERK pathway. FASEB J. 29 (6):2566-2582.
28. Sommansson A, Nylander O, Sjöblom M (2013) Melatonin decreases duodenal epithelial paracellular permeability via a nicotinic receptor-dependent pathway in rats in vivo. J. Pineal Res. 54 (3): 282-291. doi: 10.1111/jpi.12013.
29. Yasui DH, Scoles HA, Horike S, Meguro-Horike M, Dunaway KW, Schroeder DI, et al. (2011) 15q11.2-13.3 chromatin analysis reveals epigenetic regulation of CHRNA7 with deficiencies in Rett and autism brain. Hum. Mol. Genet. 20 (22): 4311-23. doi: 10.1093/hmg/ddr357.
30. Ivanov DO, Evsyukova II, Mazzoccoli G, Anderson G, Polyakova VO, Kvetnoy IM, et al. (2020) The role of prenatal melatonin in the regulation of childhood obesity. Biology (Basel) 9 (4): pii: E72. doi: 10.3390/biology9040072.
31. Huang W, Kabbani N, Brannan TK, Lin MK, Theiss MM, Hamilton JF, et al. (2019) Association of a functional polymorphism in the CHRFAM7A gene with inflammatory response mediators and neuropathic pain after spinal cord injury. J. Neurotrauma 36 (21): 3026-3033. doi: 10.1089/neu.2018.6200.
32. Bordas A, Cedillo JL, Arnalich F, Esteban-Rodriguez I, Guerra-Pastrián L, de Castro J, et al. (2017) Expression patterns for nicotinic acetylcholine receptor subunit genes in smoking- related lung cancers. Oncotarget 8 (40): 67878-67890. doi: 10.18632/oncotarget.18948.
33. Dang X, Eliceiri BP, Baird A, Costantini TW (2015) CHRFAM7A: a human-specific α7-nicotinic acetylcholine receptor gene shows differential responsiveness of human intestinal epithelial cells to LPS. FASEB J. 29 (6): 2292-302. doi: 10.1096/fj.14-268037.
34. Benfante R, Antonini RA, De Pizzol M, Gotti C, Clementi F, Locati M, et al. (2011) Expresssion of the α7 nAChR subunit duplicate form (CHRFAM7A) is down-regulated in the monocytic cell line THP-1 on treatment with LPS. J. Immunol. 230: 74–84.
35. Araud T, Graw S, Berger R, Lee M, Neveu E, Bertrand D, Leonard S (2011) The chimeric gene CHRFAM7A, a partial duplication of the CHRNA7 gene, is a dominant negative regulator of a7*nAChR function. Biochem. Pharmacol. 82: 904–914.
36. Casey JP, Magalhaes T, Conroy JM, Regan R, Shah N, Anney R, et al. (2012) A novel approach of homozygous haplotype sharing identifies candidate genes in autism spectrum disorder. Hum. Genet. 131 (4): 565-79. doi: 10.1007/s00439-011-1094-6.
37. Chan TW, Langness S, Cohen O, Eliceiri BP, Baird A, Costantini TW (2020) CHRFAM7A reduces monocyte/macrophage migration and colony formation in vitro. Inflamm. Res. 69 (7): 631-633. doi: 10.1007/s00011-020-01349-7.
38. Costantini TW, Chan TW, Cohen O, Langness S, Treadwell S, Williams E, et al. (2019) Uniquely human CHRFAM7A gene increases the hematopoietic stem cell reservoir in mice and amplifies their inflammatory response. Proc. Natl. Acad. Sci. 116 (16): 7932-7940. doi: 10.1073/pnas.1821853116.
39. Lu Y, Liu DX, Tam JP (2008) Lipid rafts are involved in SARS-CoV entry into Vero E6 cells. Biochem. Biophys. Res. Commun. 369 (2): 344-9. doi: 10.1016/j.bbrc.2008.02.023.
40. Fantini J, Di Scala C, Chahinian H, Yahi N (2020) Structural and molecular modelling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int. J. Antimicrob. Agents 55 (5): doi: 10.1016/j.ijantimicag.2020.105960.
41. Maroli A, Di Lascio S, Drufuca L, Cardani S, Setten E, Locati M, et al. (2019) Effect of donepezil on the expression and responsiveness to LPS of CHRNA7 and CHRFAM7A in macrophages: A possible link to the cholinergic anti-inflammatory pathway. J. Neuroimmunol. 332: 155-166. doi: 10.1016/j.jneuroim.2019.04.012.
42. Ramos FM, Delgado-Vélez M, Ortiz ÁL, Báez-Pagán CA, Quesada O, Lasalde-Dominicci JA (2016) Expression of CHRFAM7A and CHRNA7 in neuronal cells and postmortem brain of HIV-infected patients: considerations for HIV-associated neurocognitive disorder. J. Neurovirol. 22 (3): 327-335. doi: 10.1007/s13365-015-0401-8.
43. Franchini M, Veneri D, Lippi G (2017) Thrombocytopenia and infections. Expert Rev. Hematol. 10 (1): 99-106. doi: 10.1080/17474086.2017.1271319.
44. Liu Y, Sun W, Guo Y, Chen L, Zhang L, Zhao S, et al. (2020) Association between platelet parameters and mortality in coronavirus disease 2019: Retrospective cohort study. Platelets 16: 1-7. doi: 10.1080/09537104.2020.1754383.
45. Bi X, Su Z, Yan H, Du J, Wang J, Chen L, et al. (2020) Prediction of severe illness due to COVID-19 based on an analysis of initial fibrinogen to albumin ratio and platelet count. Platelet. 5: 1-6. doi: 10.1080/09537104.2020.1760230.
46. Zeng F, Li L, Zeng J, Deng Y, Huang H, Chen B, et al. (2020) Can we predict the severity of COVID-19 with a routine blood test? Pol. Arch. Intern. Med. 130 (5): 400-406. doi: 10.20452/pamw.15331.
47. Qu R, Ling Y, Zhang YH, Wei LY, Chen X, Li XM, et al. (2020) Platelet-to-lymphocyte ratio is associated with prognosis in patients with coronavirus disease-19. J. Med. Virol. doi: 10.1002/jmv.25767.
48. Han H, Yang L, Liu R, Liu F, Wu KL, Li J, et al. (2020) Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin. Chem. Lab. Med. pii: /j/cclm.ahead-of-print/cclm-2020-0188/cclm-2020-0188.xml. doi: 10.1515/cclm-2020-0188.
49. Wang J, Hajizadeh N, Moore EE, McIntyre RC, Moore PK, Veress LA, et al. (2020) Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): A case series. J. Thromb. Haemost. doi: 10.1111/jth.14828.
50. Avula A, Nalleballe K, Narula N, Sapozhnikov S, Dandu V, Toom S, et al. (2020) COVID-19 presenting as stroke. Brain Behav. Immun. S0889-1591 (20): 30685-1. doi: 10.1016/j.bbi.2020.04.077.
51. Anderson G, Rodriguez M, Reiter RJ (2019) Multiple sclerosis: melatonin, orexin, and ceramide interact with platelet activation coagulation factors and gut-microbiome-derived butyrate in the circadian dysregulation of mitochondria in glia and immune cells. Int. J. Mol. Sci. 20 (21). pii: E5500. doi: 10.3390/ijms20215500.
52. Zanin L, Saraceno G, Panciani PP, Renisi G, Signorini L, Migliorati K, et al. (2020) SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir. (Wien) doi: 10.1007/s00701-020-04374-x.
53. Li Z, Liu T, Yang N, Han D, Mi X, Li Y, et al. (2020) Neurological manifestations of patients with COVID-19: potential routes of SARS-CoV-2 neuroinvasion from the periphery to the brain. Front Med. doi: 10.1007/s11684-020-0786-5.
54. Kaur R, Kaur M, Singh J (2018) Endothelial dysfunction and platelet hyperactivity in type 2 diabetes mellitus: molecular insights and therapeutic strategies. Cardiovasc. Diabetol. 17 (1): 121. doi: 10.1186/s12933-018-0763-3.
55. El Haouari M. (2019) Platelet oxidative stress and its relationship with cardiovascular diseases in type 2 diabetes mellitus patients. Curr. Med. Chem. 26 (22): 4145-4165. doi: 10.2174/0929867324666171005114456.
56. Münzer P, Borst O, Walker B, Schmid E, Feijge, MA, Cosemans, JM, et al. (2014) Acid sphingomyelinase regulates platelet cell membrane scrambling, secretion, and thrombus formation. Arterioscler. Thromb. Vasc. Biol. 34: 61–71.
57. Llitjos JF, Leclerc M, Chochois C, Monsallier JM, Ramakers M, Auvray M, et al. (2020) High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost. doi: 10.1111/jth.14869.
58. Schedel A, Thornton S, Schloss P, Klüter H, Bugert P (2011) Human platelets express functional alpha7-nicotinic acetylcholine receptors. Arterioscler. Thromb. Vasc. Biol. 31 (4): 928-34. doi: 10.1161/ATVBAHA.110.218297.
59. Pombo M, Lamé MW, Walker NJ, Huynh DH, Tablin F (2015) TCDD and omeprazole prime platelets through the aryl hydrocarbon receptor (AhR) non-genomic pathway. Toxicol. Lett. 235 (1): 28-36. doi: 10.1016/j.toxlet.2015.03.005.
60. Pett SL, Kunisaki KM, Wentworth D, Griffin TJ, Kalomenidis I, Nahra R, et al. (2017) Increased indoleamine-2,3-dioxygenase activity is associated with poor clinical outcome in adults hospitalized with influenza in the INSIGHT FLU003Plus Study. Open Forum. Infect. Dis. 5 (1): ofx228. doi: 10.1093/ofid/ofx228.
61. Anderson G, Mazzoccoli G (2019) Left Ventricular Hypertrophy: Roles of Mitochondria CYP1B1 and Melatonergic Pathways in Co-Ordinating Wider Pathophysiology. Int. J. Mol. Sci. 20 (16): pii: E4068. doi: 10.3390/ijms20164068.
62. Anderson G, Reiter RJ (2019) Glioblastoma: role of mitochondria N-acetylserotonin/melatonin ratio in mediating effects of miR-451 and aryl hydrocarbon receptor and in coordinating wider biochemical changes. Int. J. Tryptophan Res. 12:1178646919855942. doi: 10.1177/1178646919855942.
63. Anderson G (2018) Linking the biological underpinnings of depression: Role of mitochondria interactions with melatonin, inflammation, sirtuins, tryptophan catabolites, DNA repair and oxidative and nitrosative stress, with consequences for classification and cognition. Prog Neuropsychopharmacol Biol. Psychiatry. 80 (Pt C): 255-266. doi: 10.1016/j.pnpbp.2017.04.022.
64. Maes M, Anderson G (2016) Overlapping the tryptophan catabolite (TRYCAT) and melatoninergic pathways in Alzheimer's disease. Curr. Pharm. Des. 22 (8):1074-1085.
65. Anderson G, Maes M (2017) How immune-inflammatory processes link CNS and psychiatric disorders: classification and treatment implications. CNS Neurol. Disord. Drug Targets 16 (3): 266-278. doi: 10.2174/1871527315666161122144659.
66. Li M, Kwok MK, Fong SSM, Schooling CM (2020) Effects of tryptophan, serotonin, and kynurenine on ischemic heart diseases and its risk factors: a Mendelian Randomization study. Eur. J. Clin. Nutr. 74 (4): 613-621. doi: 10.1038/s41430-020-0588-5.
67. Gibney SM, Fagan EM, Waldron AM, O'Byrne J, Connor TJ, Harkin A (2014) Inhibition of stress-induced hepatic tryptophan 2,3-dioxygenase exhibits antidepressant activity in an animal model of depressive behaviour. Int. J. Neuropsychopharmacol. 17 (6): 917-928. doi: 10.1017/S1461145713001673.
68. Muxel SM, Pires-Lapa MA, Monteiro AW, Cecon E, Tamura EK, Floeter-Winter LM, et al. (2012) NF-κB drives the synthesis of melatonin in RAW 264.7 macrophages by inducing the transcription of the arylalkylamine-N-acetyltransferase (AA-NAT) gene. PLoS One 7 (12): e52010. doi: 10.1371/journal.pone.0052010.
69. Pizzini A, Kurz K, Santifaller J, Tschurtschenthaler C, Theurl I, Fuchs D, et al. (2019) Assessment of neopterin and indoleamine 2,3-dioxygenase activity in patients with seasonal influenza: A pilot study. Influenza Other Respir. Viruses. 13 (6): 603-609. doi: 10.1111/irv.12677.
70. Lin YT, Lin CF, Yeh TH (2020) Influenza A virus infection induces indoleamine 2,3-dioxygenase (IDO) expression and modulates subsequent inflammatory mediators in nasal epithelial cells. Acta Otolaryngol. 140 (2): 149-156. doi: 10.1080/00016489.2019.1700304.
71. Cure E, Cumhur Cure M (2020) Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may be harmful in patients with diabetes during COVID-19 pandemic. Diabetes Metab. Syndr. 14 (4):349-350. doi: 10.1016/j.dsx.2020.04.019.
72. Grebe KM, Takeda K, Hickman HD, Bailey AL, Embry AC, Bennink JR, et al. (2010) Cutting edge: Sympathetic nervous system increases proinflammatory cytokines and exacerbates influenza A virus pathogenesis. J. Immunol. 184 (2): 540-504. doi: 10.4049/jimmunol.0903395.
73. Chen L, Li X, Chen M, Feng Y, Xiong C (2020) The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc. Res. 116 (6): 1097-1100. doi: 10.1093/cvr/cvaa078.
74. Cui WY, Zhao S, Polanowska-Grabowska R, Wang J, Wei J, Dash B, et al. (2013) Identification and characterization of poly(I:C) induced molecular responses attenuated by nicotine in mouse macrophages. Mol. Pharmacol. 83 (1): 61-72. doi: 10.1124/mol.112.081497.
75. Rastović M, Srdić-Galić B, Barak O, Stokić E, Polovina S (2019) Aging, heart rate variability and metabolic impact of obesity. Acta Clin Croat. 58 (3): 430-438. doi: 10.20471/acc.2019.58.03.05.
76. Marik PE, Kory P, Varon J (2020) Does vitamin D status impact mortality from SARS-CoV-2 infection? Med. Drug Discov. doi: 10.1016/j.medidd.2020.100041.
77. Brindicci C, Kharitonov SA, Ito M, Elliott MW, Hogg JC, Barnes PJ, et al. (2010) Nitric oxide synthase isoenzyme expression and activity in peripheral lung tissue of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 181 (1): 21-30. doi: 10.1164/rccm.200904-0493OC.
78. Akerström S, Gunalan V, Keng CT, Tan YJ, Mirazimi A (2009) Dual effect of nitric oxide on SARS-CoV replication: viral RNA production and palmitoylation of the S protein are affected. Virology 395 (1):1-9. doi: 10.1016/j.virol.2009.09.007.
79. Haberberger RV, Henrich M, Lips KS, Kummer W (2003) Nicotinic receptor alpha 7-subunits are coupled to the stimulation of nitric oxide synthase in rat dorsal root ganglion neurons. Histochem. Cell Biol. 120 (3): 173-181.
80. Brielle ES, Schneidman-Duhovny D, Linial M (2020) The SARS-CoV-2 exerts a distinctive strategy for interacting with the ACE2 human receptor. Viruses 12 (5): pii: E497. doi: 10.3390/v12050497.
81. Ji HL, Zhao R, Matalon S, Matthay MA (2020) Elevated plasmin(ogen) as a common risk factor for COVID-19 susceptibility. Physiol. Rev. 100 (3): 1065-1075. doi: 10.1152/physrev.00013.2020.
82. Arkebauer MR, Kanaparthy SS, Malayaman SN, Vosseller K, Nielsen VG (2011) Carbon monoxide and nitric oxide modulate α₂-antiplasmin and plasmin activity: role of heme. Blood Coagul. Fibrinolysis 22 (8): 712-719. doi: 10.1097/MBC.0b013e32834c73f9.
83. Zhang L, Li F, Su X, Li Y, Wang Y, Fang R, et al. (2019) Melatonin prevents lung injury by regulating apelin 13 to improve mitochondrial dysfunction. Exp. Mol. Med. 51 (7): 73. doi: 0.1038/s12276-019-0273-8.
84. Bolmatov D, Soloviov D, Zhernenkov M, Zav'yalov D, Mamontov E, Suvorov A, et al. (2020) Molecular picture of the transient nature of lipid rafts. Langmuir. 36 (18): 4887-8489 doi: 10.1021/acs.langmuir.0c00125.
85. Williams WR. (2018) Dampening of neurotransmitter action: molecular similarity within the melatonin structure. Endocr. Regul. 52 (4): 199-207. doi: 10.2478/enr-2018-0025.
86. Mutze K, Vierkotten S, Milosevic J, Eickelberg O, Königshoff M (2015) Enolase 1 (ENO1) and protein disulfide-isomerase associated 3 (PDIA3) regulate Wnt/β-catenin-driven trans-differentiation of murine alveolar epithelial cells. Dis. Model. Mech. 8 (8): 877-890. doi: 10.1242/dmm.019117.
87. Xu J, Yang J, Chen J, Luo Q, Zhang Q, Zhang H (2017) Vitamin D alleviates lipopolysaccharide-induced acute lung injury via regulation of the renin-angiotensin system. Mol. Med. Rep. 16 (5): 7432-7438. doi: 10.3892/mmr.2017.7546.
88. Li Y, Zeng Z, Cao Y, Liu Y, Ping F, Liang M, et al. (2016) Angiotensin-converting enzyme 2 prevents lipopolysaccharide-induced rat acute lung injury via suppressing the ERK1/2 and NF-κB signaling pathways. Sci. Rep. 6: 27911. doi: 10.1038/srep27911.
89. Shi YY, Liu TJ, Fu JH, Xu W, Wu LL, Hou AN, et al. (2016) Vitamin D/VDR signaling attenuates lipopolysaccharide-induced acute lung injury by maintaining the integrity of the pulmonary epithelial barrier. Mol. Med. Rep. 13 (2):1186-94. doi: 10.3892/mmr.2015.4685.
90. Zhang M, Jin F (2017) 1α,25-Dihydroxyvitamin D3 ameliorates seawater aspiration-induced lung injury by inhibiting the translocation of NF-κB and RhoA. Inflammation 40 (3): 832-839. doi: 10.1007/s10753-017-0527-3.
91. Lee SA, Mefford JA, Huang Y, Witte JS, Martin JN, Haas DW, et al. (2016) Host genetic predictors of the kynurenine pathway of tryptophan catabolism among treated HIV-infected Ugandans. AIDS 30 (11): 1807-1815. doi: 10.1097/QAD.0000000000001124.
92. Eid A, Khoja S, AlGhamdi S, Alsufiani H, Alzeben F, Alhejaili N, et al. (2019) Vitamin D supplementation ameliorates severity of generalized anxiety disorder (GAD). Metab. Brain Dis. 34 (6): 1781-1786. doi: 10.1007/s11011-019-00486-1.
93. La Vignera S, Cannarella R, Condorelli RA, Torre F, Aversa A, Calogero AE (2020) Sex-specific SARS-CoV-2 mortality: among hormone-modulated ACE2 expression, risk of venous thromboembolism and hypovitaminosis D. Int. J. Mol. Sci. 21 (8): pii: E2948. doi: 10.3390/ijms21082948.
94. Mocayar Marón FJ, Ferder L, Reiter RJ, Manucha W (2020) Daily and seasonal mitochondrial protection: Unraveling common possible mechanisms involving vitamin D and melatonin. J. Steroid. Biochem. Mol. Biol. 199: 105595. doi: 10.1016/j.jsbmb.2020.105595.
95. Fang N, Hu C, Sun W, Xu Y, Gu Y, Wu L, et al. (2020) Identification of a novel melatonin- binding nuclear receptor: Vitamin D receptor. J. Pineal Res. 68 (1): e12618. doi: 10.1111/jpi.12618.
96. Schrumpf JA, Amatngalim GD, Veldkamp JB, Verhoosel RM, Ninaber DK, Ordonez SR, et al. (2017) Proinflammatory cytokines impair vitamin D-induced host defense in cultured airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 56 (6): 749-761. doi: 10.1165/rcmb.2016-0289OC.
97. Kida Y, Shimizu T, Kuwano K (2006) Sodium butyrate up-regulates cathelicidin gene expression via activator protein-1 and histone acetylation at the promoter region in a human lung epithelial cell line, EBC-1. Mol. Immunol. 43 (12): 1972-1981.
98. Liu Q, Liu J, Roschmann KIL, van Egmond D, Golebski K, Fokkens WJ, et al. (2013) Histone deacetylase inhibitors up-regulate LL-37 expression independent of toll-like receptor mediated signalling in airway epithelial cells. J. Inflamm. (Lond) 10 (1): 15. doi: 10.1186/1476-9255-10-15.
99. Budden KF, Gellatly SL, Wood DL, Cooper MA, Morrison M, Hugenholtz P, et al. (2017) Emerging pathogenic links between microbiota and the gut-lung axis. Nat. Rev. Microbiol. 15 (1): 55-63. doi: 10.1038/nrmicro.2016.142.
100. Xu X, Shi L, Ma X, Su H, Ma G, Wu X, et al. (2019) RhoA-Rho associated kinase signaling leads to renin-angiotensin system imbalance and angiotensin converting enzyme 2 has a protective role in acute pulmonary embolism. Thromb. Res. 176: 85-94. doi: 10.1016/j.thromres.2019.02.016. Epub 2019 Feb 15. PMID: 30784777.
101. Nordlund JJ, Lerner AB (1997) The effects of oral melatonin on skin color and on the release of pituitary hormones. J. Clin. Endocrinol. Metab. 45: 768–774, https://doi.org/10.1210/jcem-45-4-768.
102. Ferry G, Ubeaud C, Lambert PH, Bertin S, Cogé F, Chomarat P, et al. (2005) Molecular evidence that melatonin is enzymatically oxidized in a different manner than tryptophan: investigations with both indoleamine 2,3-dioxygenase and myeloperoxidase. Biochem. J. 388 (1): 205-215.
103. NaveenKumar SK, Hemshekhar M, Kemparaju K, Girish KS (2019) Hemin-induced platelet activation and ferroptosis is mediated through ROS-driven proteasomal activity and inflammasome activation: Protection by melatonin. Biochim. Biophys. Acta Mol. Basis Dis. 1865 (9): 2303-2316. doi: 10.1016/j.bbadis.2019.05.009.
104. Chen S, Qi Y, Wang S, Xu Y, Shen M, Hu M, et al (2020) Melatonin enhances thrombopoiesis through ERK1/2 and Akt activation orchestrated by dual adaptor for phosphotyrosine and 3-phosphoinositides. J. Pineal Res. 68 (3): e12637. doi: 10.1111/jpi.12637.
105. Abedi F, Hayes AW, Reiter R, Karimi G (2020) Acute lung injury: The therapeutic role of Rho kinase inhibitors. Pharmacol. Res. 155: 104736. doi: 10.1016/j.phrs.2020.104736.
106. Ding R, Zhao D, Li X, Liu B, Ma X (2017) Rho-kinase inhibitor treatment prevents pulmonary inflammation and coagulation in lipopolysaccharide-induced lung injury. Thromb. Res. 150: 59-64. doi: 10.1016/j.thromres.2016.12.020.
107. Xu Y, Cui K, Li J, Tang X, Lin J, Lu X, et al. (2020) Melatonin attenuates choroidal neovascularization by regulating macrophage/microglia polarization via inhibition of RhoA/ROCK signaling pathway. J. Pineal Res. e12660. doi: 10.1111/jpi.12660.
108. Wolf JJ, Studstill CJ, Hahm B (2019) Emerging Connections of S1P-Metabolizing Enzymes with Host Defense and Immunity During Virus Infections. Viruses 11 (12): pii: E1097. doi: 10.3390/v11121097.
109. Anderson G, Maes M (2014) Reconceptualizing adult neurogenesis: role for sphingosine-1-phosphate and fibroblast growth factor-1 in co-ordinating astrocyte-neuronal precursor interactions. CNS Neurol. Disord. Drug Targets 13 (1): 126-36.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


