Enterochromaffin cells as the source of melatonin: key findings and functional relevance in mammals
Melatonin and enterochromaffin cells
Abstract
The enteroendocrine cells in gastrointestinal (GI) tract synthesize more than thirty hormones in mammals. Among these cells, the enterochromaffin (EC) cells are probably the most important one due to the fact that they produce melatonin. The rate-limiting enzymes for melatonin synthesis including arylalkylamine-N-acetyltransferase (AANAT, currently the SNAT) and hydroxyindole-O-methyltransferase (HIOMT, currently the ASMT) have been identified in EC cells and this has confirmed the local melatonin production in GI tract by these cells. EC cells play a critical role in regulation of gastrointestinal physiology, particularly, in protection of the GI tract from free radical attack and inflammatory reaction. GI tract is the major site exposed to the oxidative stress and inflammation because of the food residue metabolism and the presence of trillions of microbes including the pathological bacteria. Thus, it requires strong protection. Melatonin synthesized by the EC cells provides the onsite protection in GI tract since this molecule is the potent free radical scavenger and effective ant-inflammatory agent. In this review we summarize the available information regarding the structural and functional variability of the EC cells as well as their pathophysiological roles in the GI tract. The focus is given to the protective effects of melatonin produced by the EC cells on the oxidative stress, inflammation and microbiota balance in GI tract.
References
2. Ahlman H, Nilsson O (2003) The gut as the largest endocrine organ in the body. Ann. Oncol. 12 (Suppl. 2): S63–S68. DOI: 10.1093/annonc/12.suppl2.s63.
3. Raikhlin NT, Kvetnoy IM (1974) Lightening effect of the extract of human appendix mucosa on frog skin melanophores. Bull. Exp. Biol. Med. 8: 114–116.
4. Raikhlin NT, Kvetnoy IM, Tolkachev VN (1975) Melatonin may be synthesized in enterochro-maffin cells. Nature 255: 344–345. DOI: 10.1038/255344a0.
5. Bubenik GA, Brown G, Grota L (1977) Immuno-histological localization of melatonin in the rat digestive system. Experientia. 33: 662–663. https://doi.org/10.1007/BF01946561.
6. Bubenik GA (2001) Localization, physiological significance and possible clinical implication of gastrointestinal melatonin. Biol. Signals Recept. 10: 350–366. DOI: 10.1159/000046903.
7. Huether G, Poeggeler B, Reimer A, George A (1992) Effect of tryptophan administration on circulating melatonin levels in chicks and rats: evidence for stimulation of melatonin synthesis and release in the gastrointestinal tract. Life Sci. 51: 945–953. DOI: 10.1016/0024-3205(92)90402-b.
8. Huether G (1994) Melatonin synthesis in the gastrointestinal tract and the impact of nutritional factors on circulating melatonin. Ann. NY. Acad. Sci. 719: 146–158. DOI: 10.1111/j.1749-6632.1994.tb56826.x.
9. Bubenik GA, Pang SF, Hacker RR, Smith PS (1996) Melatonin concentrations in serum and tissues of porcine gastrointestinal tract and their relationship to the intake and passage of blood. J. Pineal Res. 21: 251–256. DOI:10.1111/j.1600-079x.1996.tb00294.x.
10. Bubenik GA, Brown GM (1997) Pinealectomy reduces melatonin levels in the serum but not in the gastrointestinal tract of the rat. Biol. Signals. 6: 40–44.DOI: 10.1159/000109107.
11. Bubenik GA (1999) Localization and physiological significance of gastrointestinal melatonin. In: R Watson (ed) Melatonin in Health Promotion. CRC press, Boca Raton, Florida, pp. 21–39.
12. Pal PK, Hasan KN, Maitra SK (2016) Gut melatonin response to microbial infection in carp Catla catla. Fish Physiol. Biochem. 42: 579–592. DOI: 10.1007/s10695-015-0161-7.
13. Kennaway DJ, Firth RG, Philipou G, Matthews CD, Seamark RF (1977) A specific radioimmunoassay for melatonin in biological tissue and fluids and its validation by gas chromatography-mass spectrometry. Endocrinology. 101: 119–127. DOI: 10.1210/endo-101-1-119.
14. Vaughan GM, Reiter RJ (1986) Pineal dependence of the Syrian hamster’s nocturnal serum melatonin surge. J. Pineal Res. 3: 9–14. https://doi.org/10.1111/j.1600-079X.1986.tb00721.x.
15. Huether G, Poegeller B, Reimer R, George A (1992) Effect of tryptophan administration on circulating melatonin levels in chicks and rats: evidence for stimulation of melatonin synthesis and release in the gastrointestinal tract. Life Sci. 51: 945–953. DOI: 10.1016/0024-3205(92)90402-b.
16. Kezuka H, Iigo I, Furukawa K, Aida K, Hanyu I (1992) Effects of photoperiod, pinealectomy and ophtalmectomy on circulating melatonin rhythms in the goldfish (Carassius auratus). Zool. Sci. 9: 1147–1153.
17. Klein DC (1985) Photoneural regulation of the mammalian pineal gland. In: Everet D, Clark E. (eds) Photoperiodism, melatonin and the pineal. Ciba Found Symp. 117: 38–56.
18. Klein DC, Roseboom PH, Coon SL (1996) New light is shining on the melatonin rhythm enzyme: the Wrst postcloning view. Trends Endocrinol. Metab. 7: 106–112. https://doi.org/10.1016/1043-2760(96)00033-1.
19. Mukherjee S, Moniruzzaman M, Maitra SK (2014) Daily and seasonal profiles of gut melatonin and their temporal relationship with pineal and serum melatonin in carp Catla catla under natural photo-thermal conditions. Biol. Rhythm Res. 45: 301–315. DOI: 10.1080/09291016.2013.817139.
20. Pal PK, Bhattacharjee B, Ghosh AK, Chattopadhyay A, Bandyopadhyay D (2019) Adrenaline induced disruption of endogenous melatonergic system, antioxidant and inflammatory responses in the gastrointestinal tissues of male Wistar rat: an in vitro study. Melatonin Res. 1: 109-131. DOI: 10.32794/mr11250007.
21. Zhao F, Ma C, Zhao G, Wang G, Li X, Yang K (2019) Rumen-protected 5-hydroxytryptophan improves sheep melatonin synthesis in the pineal gland and intestinal tract. Med. Sci. Monit. 25: 3605-3616. DOI: 10.12659/MSM.915909.
22. Huether G (1993) The contribution of extrapineal sites of melatonin synthesis to circulating melatonin levels in higher vertebrates. Experientia 49: 665–670. DOI: 10.1007/bf01923948.
23. Bubenik GA, Pang SF (1997) Melatonin level in the gastrointestinal tissue of fish, amphibians, and a reptile. Gen. Comp. Endocrinol. 106: 415–419. DOI: 10.1006/gcen.1997.6889.
24. Boujard T, Leatherland JF (1992) Circadian rhythms and feeding time in fishes. Environ. Biol. Fish 35: 109–131. https://doi.org/10.1007/BF00002186.
25. Escobar C, Díaz-Muñoz M, Encinas F, Aguilar-Roblero R (1998) Persistence of metabolic rhythmicity during fasting and its entrainment by restricted feeding schedule in rats. Am. J. Physiol. 274:1309–1316. DOI: 10.1152/ajpregu.1998.274.5.R1309.
26. Polakof S, Ceinos RM, Fernández-Durán B, Míguez JM, Soengas JL (2007) Daily changes in parameters of energy metabolism in brain of rainbow trout: dependence on feeding. Comp. Biochem. Physiol. 146: 265–273. DOI: 10.1016/j.cbpa.2006.10.026.
27. Polakof S, Míguez JM, Soengas JL (2007) Daily changes in parameters of energy metabolism in liver, white muscle, and gills of rainbow trout: dependence on feeding. Comp. Biochem. Physiol. A. 147: 363–374. DOI: 10.1016/j.cbpa.2007.01.009.
28. Chow PH, Lee PN, Poon AMS, Shiu SYW, Pang SF (1996) The gastrointestinal system: A site of melatonin paracrine action. In: Tang PL, Pang SF, Reiter RJ (eds.) Melatonin: A universal photoperiodic signal with diverse action. Front. Horm. Res. Basel, Karger. 21: 123–132. https://doi.org/10.1159/000425610.
29. Maitra SK, Pal PK (2017) Structural diversity and functional integrity of the fish pineal gland. In: Catalá A. (Ed.) Pineal gland: Research advances and clinical challenges. Nova Science Publishers, Inc. New York, USA. Pp. 51–92. https://www.novapublishers.com/ catalog/product_ info.php? products_id=62459.
30. Maitra SK, Pal PK (2017) Melatonin rhythms in the pineal and non-pineal tissues and their physiological implications in subtropical fish. Biol. Rhythm Res. 48: 757–776. https://doi.org/10.1080/09291016.2017.1345453.
31. Lee PPN, Pang SF (1993) Melatonin and its receptors in the gastrointestinal tract. Neurosignals 2: 181–193. DOI: 10.1159/000109491.
32. Lee PPN, Shiu SYU, Chow PH, Pang SF (1995) Regional and diurnal studies on melatonin binding sites in the duck gastrointestinal tract. Biol. Signals 4: 212–224. DOI:10.1159/000109445.
33. Bubenik GA, Niles LP, Pang SF, Pentney PJ (1993) Diurnal variation and binding characteristics of melatonin in the mouse brain and gastrointestinal tissues. Comp. Biochem. Physiol. C. 104: 221–224. DOI: 10.1016/0742-8413(93)90027-i.
34. Barajas-Lopez C, Pereso AL, Espinos-Luna R, Reyes-Vazquez C, Prieto-Gomez B (1996) Melatonin modulates cholinergic-transmission blocking nicotinic channels in the guinea-pig sub-mucous-plexus. Eur. J. Pharmacol. 312: 319–325. DOI: 10.1016/0014-2999(96)00481-5.
35. Poon AMS, Chow PH, Mak ASY, Pang SF (1997) Autoradiographic localization of [125I] iodomelatonin binding sites in the gastrointestinal tract of mammals including humans and birds. J. Pineal Res. 23: 5–14. https://doi.org/10.1111/j.1600-079X.1997.tb00328.x.
36. Kulczykowska E, Kalamarz H, Warne J, Balment R (2006) Day-night specific binding 2[I125]iodomelatonin and melatonin in chronically cannulated Xounder (Platichthys xesus). Comp. Biochem. Physiol.176: 277–285.
37. Pal PK, Maitra SK (2018) Response of gastrointestinal melatonin, antioxidants, and digestive enzymes to altered feeding conditions in carp (Catla catla). Fish Physiol. Biochem. 44: 1061–1073. https://doi.org/10.1007/s10695-018-0494-0.
38. Pal PK, Bhattacharjee B, Chattopadhyay A, Bandyopadhyay D (2019) Pleiotropic roles of melatonin against oxidative stress mediated tissue injury in the gastrointestinal tract: An overview. Melatonin Res.2: 158-184. DOI: 10.32794/mr11250027.
39. Khizhkin EA, Ilyukha VA, Vinogradova IA, Anisimov VN (2019) Absence of photoperiodism and digestive enzymes in rats: the role of the age and endogenous melatonin level. Adv. Gerontol. 32: 347-356.
40. Forssmann WG, Orci L, Pictet R, Renold AE, Rouiller C (1969) The endocrine cells in the epithelium of the gastrointestinal mucosa of the rat: An electron microscope study. J. Cell Biol. 40: 692-715. DOI: 10.1083/jcb.40.3.692.
41. Heidenhain R (1870) Untersuchungenüber den Bau der Labdrüsen. Archiv. Fürmikroskopische Anatomie. 6: 368-406. https://doi.org/10.1007/BF02933955.
42. Vialli M (1966) Histology of the enterochromaffin cell system. In 5-Hydroxytryptamine and related indolealkylamines (pp. 1-65). Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-85467-5_1.
43. Wade PR, Westfall JA (1985) Ultrastructure of enterochromaffin cells and associated neural and vascular elements in the mouse duodenum. Cell Tissue Res. 241: 557-563. https://doi.org/10.1007/BF00214576.
44. Erspamer V, Asero B (1952) Identification of enteramine, the specific hormone of the enterochromaffin cell system, as 5-hydroxytryptamine. Nature 169: 800-801. DOI: 10.1038/169800b0.
45. Barter R, Pearse AE (1953) Detection of 5-hydroxytryptamine in mammalian enterochromaffin cells. Nature.172: 810. DOI: 10.1038/172810a0.
46. Holcenberg J, Benditt E (1959) A new histochemical technique for demonstration of enterochromaffin cells- a reaction for indoleethylamines. J. Histochem. Cytochem. 7: 303-304.
47. Pearse AG (1968) Histochemistry: theoretical and applied. 3rd Edition. London, Churchill.
48. Sjölund K, Sanden G, Håkanson R, Sundler F (1983) Endocrine cells in human intestine: an immunocytochemical study. Gastroenterol. 85: 1120-1130. https://doi.org/10.1016/ S0016-5085(83)80080-8.
49. Pearse AG, Polak JM, Bloom SR, Adams C, Dryburgh JR, Brown JC (1974) Enterochromaffin cells of the mammalian small intestine as the source of motilin. Virchows Archiv. B Cell. Pathol. 16: 111-120. https://doi.org/10.1007/BF02894069.
50. Heitz P, Polak JM, Timson CM, Pearse AG (1976) Enterochromaffin cells as the endocrine source of gastrointestinal substance P. Histochem. 49: 343-347. DOI: 10.1007/bf00496138.
51. Alumets J, Håkanson R, Sundler F, Chang KJ (1978) Leu-enkephalin-like material in nerves and enterochromaffin cells in the gut. Histochem. 56: 187-196. https://doi.org/ 10.1007/BF00495979.
52. Rhee SH, Pothoulakis C, Mayer EA (2009) Principles and clinical implications of the brain–gut–enteric microbiota axis. Nature Rev. Gastroenterol. Hepatol. 6: 306-314. DOI: 10.1038/nrgastro.2009.35.
53. Shajib MS, Khan WI (2015) The role of serotonin and its receptors in activation of immune responses and inflammation. Acta Physiologica 213: 561-574. DOI: 10.1111/apha.12430.
54. Linan-Rico A, Ochoa-Cortes F, Beyder A, Soghomonyan S, Zuleta-Alarcon A, Coppola V, Christofi FL (2016) Mechanosensory signaling in enterochromaffin cells and 5-HT release: potential implications for gut inflammation. Frontiers Neurosci.10: 564. DOI: 10.3389/fnins.2016.00564.
55. Modlin IM, Kidd M, Pfragner R, Eick GN, Champaneria MC (2006) The functional characterization of normal and neoplastic human enterochromaffin cells. J. Clinic. Endocrinol. Metabol. 91: 2340-2348. https://doi.org/10.1210/jc.2006-0110.
56. Hansen MB, Witte AB (2008) The role of serotonin in intestinal luminal sensing and secretion. Acta Physiologica 193: 311-323. DOI: 10.1111/j.1748-1716.2008.01870.x.
57. Bellono NW, Bayrer JR, Leitch DB, Castro J, Zhang C, O’Donnell TA, Brierley SM, Ingraham HA, Julius D (2017) Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell 170: 185-198. DOI: 10.1016/j.cell.2017.05.034.
58. Braun T, Voland P, Kunz L, Prinz C, Gratzl M (2007) Enterochromaffin cells of the human gut: sensors for spices and odorants. Gastroenterol. 132: 1890-1901. DOI: 10.1053/j.gastro.2007.02.036.
59. Linan-Rico A, Ochoa-Cortes F, Zuleta-Alarcon A, Alhaj M, Tili E, Enneking J, Harzman A, Grants I, Bergese S, Christofi FL (2017) UTP–Gated signaling pathways of 5-HT release from BON cells as a model of human enterochromaffin cells. Front. Pharmacol. 8: 429. DOI: 10.3389/fphar.2017.00429.
60. Kim M, Cooke HJ, Javed NH, Carey HV, Christofi F, Raybould HE (2001) D-glucose releases 5-hydroxytryptamine from human BON cells as a model of enterochromaffin cells. Gastroenterol. 121:1400-1406. DOI: 10.1053/gast.2001.29567.
61. Cooke HJ, Wunderlich J, Christofi FL (2003) “The force be with you”: ATP in gut mechanosensory transduction. News Physiol. Sci. 18: 43-49. https://doi.org/10.1152/nips.01411.2002.
62. Haugen M, Dammen R, Svejda B, Gustafsson BI, Pfragner R, Modlin I, Kidd M (2012) Differential signal pathway activation and 5-HT function: the role of gut enterochromaffin cells as oxygen sensors. Am. J. Physiol-Gastr. L. 303: G1164-1173. DOI: 10.1152/ajpgi.00027.2012.
63. Costedio MM, Hyman N, Mawe GM (2007) Serotonin and its role in colonic function and in gastrointestinal disorders. Dis. Colon Rectum. 50: 376-388. DOI: 10.1007/s10350-006-0763-3.
64. Gershon MD, Tack J (2007) The serotonin signalling system: from basic understanding to drug development for functional GI disorders. Gastroenterol. 132: 397-414. DOI: 10.1053/j.gastro.2006.11.002.
65. Björnsson ES, Chey WD, Hooper F, Woods ML, Owyang C, Hasler WL (2002) Impaired gastrocolonic response and peristaltic reflex in slow-transit constipation: role of 5-HT3 pathways. Am. J. Physiol-Gastr. L. 283: G400-407. DOI: 10.1152/ajpgi.00082.2001.
66. Alcaino C, Knutson KR, Treichel AJ, Yildiz G, Strege PR, Linden DR, Li JH, Leiter AB, Szurszewski JH, Farrugia G, Beyder A (2018) A population of gut epithelial enterochromaffin cells is mechanosensitive and requires Piezo2 to convert force into serotonin release. Proc. Natl. Acad. Sci. 115: E7632-7641. DOI: 10.1073/pnas.1804938115.
67. Khan WI, Ghia JE (2010) Gut hormones: emerging role in immune activation and inflammation. Clin. Exp. Immunol. 161: 19-27. DOI: 10.1111/j.1365-2249.2010.04150.x.
68. Bogunovic M, Davé SH, Tilstra JS, Chang DT, Harpaz N, Xiong H, Mayer LF, Plevy SE (2007) Enteroendocrine cells express functional Toll-like receptors. Am. J. Physiol-Gastr. L. 292: G1770-1783. DOI: 10.1152/ajpgi.00249.2006.
69. Wang H, Steeds J, Motomura Y, Deng Y, Verma-Gandhu M, El-Sharkawy RT, McLaughlin JT, Grencis RK, Khan WI (2007) CD4+ T cell-mediated immunological control of enterochromaffin cell hyperplasia and 5-hydroxytryptamine production in enteric infection. Gut 56: 949-957. DOI: 10.1136/gut.2006.103226.
70. Spiller R (2008) Serotonin and GI clinical disorders. Neuropharmacol. 55: 1072-1080. DOI: 10.1016/j.neuropharm.2008.07.016.
71. Jun S, Kohen R, Cain KC, Jarrett ME, Heitkemper MM (2011) Associations of tryptophan hydroxylase gene polymorphisms with irritable bowel syndrome. Neurogastroent. Motil. 23: 233-e116. DOI: 10.1111/j.1365-2982.2010.01623.x.
72. Bischoff SC, Mailer R, Pabst O, Weier G, Sedlik W, Li Z, Chen JJ, Murphy DL, Gershon MD (2009) Role of serotonin in intestinal inflammation: knockout of serotonin reuptake transporter exacerbates 2, 4, 6-trinitrobenzene sulfonic acid colitis in mice. Am. J. Physiol-Gastr. L. 296: G685-695. DOI: 10.1152/ajpgi.90685.2008.
73. Pal PK, Maitra SK (2017) Neuronal control of gut melatoninergic system in carp. BAOJ Neuro. 2: 024. https://bioaccent.org/neurology/neurology24.pdf.
74. Bubenik GA, Brown G, Grota L (1977) Immuno-histological localization of melatonin in the rat digestive system. Experientia 33: 662–663. https://doi.org/10.1007/BF01946561.
75. Huether G (1996) Melatonin as an antiaging drug: Between facts and fantasy. Gerontolog. 42: 87–96. DOI: 10.1159/000213777.
76. Bubenik GA (1999) Localization and physiological significance of gastrointestinal melatonin. In: R Watson (ed.) Melatonin in Health Promotion. CRC press, Boca Raton, Florida, pp. 21–39.
77. Menendez-Pelaez A, Buzzell GR (1992) Harderian gland indoles. In: Webb SM, Hoffman RA, Puig-Domingo ML, Reiter RJ (eds.) Harderian glands: Porphyrin metabolism, behavioral, and endocrine effects. Springer, Berlin, pp. 219–234.
78. Raikhlin NT, Kvetnoy IM, Kadagidze ZG, Soko-lov AA (1976) Immunohistochemical evidence of lo-calization of melatonin and N-acetylserotonin in enterochromaffin cells. Bull Exp. Biol. Med. 82:1400–1401.
79. Raikhlin NT, Kvetnoy IM, Kadagidze ZG, Sokolov AA (1978) Immunomorphological studies on synthesis of melatonin in enterochromaffin cells. Acta Histochem. Cytochem. 11: 75–77.
80. Kvetnoy IM, Ingel IE, Kvetnaia TV Malinovskaya NK, Rapoport SI, Raikhlin NT, Trofimov AV, Yuzhakov VV (2002) Gastrointestinal melatonin: cellular identification and biological role. Neuro. Endocrinol. Lett. 23: 121–132.
81. Quay WB, Ma YH (1976) Demonstration of gastro-intestinal hydroxyindole-O-methyltransferase. IRCS Med. Sci. 4:563.
82. Stefulj J, Hörtner M, Ghosh M, Schauenstein K, Rinner I, Wölfler A, Semmler J, Liebmann PM (2001) Gene expression of the key enzymes of melatonin synthesis in extrapineal tissues of the rat. J. Pineal Res. 30: 243–247.
83. Kvetnoy IM, Yuzhakov VV (1993) Extrapinealmelato-nin: advances in microscopical identification of hormones in endocrine and non-endocrine cells. Microsc. Anal. 21: 27–29.
84. Lovenberg W, Jequier E, Sjoerdsma A (1967) Tryptophan hydroxylation: Measurement in pineal gland, brain stem and carcinoid tumor. Science 155: 217–219.
85. Balemans MGM, Bary FAM, Legerstee WC, van Benthem J (1978) Estimation of the methylating capacity in the pineal gland of the rat with special reference to the methylation of N-acetylserotonin and 5-hydroxytryptophol separately. Experientia 34: 1434–1435.
86. Voisin P, Namboodiri MAA, Klein DC (1984) Arylamine N-acetyltransferase and arylalkylamine N-acetyltransferase in the mammalian pineal gland. J. Biol. Chem. 259: 10913–10918.
87. Axelrod J, Weissbach H (1960) Enzymatic O-methylation of N-acetylserotonin to melatonin. Science 131: 1312–1312.
88. Tan DX, Manchester LC, Liu X, Rosales-Corral SA, Acuna-Castroviejo D, Reiter RJ (2013) Mitochondria and chloroplasts as the original sites of melatonin synthesis: a hypothesis related to melatonin's primary function and evolution in eukaryotes. J. Pineal Res. 54 (2): 127-38. DOI: 10.1111/jpi.12026).
89. He C, Wang J, Zhang Z, Yang M, Li Y, Tian X, Ma T, Tao J, Zhu K, Song Y, Ji P, Liu G (2016) Mitochondria synthesize melatonin to ameliorate its function and improve mice oocyte's quality under in vitro conditions. Int. J. Mol. Sci. 14: 17(6). pii: E939. DOI: 10.3390/ijms17060939.
90. Suofu Y, Li W, Jean-Alphonse FG, Jia J, Khattar NK, Li J, Baranov SV, Leronni D, Mihalik AC, He Y, Cecon E, Wehbi VL, Kim J, Heath BE, Baranova OV, Wang X, Gable MJ, Kretz ES, Di Benedetto G, Lezon TR, Ferrando LM, Larkin TM, Sullivan M, Yablonska S, Wang J, Minnigh MB, Guillaumet G, Suzenet F, Richardson RM, Poloyac SM, Stolz DB, Jockers R, Witt-Enderby PA, Carlisle DL, Vilardaga JP, Friedlander RM (2017) Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc. Natl. Acad. Sci. 114 (38): E7997-E8006. DOI: 10.1073/pnas.1705768114.
91. Dobocovich M, Mankowsha M (2005) Functional MP1 and MP2 melatonin receptors in mammals. Endocrine 27: 101–110. DOI: 10.1385/ENDO:27:2:101.
92. Boutin JA, Audinot V, Ferry G, Delagrange P (2005) Molecular tools to study melatonin pathways and actions. Trends Pharmacol. Sci. 26: 412–419. DOI:10.1016/j.tips. 2005.06.006.
93. Srinivasan V, Lauterbach EC, Ho KY, Acuña-Castroviejo D, Zakaria R, Brzezinski A (2012) Melatonin in antinociception: Its therapeutic applications. Curr. Neuropharmacol. 10: 167–178. DOI: 10.2174/157015912800604489.
94. Wang RX, Liu H, Xu L, Zhang H, Zhou RX (2015) Involvement of nuclear receptor RZR/RORγ in melatonin-induced HIF-1α inactivation in SGC-7901 human gastric cancer cells. Oncol. Rep. 34 (5): 2541-2546. DOI: 10.3892/or.2015.4238.
95. Hardeland R (2018) Review Melatonin and retinoid orphan receptors: Demand for new interpretations after their exclusion as nuclear melatonin receptors. Melatonin Res.1: 78-93. DOI: 10.32794/mr11250005.
96. Witt-Enderby PA, Radio NM, Doctor JS, Davis VL (2006) Therapeutic treatments potentially mediated by melatonin receptors: potential clinical uses in the prevention of osteoporosis, cancer and as an adjuvant therapy. J. Pineal Res. 41: 297–305. DOI: 10.1111/j.1600-079X.2006.00369.x.
97. Ahluwalia A, Brzozowska IM, Hoa N, Jones MK, Tarnawski AS (2018) Melatonin signaling in mitochondria extends beyond neurons and neuroprotection: Implications for angiogenesis and cardio/gastroprotection. Proc. Natl. Acad. Sci. 115 (9): E1942-E1943. DOI: 10.1073/pnas.1722131115.
98. Hevia D, González-Menéndez P, Quiros-González I, Miar A, Rodríguez-García A, Tan DX, Reiter RJ, Mayo JC, Sainz RM (2015) Melatonin uptake through glucose transporters: a new target for melatonin inhibition of cancer. J. Pineal Res. 58 (2): 234-250. DOI: 10.1111/jpi.12210.
99. Tan DX, Manchester LC, Qin L, Reiter RJ (2016) Melatonin: A mitochondrial targeting molecule involving mitochondrial protection and dynamics. Int. J. Mol. Sci. 17 (12): 2124. DOI: 10.3390/ijms17122124.
100. Huo X, Wang C, Yu Z, Peng Y, Wang S, Feng S, Zhang S, Tian X, Sun C, Liu K, Deng S, Ma X (2017) Human transporters, PEPT1/2, facilitate melatonin transportation into mitochondria of cancer cells: An implication of the therapeutic potential. J. Pineal Res. 62 (4). DOI: 10.1111/jpi.12390.
101. Mayo JC, Aguado A, Cernuda-Cernuda R, Álvarez-Artime A, Cepas V, Quirós-González I, Hevia D, Sáinz RM (2018) Melatonin Uptake by Cells: An Answer to Its Relationship with Glucose? Molecules 23 (8). pii: E1999. DOI: 10.3390/molecules23081999.
102. Reiter RJ (2000) Melatonin: Lowering the high price of free radicals. News Physiol. Sci. 15: 246–250.
103. Rodriguez C, Mayo JC, Sainz RM, Antolin I, Herrera F, Martin V, Reiter RJ (2004) Regulation of antioxidant enzymes: A significant role for melatonin. J. Pineal Res. 36: 1–9.
104. Pal PK, Hasan NK and Maitra SK (2016b) Temporal relationship between the daily profiles of gut melatonin, oxidative status and major digestive enzymes in carp Catla catla. Biol. Rhythm Res. 47: 755–771. DOI: 10.1080/09291016.2016.1191697.
105. Martín M, Macías M, León J, Escames G, Khaldy H, Acuña- Castroviejo D (2002) Melatonin increases the activity of the oxidative phosphorylation enzymes and the production of ATP in rat brain and liver mitochondria. Int. J. Biochem. Cell. Biol. 34: 348–357. https://doi.org/10.1016/S1357-2725(01)00138-8.
106. Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre-Jimenez M, Qin L (2016) Melatonin as an antioxidant: under promises but over delivers. J. Pineal Res. 61: 253–278. DOI: 10.1111/jpi.12360.
107. Reiter RJ, Tan DX, Manchester LC, Qi W (2001) Biochemical reactivity of melatonin with reactive oxygen and reactive nitrogen species: A review of the evidence. Cell. Biochem. Biophys. 34: 237–256. DOI: 10.1385/CBB:34:2:237.
108. Tan DX, Reiter RJ, Manchester LC, Yan MT, El-Sawi M, Sainz RM, Mayo JC, Kohen R, Allegra M, Hardeland R (2002) Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr. Top. Med. Chem. 2: 181-197. https://www.ncbi.nlm.nih.gov/pubmed/11899100.
109. Cuzzocrea S, Reiter JR (2002) Pharmacological action of melatonin in acute and chronic inflammation. Curr. Top. Med. Chem. 2: 153–165. DOI: 10.2174/ 1568026023394425.
110. Manchester LC, Coto-Montes A, Boga JA, Andersen LP, Zhou Z, Galano A, Vriend J, Tan DX, Reiter RJ (2015) Melatonin: an ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 59: 403–419. DOI: 10.1111/jpi.12267.
111. Tan DX, Reiter RJ (2019) Mitochondria: the birth place, battle ground and the site of melatonin metabolism in cells. Melatonin Res. 2: 44-66. DOI: https://doi.org/https:// doi.org/10.32794/mr11250011.
112. Torres-Farfan C, Richter HG, Rojas-García P, Vergara M, Forcelledo ML, Valladares LE, Torrealba F, Valenzuela GJ, Seron- Ferre M (2003) mt1 Melatonin receptor in the primate adrenal gland: inhibition of adrenocorticotropin-stimulated cortisol production by melatonin. J. Clin. Endocrinol. Metab. 88: 450–458. DOI: 10.1210/jc.2002-021048.
113. Saito S, Tachibana T, Choi YH, Denbow DM, Furuse M (2005) ICV melatonin reduces stress responses in neonatal chicks. Behav. Brain Res. 165: 197–203. DOI: 10.1016/j.bbr.2005.06.045.
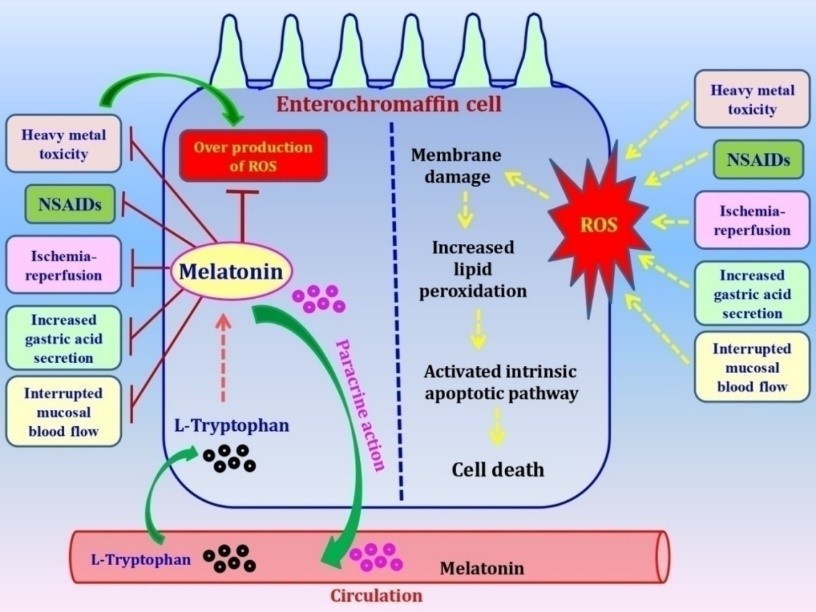
114. Brozozowski T, Konturek PC, Konturek SJ, Bubenik GA (1997) The role of melatonin and L-tryptophan in prevention of acute gastric lesions induced by stress, ethanol, ischemia and aspirin. J. Pineal Res. 23: 79–89. https://pdfs.semanticscholar.org/ e851/bd31b7bf03f282a83ade3193e1f76aa6338d.pdf.
115. Konturek PC, Konturek SJ, Majka J, Zembala M, Hahn EG (1997) Melatonin affords protection against gastric lesions induced by ishemia-reperfu-sion possibly due to its antioxidant and muco-sal microcirculatory effects. Eur. J. Pharmacol. 322: 73–77.DOI: 10.1016/S0014-2999(97)00051-4.
116. Cabeza J, Alarcón-de-la-Lastra C, Jiménez D, Martín MJ, Motilva V (2003) Melatonin modulates the effects of gastric injury in rats: role of prostaglandins and nitric oxide. Neurosignals 12: 71–77. DOI: 10.1159/000071816.
117. Ates B, Yilmaz I, Geckil H, Iraz M, Birincioglu M, Fiskin K (2004) Protective role of melatonin given either before ischemia or prior to reperfusion on intestinal ischemia-reperfusion damage. J. Pineal Res. 37: 149–152. DOI:10.1111/j.1600079X.2004. 00148.x.
118. Sileri P, Sica GS, Gentileschi P, Venza M, Benavoli D, Jarzembowski T, Manzelli A, Gaspari AL (2004) Melatonin reduces bacterial translocation after intestinal ischemia-reperfusion injury. Transplant Proc. 36: 2944–2946. DOI:10.1016/j.transproceed. 2004.10.085.
119. Ustundag B, Kazez A, Demirbag M, Canatan H, Halifeoglu I, and Ozercan IH (2000) Protective effect of melatonin on antioxidative system in experimental ischemia-reperfusion of rat small intestine. Cell Physiol. Biochem. 10: 229–236. DOI: 10.1159/000016354.
120. Ozacmak VH, Sayan H, Arslan SO, Altaner S, Aktas RG (2005) Protective effect of melatonin on contractile activity and oxidative injury induced by ischemia and reperfusion of rat ileum. Life Sci. 76: 1575–1588. DOI: 10.1016/j.lfs.2004.08.031.
121. Ganguly K, Maity P, Reiter RJ, Swarnakar S (2005) Effect of melatonin on secreted and induced matrix metalloproteinase -9 and -2 activity during prevention of indomethacin-induced gastric ulcer. J. Pineal Res. 39: 307–315. DOI: 10.1111/j.1600-079x.2005.00250.x.
122. Ganguly K, Kundu P, Banerjee A, Reiter RJ, Swarnakar S (2006) Hydrogen peroxide-mediated down regulation of matrixmetalloprotease-2 in indomethacin-induced acute gastric ulceration is blocked by melatonin and other antioxidants. Free Radic. Biol. Med. 41: 911–925. https://doi.org/10.1016/j.freeradbiomed.2006.04.022.
123. Poeggeler B, Reiter RJ, Hardeland R, Sewerynek E, Melchiorri D, Barlow-Walden LR (1995) Melatonin, a mediator of electron transfer and repair reactions acts synergistically with the chain breaking antioxidants ascorbate trolox and glutathione. Neuroendocrinol. Lett. 17: 87-92. DOI: 10.1186/1743-7075-2-22.
124. Taslidere E, Vardi N, Parlakpinar H, Yıldız A, Taslidere B, Karaaslan MG (2018) Effects of melatonin on acetylsalicylic acid induced gastroduodenal and jejunal mucosal injury. Biotech. Histochem. 93: 485-495. DOI: 10.1080/10520295.2018.1442020.
125. Tan DX, Manchester LC, Terron MP, Flores LJ, Reiter RJ (2007) One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 42 (1): 28-42. DOI: 10.1111/j.1600-079X.2006.00407.x.
126. Tordjman S, Chokron S, Delorme R, Charrier A, Bellissant E, Jaafari N, Fougerou C (2017) Melatonin: Pharmacology, functions and therapeutic benefits. Curr. Neuropharmacol. 15 (3): 434–443. DOI: 10.2174/1570159X14666161228122115.
127. Reiter RJ, Tan DX, Rosales-Corral S, Galano A, Zhou XJ, Xu B (2018) Mitochondria: Central organelles for melatonin's antioxidant and anti-aging actions. Molecules 23 (2). pii: E509. DOI: 10.3390/molecules23020509.
128. Jenwitheesuk A, Nopparat C, Mukda S, Wongchitrat P, Govitrapong P (2014) Melatonin regulates aging and neurodegeneration through energy metabolism, epigenetics, autophagy and circadian rhythm pathways. Int. J. Mol. Sci. 15: 16848-16884. DOI:10.3390/ijms150916848.
129. Rehman SU, Ikram M, Ullah N, Alam SI, Park HY, Badshah H, Choe K, Kim MO (2019) Neurological enhancement effects of melatonin against brain injury-induced oxidative stress, neuroinflammation, and neurodegeneration via AMPK/CREB signaling. Cells 8: 760. DOI:10.3390/cells8070760.
130. Bonnefont-Rousselot D, Collin F (2010) Melatonin: action as antioxidant and potential applications in human disease and aging. Toxicology 278 (1): 55-67. DOI: 10.1016/j.tox.2010.04.008.
131. Hacışevki A, Baba B (2018) An overview of melatonin as an antioxidant molecule: a biochemical approach. In: Dragoi CM (Ed.) Melatonin molecular biology, clinical and pharmaceutical approaches Pp. 59-85. DOI: 10.5772/intechopen.79421.
132. Faixi S, Faixova Z, Boldizarova K, Javorsky P (2005) The effect of long-term high heavy metal intake on lipid peroxidation of gastrointestinal tissue in sheep. Vet. Med. Czech. 50 (9): 401–405.
133. Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE (2014) Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 94: 329–354. DOI:10.1152/physrev.00040.2012.
134. León J, Acuña-Castroviejo D, Escames G, Tan DX, Reiter RJ (2005) Melatonin mitigates mitochondrial malfunction. J. Pineal Res. 38: 1–9. DOI: 10.1111/j.1600-079x.2004.00181.x.
135. Xu S, Pi H, Zhang L, Zhang N1, Li Y, Zhang H, Tang J, Li H, Feng M, Deng P, Guo P, Tian L, Xie J, He M, Lu Y, Zhong M, Zhang Y, Wang W, Reiter RJ, Yu Z, Zhou Z (2016) Melatonin prevents abnormal mitochondrial dynamics resulting from the neurotoxicity of cadmium by blocking calcium-dependent translocation of Drp1 to the mitochondria. J. Pineal Res. 60: 291-302. DOI: 10.1111/jpi.12310.
136. Mitra E, Bhattacharjee B, Pal PK, Ghosh AK, Mishra S, Chattopadhyay A, Bandyopadhyay D (2019) Melatonin protects against cadmium-induced oxidative damage in different tissues of rat: a mechanistic insight. Melatonin Res. 2: 1-21. DOI: 10.32794/mr11250018.
137. Li M, Pi H, Yang Z, Reiter RJ, Xu S, Chen X, Chen C1, Zhang L, Yang M, Li Y, Guo P1, Li G, Tu M, Tian L, Xie J, He M, Lu Y, Zhong M, Zhang Y, Yu Z, Zhou Z (2016) Melatonin antagonizes cadmium-induced neurotoxicity by activating the transcription factor EB-dependent autophagy-lysosome machinery in mouse neuroblastoma cells. J. Pineal Res. 61: 353-369. DOI: 10.1111/jpi.12353.
138. Sener G, Sehirli AO, Ayanoglu-Dülger G (2003) Melatonin protects against mercury (II)-induced oxidative tissue damage in rats. Pharmacol. Toxicol. 93: 290-296. DOI: 10.1111/j.1600-0773.2003.pto930607.x.
139. Zhang Y, Wei Z, Liu W, Wang J, He X, Huang H, Zhang J, Yang Z (2017) Melatonin protects against arsenic trioxide-induced liver injury by the upregulation of Nrf2 expression through the activation of PI3K/AKT pathway. Oncotarget 8: 3773–3780. DOI: 10.18632/oncotarget.13931.
140. Mishra S, Ghosh D, Dutta M, Chattopadhyay A, Bandyopadhyay D (2013) Melatonin protects against lead-induced oxidative stress in stomach, duodenum and spleen of male Wistar rats. J. Pharm. Res.1: 997-1004.
141. Pandi-Perumal SR, Srinivasan V, Maestroni GJ, Cardinali DP, Poeggeler B, Hardeland R (2006) Melatonin: Nature's most versatile biological signal? FEBS J. 273: 2813–2838. DOI: 10.1111/j.1742-4658.2006.05322.x.
142. Konturek PC, Konturek SJ, Burnat G, Brzozowski T, Brzozowska I, Reiter RJ (2008) Dynamic physiological and molecular changes in gastric ulcer healing achieved by melatonin and its precursor L-tryptophan in rats. J. Pineal Res. 45: 180–190. DOI: 10.1111/j.1600-079x.2008.00574.x.
143. Kusuhura H, Komatsu H, Sumichika H, Sugahara K (1999) Reactive oxygen species are involved in the apoptosis induced by nonsteroidal anti-inflammatory drugs in cultured gastric cells. Eur. J. Pharmacol. 383: 331–337. https://doi.org/10.1016/S0014-2999(99)00599-3.
144. Mei Q, Diao L, Xu J, Liu X, Jin J (2011) A protective effect of melatonin on intestinal permeability is induced by diclofenac via regulation of mitochondrial function in mice. Acta Pharmacol. Sin. 32: 495–502. DOI: 10.1038/aps.2010.225.
145. Bandyopadhyay D, Ghosh G, Bandyopadhyay A, Reiter RJ (2004) Melatonin protects against piroxicam-induced gastric ulceration. J. Pineal Res. 36: 195–203. https://doi.org/ 10.1111/j.1600-079X.2004.00118.x.
146. Brzozowska I, Konturek PC, Brzozowski T, Konturek SJ, Kwiecien S, Pajdo R, Drozdowicz D, Pawlik M, Ptak A, Hahn EG (2002) Role of prostaglandins, nitric oxide, sensory nerves and gastrin in acceleration of ulcer healing by melatonin and its precursor, L-tryptophan. J. Pineal Res. 32: 149–162. https://doi.org/10.1034/j.1600-079x.2002.1o811.x.
147. Brzozowski T, Konturek PC, ZwirskaKorczala K, Konturek SJ, Brzozowska I, Drozdowicz D, Sliwowski Z, Pawlik M, Pawlik WW, Hahn EG (2005) Importance of the pineal gland, endogenous prostaglandins and sensory nerves in the gastroprotective actions of central and peripheral melatonin against stress-induced damage. J. Pineal Res. 39: 375–385. https://doi.org/10.1111/j.1600-079X.2005.00264.x.
148. Lissoni P, Rovelli F, MeregalliS, Fumagalli L, Musco F, Brivio F, Brivio O, Esposti G (1997) Melatonin as a new possible anti-inflammatory agent. J. Biol. Regul. Homeost. Agents. 11: 157–159.
149. Cuzzocrea S, Reiter JR (2002) Pharmacological action of melatonin in acute and chronic inflammation. Curr. Top. Med. Chem. 2: 153–165. DOI: 10.2174/1568026023394425.
150. Galano A, Tan DX, Reiter RJ (2011) Melatonin as a natural ally against oxidative stress: a physicochemical examination. J. Pineal Res. 51: 1–16. DOI: 10.1111/j.1600-079X.2011.00916.x.
151. Carrillo-Vico A, Lardone PJ, Alvarez-Sánchez N, Rodríguez-Rodríguez A, Guerrero JM (2013) Melatonin: buffering the immune system. Int. J. Mol. Sci. 14: 8638–8683. DOI: 10.3390/ijms14048638.
152. Agil A, Reiter RJ, Jiménez-Aranda A, Ibán-Arias R, Navarro-Alarcón M, Marchal JA, Adem A, Fernández-Vázquez G (2013) Melatonin ameliorates low-grade inflammation and oxidative stress in young Zucker diabetic fatty rats. J. Pineal Res. 54: 381–388. DOI: 10.1111/jpi.12012.
153. Dong Y, Fan C, Hu W, Jiang S, Ma Z, Yan X, Deng C, Di S, Xin Z, Wu G, Yang Y, Reiter RJ, Liang G (2016) Melatonin attenuated early brain injury induced by subarachnoid hemorrhage via regulating NLRP3 inflammasome and apoptosis signalling. J. Pineal Res. 60: 253–262. DOI: 10.1111/jpi.12300.
154. Cuzzocrea S, Reiter RJ (2001) Pharmacological action of melatonin in shock, inflammation and ischemia/reperfusion injury. Eur. J. Pharmacol. 426: 1–10. https://doi.org/10.1016/S0014-2999(01)01175-X.
155. Li JH, Yu JP, Yu HG Xi-Ming Xu, Liang-Liang Yu, Jin Liu, He-Sheng Luo (2005) Melatonin reduces inflammatory injury through inhibiting NF-kappaB activation in rats with colitis. Mediators Inflamm. 2005: 185–193. DOI: 10.1155/MI.2005.185.
156. Mauriz JL, Collado PS, Veneroso C, Reiter RJ, González-Gallego J (2013) A review of the molecular aspects of melatonin’s anti-inflammatory actions: recent insights and new perspectives. J. Pineal Res. 54: 1–14. DOI: 10.1111/j.1600-079X.2012.01014.x.
157. Vriend J, Reiter RJ (2014) Melatonin as a proteasome inhibitor. Is there any clinical evidence? Life Sci. 115: 8–14. DOI: 10.1016/j.lfs.2014.08.024.
158. Najafi M, Shirazi A, Motevaseli E, Rezaeyan AH, Salajegheh A, Rezapoor S (2017) Melatonin as an anti-inflammatory agent in radiotherapy. Inflammopharmacol. 25: 403-413. DOI 10.1007/s10787-017-0332-5.
159. Hu ZP, Fang XL, Fang N, Wang XB, Qian HY, Cao Z, Cheng Y, Wang BN, Wang Y (2013) Melatonin ameliorates vascular endothelial dysfunction, inflammation, and atherosclerosis by suppressing the TLR4/NF-kB system in high-fat-fed rabbits. J. Pineal Res. 55: 388–398. DOI: 10.1111/jpi.12085.
160. Akinrinmade FJ, Akinrinde AS, Amid A (2016) Changes in serum cytokine levels, hepatic and intestinal morphology in aflatoxin B1-induced injury: modulatory roles of melatonin and flavonoid-rich fractions from Chromolenaodorata. Mycotoxin Res. 32:53-60.DOI 10.1007/s12550-016-0239-9.
161. Carrillo-Vico A, García-Mauriño S, Calvo JR, Guerrero JM (2003) Melatonin counteracts the inhibitory effect of PGE2 on IL-2 production in human lymphocytes via its mt1 membrane receptor. FASEB J. 17: 755–757.
162. Favero G, Franceschetti L, Bonomini F, Rodella LF, Rezzani R (2017) Melatonin as an anti-inflammatory agent modulating inflammasome activation. Int. J. Endocrinol. 2017: 1835195. https://doi.org/10.1155/2017/1835195.
163. Hardeland R, Cardinali DP, Srinivasan V, SpenceDW, Brown GM, Pandi-Perumal SR, (2011) Melatonin—a pleiotropic, orchestrating regulator molecule. Progress Neurobiol. 93: 350–384.
164. Park YS, Chung SH, Lee SK, Kim JH, Kim JB, Kim TK, Kim DS, Baik HW (2015) Melatonin improves experimental colitis with sleep deprivation. Int. J. Mol. Med. 35: 979-986. DOI: 10.3892/ijmm.2015.2080.
165. Tahan G, Gramignoli R, Marongiu F, Aktolga S, Cetinkaya A, Tahan V, Dorko K (2011) Melatonin expresses powerful anti-inflammatory and antioxidant activities resulting in complete improvement of acetic-acid-induced colitis in rats. Dig. Dis. Sci. 56: 715-720. DOI: 10.1007/s10620-010-1364-5.
166. Zhu D, Ma Y, Ding S, Jiang H, Fang J (2018) Effects of melatonin on intestinal microbiota and oxidative stress in colitis mice. Biomed. Res. 2018: 2607679. DOI: 10.1155/2018/2607679.
167. Paulose JK, Cassone VM (2016) The melatonin-sensitive circadian clock of the enteric bacterium Enterobacter aerogenes. Gut Microbes. 7: 424-427. DOI: 10.1080/19490976.2016.1208892.
168. Yin J, Li Y, Han H, Chen S, Gao J, Liu G, Wu X, Deng J, Yu Q, Huang X, Fang R (2018) Melatonin reprogramming of gut microbiota improves lipid dysmetabolism in highfat dietfed mice. J. Pineal Res. 65: e12524. DOI: 10.1111/jpi.12524.
169. Bang CS, Yang YJ, Baik GH (2019) Melatonin for the treatment of gastroesophageal reflux disease; protocol for a systematic review and meta-analysis. Medicine (Baltimore). 98: e14241. DOI: 10.1097/MD.0000000000014241.
170. Gao J, Xu K, Liu H, Liu G, Bai M, Peng C, Li T and Yin Y (2018) Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front. Cell Infect. Microbiol. 8: 13. DOI: 10.3389/fcimb.2018.00013.
171. Chojnacki C, Wiśniewska-Jarosińska M, Kulig G, Majsterek I, Reiter RJ, Chojnacki J (2013) Evaluation of enterochromaffin cells and melatonin secretion exponents in ulcerative colitis. World J. Gastroenterol. 19: 3602-3607. DOI:10.3748/wjg.v19.i23.3602.
172. Brzezinski A (1997) Melatonin in humans. N. Engl. J. Med. 336: 186–195. DOI: 10.1056/NEJM199701163360306.
173. Bubenik GA, Dhanvantari S (1989) Influence of serotonin and melatonin on some parameters of gastrointestinal activity. J. Pineal Res. 7: 333–344. DOI: 10.1111/j.1600-079x.1989.tb00909.x
174. Lewinski A, Rybicka I, Wajs E, Szkudlinski M, Pawlikowski M (1991) Influence of pineal indol-amines on the mitotic activity of gastric and colonic mucosa epithelial cells in the rat: Interaction with omeprazole. J. Pineal Res. 10: 104–108.DOI: 10.1111/j.1600-079x.1991.tb00018.x.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


