Melatonin protects against cadmium-induced oxidative damage in different tissues of rat: a mechanistic insight
Melatonin prevents against cadmium-induced tissue damage
Abstract
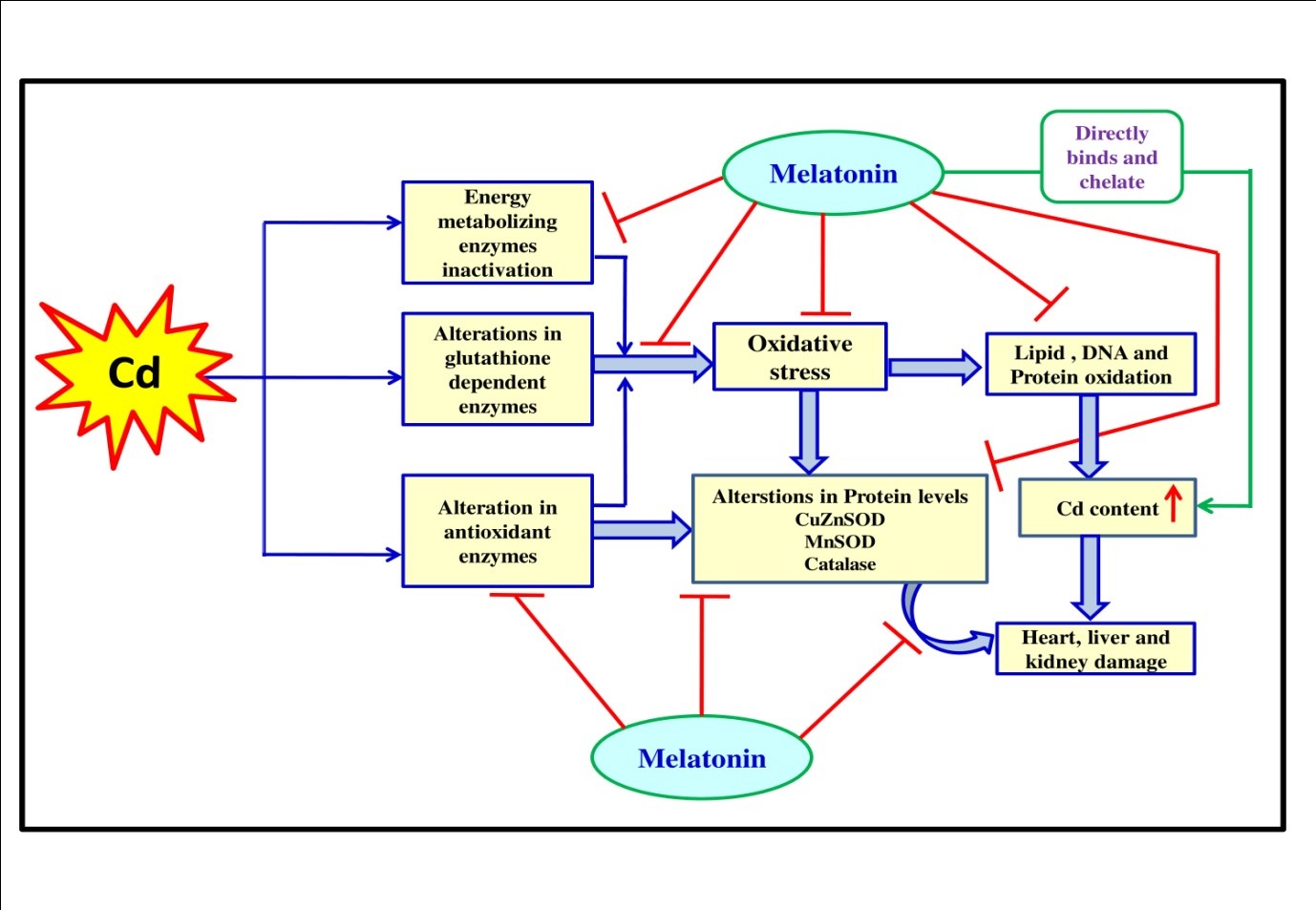
Cadmium (Cd) is a notorious environmental pollutant known for its wide range of toxicities to organisms. Thus, the present study is designed to examine whether melatonin, a potent antioxidant, protects against Cd-induced oxidative damage in the heart, liver and kidney of rats. Cd treatment at a dose of 0.44 mg/kg for 15 days caused severe damage in all these organs. These included significantly increased activities of SGPT, SGOT, lactate dehydrogenase- 1 and 5 and ALP and levels of total lactate, creatinine, lipid peroxidation, protein carbonyl content and reduced glutathione while the activities of superoxide dismutases, catalase, glutathione peroxidase, glutathione reductase and glutathione-S-transferase along with mitochondrial pyruvate dehydrogenase, isocitrate dehydrogenase, α-keto glutarate dehydrogenase, succinate dehydrogenase, NADH-cytochrome-c-oxidoreductase and cytochrome-c-oxidase were significantly reduced by Cd. However, if melatonin was given orally 30 min before Cd injection, all these alterations induced by Cd were significantly preserved by melatonin. Histological observations also demonstrated that Cd exposure caused cellular lesions, promoting necrotic or apoptotic changes. Notably, all these changes were significantly protected by melatonin. The results suggest that melatonin is a beneficial molecule to ameliorate Cd-induced oxidative damage in the heart, liver and kidney tissues of rats with its powerful antioxidant capacity, heavy metal chelating activity and competition of binding sites with Cd to the GSH and catalase.
References
2. Goering PL, Waalkes MP, Klaassen CD (1964) Toxicology of cadmium. In: Goyer RA, Cherian MG, eds. Handbook of experimental pharmacology: toxicology of metals, Springer-Verlag, New York, 115: 189-214.
3. International Agency for Research on Cancer (IARC) (1993) IARC cancer monographs on the evaluation of the carcinogenic risks to humans, vol. 58. IARC, Lyon, France. 119238.
4. Nair AR, Degheselle O, Smeets K, Van Kerkhove E, Cuypers A (2013) Cadmium-Induced Pathologies: Where Is the Oxidative Balance Lost (or Not)? Int. J. Mol. Sci. 14: 6116-6143. doi: 10.3390/ijms14036116.
5. Chwełatiuk E, Włostowski T, Krasowska A, Bonda E (2005) Melatonin increases tissue accumulation and toxicity of cadmium in the bank vole (Clethrionomysglareolus). BioMetals. 18: 283–291. DOI 10.1007/s10534-005-1720-7.
6. Chwełatiuk E, Włostowski T, Krasowska A, Bonda E (2006) The effect of orally administered melatonin on tissue accumulation and toxicity of cadmium in mice. J. Trace Elem. Med. Biol. 19: 259–265. doi:10.1016/j.jtemb.2005.10.006.
7. Valko M1, Jomova K, Rhodes CJ, Kuča K, Musílek K (2016) Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch. Toxicol. 90: 1-37. doi: 10.1007/s00204-015-1579-5.
8. El-Sokkary GH, Nafady AA, Shabash EH (2009) Melatonin ameliorates cadmium-induced oxidative damage and morphological changes in the kidney of rat. Open Neuroendocrinol. J. 2: 1-9.
9. El-Sokkary GH, Nafady AA, Shabash EH (2010) Melatonin administration ameliorates cadmium-induced oxidative stress and morphological changes in the liver of rat. Ecotox. Environ. Safe 73: 456–463. doi:10.1016/j.ecoenv.2009.09.014.
10. Romero A, Caride A, Pereiro N, Lafuente A (2011) Modulatory effects of melatonin on cadmium-induced changes in biogenic amines in rat hypothalamus. Neurotox. Res. 20: 240-249. doi: 10.1007/s12640-010-9237-4.
11. Jiménez-Ortega V, Barquilla PC, Fernández-Mateos P, Cardinali DP, Esquifino A (2012) Cadmium as an endocrine disruptor: Correlation with anterior pituitary redox and circadian clock mechanisms and prevention by melatonin. Free Radic. Biol. Med. 53: 2287–2297. http://dx.doi.org/10.1016/j.freeradbiomed.2012.10.533.
12. Li Y, Wang H, Meng C, Zhao XF, Zhang C, Zhang Y, Zhao M, Chen YH, Meng XH, Xu DX (2012) Melatonin alleviates cadmium-induced cellular stress and germ cell apoptosis in testes. J. Pineal Res. 52: 71–79. Doi:10.1111/j.1600-079X.2011.00921.x.
13. Miura N, Yanagiba Y, Ohtani K, Mita M, Togawa M, Hasegawa T (2012) Diurnal variation of cadmium-induced mortality in mice. J. Toxicol. Sci. 37: 191-196.
14. Cuypers A, Plusquin M, Remans T, Jozefczak M, Keunen E, Gielen H, Opdenakker K, Nair AR, Munters E, Artois TJ, Nawrot T, Vangronsveld J, Smeets K (2010) Cadmium stress: an oxidative challenge. Biometals. 23: 927-940. DOI: 10.1007/s10534-010-9329-x
15. Birben E1, Sahiner UM, Sackesen C, Erzurum S, Kalayci O (2012) Oxidative stress and antioxidant defense. World Allergy Organ J. 5: 9. doi: 10.1097/WOX. 0b013e31 82439613.
16. Tan DX, Manchester LC, Esteban-Zubero E, Zhou Z, Reiter RJ (2015) Melatonin as a Potent and Inducible Endogenous Antioxidant: Synthesis and Metabolism. Molecules. 20:18886-18906. doi: 10.3390/molecules201018886.
17. Tan DX, Zheng X, Kong J, Manchester LC, Hardeland R, Kim SJ, Xu X, Reiter RJ (2014) Fundamental issues related to the origin of melatonin and melatonin isomers during evolution: relation to their biological functions. Int. J. Mol. Sci. 15:15858-15890. doi: 10.3390/ijms150915858.
18. Tan DX, Manchester LC, Qin L, Reiter RJ (2016) Melatonin: A Mitochondrial Targeting Molecule Involving Mitochondrial Protection and Dynamics. Int. J. Mol. Sci. 17: 2124. doi: 10.3390/ijms17122124.
19. Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre JM, Qin L (2016) Melatonin as an antioxidant: under promises but over delivers. J. Pineal Res. 61: 253-278. doi: 10.1111/jpi.12360.
20. Hardeland R (2017) Melatonin and the electron transport chain. Cell Mol. Life Sci. 74: 3883-3896. doi: 10.1007/s00018-017-2615-9.
21. Mitra E, Ghosh AK, Ghosh D, Mukherjee D, Chattopadhyay A, Dutta S, Pattari SK, Bandyopadhyay D (2012) Protective effect of aqueous Curry leaf (Murrayakoenigii) extract against cadmium-induced oxidative stress in rat heart. Food Chem. Toxicol. 50: 1340-53. doi: 10.1016/j.fct.2012.01.048.
22. Mitra E, Ghosh AK, Ghosh D, Firdaus SB, Mukherjee D, Chattopadhyay A, Pattari SK, Datta S, Bandyopadhyay D (2014) Ameliorative effect of aqueous tulsi leaf (Ocimum sanctum) extract against cadmium-induced oxidative stress in rat liver. Int. J. Pharm. Pharm. Sci. 5: 557-568.
23. Bhattacharjee B, Ghosh AK, Mishra S, Das J, Chattopadhyay A, Bandyopadhyay D (2016) Terminalia arjuna aqueous bark extract protects against cadmium acetate-induced injury to rat liver and heart through antioxidant mechanisms: a dose response study. J. Pharma. Res.10: 771-792.
24. Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 28: 56-63.
25. Strittmatter CF (1965) Studies on avian xanthine dehydrogenases: Properties and patterns of appearance during development. J. Biol. Chem. 240: 2557–2564.
26. Varcoe JS (2001) Clinical Biochemistry: Techniques and Instrumentation-A practical approach, first ed. World Scientific Publishing Company, pp. 40-43.
27. Folin O, WU H (1919) A system of blood analysis. J. Biol. Chem. 38: 81.
28. Kind PRN, King EJ (1954) Estimation of plasma phosphatase by determination of hydrolysed phenol with anti-pyrine. J. Clin. Pathol. 7: 322–326. doi:10.1136/jcp.7.4.322.
29. Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound, nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 25: 192-205. doi:org/10.1016/ 0003-2697 (68)90092-4.
30. Bandyopadhyay D, Ghosh G, Bandyopadhyay A, Reiter RJ (2004) Melatonin protects against piroxicam-induced gastric ulceration. J. Pineal Res. 36: 195–203. doi:org/ 10.1111/j.1600-079X.2004.00118.x
31. Buege JA, Aust SG (1978) Microsomal Lipid Peroxidation. Methods Enzymol. 52: 302-310. doi: org/10.1016/S0076-6879(78)52032-6.
32. Levine RL, Williams JA, Stadtman ER, Shacter E (1994) Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol. 233: 346-357. doi:org/10.1016/S0076-6879(94)33040-9
33. Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyragallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 47: 469-474. doi:org/10.1111/j.1432-1033.1974.tb03714.x.
34. Beers Jr. RF, Sizer IW (1952) A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 195: 133-140.
35. Chattopadhyay A, Biswas S, Bandyopadhyay D, Sarkar C, Datta AG (2003) Effect of isoproterenol on lipid peroxidation and antioxidant enzymes of myocardial tissue of mice and protection by quinidine. Mol. Cell. Biochem. 245: 43-49. doi: 10.1023/A: 1022808224917.
36. Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 27: 680–685. doi: 10.1038/227680a0.
37. Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 70: 158-169.
38. Krohne-Ehrich G, Schirmer RH, Untucht-Grau R (1977) Glutathione reductase from human erythrocytes. Isolation of the enzyme and sequence analysis of the redox-active peptide. Eur. J. Biochem. 80: 65-71. doi:org/10.1111/j.1432-1033.1977.tb11856.x
39. Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione -S- transferases, the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 249: 7130 -7139.
40. Chretien D, Pourrier M, Bourgeron T, Séné M, Rötig A, Munnich A, Rustin P (1995) An improved spectrophotometric assay of pyruvate dehydrogenase in lactate dehydrogenase contaminated mitochondrial preparations from human skeletal muscles. Clin. Chim. Acta 240: 129-136. doi:org/10.1016/0009-8981(95)06145-6.
41. Mukherjee D, Ghosh AK, Dutta M, Mitra E, Mallick S, Saha B, Reiter RJ, Bandyopadhyay D (2015) Mechanism of isoproterenol induced cardiac mitochondrial damages: protective action of melatonin. J. Pineal Res. 58: 275-290. doi: 10.1111/ jpi.12213
42. Duncan MJ, Fraenkel DG (1979) Alpha-ketoglutarate dehydrogenase mutant of Rhizobium meliloti. J. Bacteriol. 137: 415-419.
43. Veeger C, DerVartanian DV, Zeylemaker WP (1969) Succinate dehydrogenase. Methods Enzymol. 13:81-90. doi:org/10.1016/0076-6879 (69)13020-7.
44. Goyal N, Srivastava VM (1995) Oxidation and reduction of cytochrome c by mitochondrial enzymes of Setariacervi. J. Helminthol. 69: 13-17.
45. Greenlee L, Handler P (1964) Xanthine oxidase. IV. Influence of pH on substrate specificity. J. Biol. Chem. 239: 1090-1095.
46. Mukherjee D, Ghosh Roy S, Bandopadhyay A, Chattopadhyay A, Basu A, Mitra E, Ghosh AK, Reiter RJ, Bandhyopadhyay D (2010) Melatonin protects against isoproterenol induced myocardial injury in the rat: antioxidative mechanism. J. Pineal. Res. 48: 251-262. doi: 10.1111/j.1600-079X.2010.00749.x.
47. Roy SG, De P, Mukherjee D, Chander V, Konar A, Bandyopadhyay D, Bandyopadhyay A (2009) Excess of glucocorticoid induces cardiac dysfunction via activating angiotensin II pathway. Cell. Physiol. Biochem. 24: 1–10.
48. Watanabe I, Ogawa K, Yamada E (1988) Taste buds of rabbits foliate papillae. A scanning electron microscopy study. Cienc. Cult. 40: 787–790.
49. Bhattacharjee B, Pal PK, Ghosh AK, Mishra S, Chattopadhyay A, Bandyopadhyay D (2019) Aqueous bark extract of Terminalia arjuna protects against cadmium-induced hepatic and cardiac injuries in male Wistar rats through antioxidative mechanisms. Food Chem. Toxicol. 124: 249-264. doi: 10.1016/j.fct.2018.12.008.
50. Mitra E, Basu A, Ghosh D, Ghosh AK, Chattopadhyay A, Pattari SK, Datta S, Bandyopadhyay D (2013) Ameliorative effect of aqueous tulsi leaf (Ocimum sanctum) extract against cadmium-induced oxidative stress in rat liver. Int. J. Pharm. Pharm. Sci. 5: 557-568.
51. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193: 265-275.
52. Ni J, Wang Q, Shah FA, Liu W, Wang D, Huang S, Fu S, Wu L (2018) Exogenous melatonin confers cadmium tolerance by counterbalancing the hydrogen peroxide homeostasis in wheat seedlings. Molecules 23: pii: E799. doi: 10.3390/ molecules 23040799.
53. Gu Q, Chen Z, Yu X, Cui W, Pan J, Zhao G, Xu S, Wang R, Shen W (2017) Melatonin confers plant tolerance against cadmium stress via the decrease of cadmium accumulation and reestablishment of microRNA-mediated redox homeostasis. Plant Sci. 261: 28-37. doi: 10.1016/j.plantsci.2017.05.001.
54. Cuypers A, Plusquin M, Remans T, Jozefczak M, Keunen E, Gielen H, Opdenakker K, Nair AR, Munters E, Artois TJ, NawrotT, Vangronsveld J, Smeets K (2010) Cadmium stress: an oxidative challenge. Biometals 23: 927-940. doi: 10.1007/s10534-010-9329-x.
55. Zhang HM, Zhang Y, Zhang BX (2011) The role of mitochondrial complex III in melatonin-induced ROS production in cultured mesangial cells. J. Pineal. Res. 50: 78-82. doi: 10.1111/j.1600-079X.2010.00815.x.
56. Tan DX, Reiter RJ (2019) Mitochondria: the birth place, battle ground and the site of melatonin metabolism in cells. Melatonin Res. 2: 44-66. doi: https://doi.org/https:// doi.org/10.32794/mr11250011.
57. Galano A, Reiter RJ (2018) Melatonin and its metabolites vs oxidative stress: From individual actions to collective protection. J. Pineal. Res. 65: e12514. doi: 10.1111/jpi.12514.
58. Chekmeneva E, Prohens R, Díaz-Cruz JM, Ariño C, Esteban M (2008) Thermodynamics of Cd2+ and Zn2+ binding by the phytochelatin (gamma-Glu-Cys) 4-Gly and its precursor glutathione. Anal. Biochem. 375: 82-89. doi: 10.1016/j.ab.2008.01.008.
59. Wang Y, Fang J, Leonard SS, Rao KM (2004) Cadmium inhibits the electron transfer chain and induces reactive oxygen species. Free Radic. Biol. Med. 36: 1434–1443.
60. Wang J, Zhang H, Zhang T, Zhang R, Liu R, Chen Y (2015) Molecular mechanism on cadmium-induced activity changes of catalase and superoxide dismutase. Int. J. Biol. Macromol. 77: 59-67. doi: 10.1016/j.ijbiomac.2015.02.037.
61. Limson J, Nyokong T, Daya S (1998) The interaction of melatonin and its precursors with aluminium, cadmium, copper, iron, lead, and zinc: An adsorptive voltammetric study. J. Pineal Res. 24: 15-21.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


